Abstract
Masking, measured as a decrease in nocturnal rodent wheelrunning, is a visual system response to rod/cone and retinal ganglion cell photoreception. Here, we show that a few milliseconds of light are sufficient to initiate induce masking which continues for many minutes without additional photic stimulation. C57J/B6 mice were tested using flash stimuli previously shown to elicit large circadian rhythm phase shifts. Ten flashes, 2 msec each and equally distributed over 5 min, activate locomotor suppression that endures for an additional 25-35 min in the dark and does not differ in magnitude or duration from that elicited by 5 min saturating light pulse. Locomotor activity by mice without access to running wheels is also suppressed by light flashes. The effectiveness of various light flash patterns on mouse locomotor suppression are similar to those previously described for hamster phase shifts. Video analysis of active mice indicates that light flashes initiated at ZT13 rapidly induce an interval of behavioral quiescence that lasts about 10 min at which time the animals assume a typical sleep posture that is maintained for an additional 25 min. Thus, the interval coinciding with light-induced wheelrunning suppression appears to consist of two distinct behavioral states, one interval during which locomotor quiescence is initiated and maintained, followed by a second interval characterized by behavioral sleep. Given this sequence effected by light stimulation, we suggest that it be referred to as “photosomnolence,” the term reflecting upon both nature of the stimulus and the associated behavioral change.
Keywords: circadian, locomotion, wheelrunning, sleep, wake, retinohypothalamic
“Masking” refers to a change in magnitude of a rhythmic variable induced by an environmental stimulus (Minors and Waterhouse, 1989;Mrosovsky, 1999;Redlin, 2001;Rensing, 1989). Negative masking is one variant typically documented in nocturnal rodents as a light-induced reduction of wheelrunning (Mrosovsky et al., 1999;Redlin and Mrosovsky, 1999b). Such suppression of locomotion has generally been considered an acute inhibitory response “confined to the time when the light is present” (Redlin and Mrosovsky, 1999b) and is thought to represent an effect of the stimulus on a rhythmic output, specifically not involving a central pacemaker (Aschoff, 1960;Mrosovsky, 1999;Redfern et al., 1994). Masking has also been considered an extraneous impediment to the understanding of circadian rhythms. For example, masking can interfere with the interpretation of gene knockout effects or results from studies involving photic and nonphotic entrainment (e.g., Albers et al., 1982;Mrosovsky, 1989;Van der Horst et al., 1999).
With the notable exception of Mrosovsky and colleagues, few investigators have considered the biological basis of masking. The phenomenon is itself expressed as a circadian rhythm with maximal response early in the subjective night (Redlin and Mrosovsky, 1999b). In a typical test, animals are exposed to a 1 hr light pulse shortly after lights off, with locomotor activity during the pulse expressed relative to an earlier baseline (Mrosovsky et al., 1999). Light-induced masking and circadian rhythm phase control are accomplished via photic input pathways involving rod and cone photoreceptors in the outer retina and intrinsically photoreceptive retinal ganglion cells (ipRGCs) in the inner retina (Hattar et al., 2003;Mrosovsky et al., 2001;Mrosovsky and Hattar, 2003;Thompson et al., 2008). Both masking and circadian rhythm phase control by light can be elicited in the absence of either rods/cones or functional ipRGCs, but not both (Hattar et al., 2003;Panda et al., 2003). Moreover, both behaviors are eliminated by physical loss of ipRGCs despite functioning rod/cone photoreception (Gõz et al., 2008;Guler et al., 2008;Hatori et al., 2008).
The prevailing view that phase shifting effects of light occur only in response to stimulus durations for which there is reciprocity between intensity and duration (Dkhissi-Benyahya et al., 2000;Muscat and Morin, 2005;Nelson and Takahashi, 1991) has been challenged by studies employing millisecond (flash) light stimuli (Vidal and Morin, 2007). Such stimuli elicit circadian rhythm phase shifts of 60-80 min. In hamsters, they also elicit robust masking that endures many minutes beyond the last flash. Flash stimuli that effectively induce both masking and phase shifts also elicit abundant FOS protein induction in SCN neurons (Vidal and Morin, 2007).
A fundamental issue emerges from the functional and anatomical similarities between the input pathways underlying light-induced masking and circadian rhythm phase shifts. This concerns the separability of the two phenomena because, at the present time, it is not possible to identify a point of divergence in the pathway(s) regulating them. As an initial approach to this issue, we have conducted parametric investigations with flash stimulus patterns to determine how the light-induced locomotor suppression varies with flash number, inter-flash interval, or when flashes are added to a 5 min masking pulse. The data show that light rapidly elicits locomotor suppression which continues for many minutes beyond the eliciting stimulus. Analysis of behavior during the interval of reduced locomotion indicates that light-induced sleep is a likely component of the masking response.
METHODS
Adult male C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed in individual plastic cages under a 12-h light /12-h dark photoperiod (LD 12:12) with free access to food and water for at least 2 wks before being assigned to an experiment. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Stony Brook University.
For all experiments, each animal was placed in a 45 L × 20 W × 20 H cm clear polycarbonate home cage containing sawdust bedding and a 16.5 cm diameter stainless steel running wheel with an axle that closed a microswitch once per revolution. Some cages had a Coral Plus passive infrared (PIR) detection device (Visonic Inc, Bloomfield, CT) mounted to detect general activity in the cage. Mice were allowed to stably entrain to a LD 12:12 photoperiod at least 7 days before their first test. The running wheel cages were placed on a 5 shelf stainless steel rack, 4 or 5 cages per shelf, with the lowest shelf 20 cm above the floor and 34 cm between shelves.
As indicated below, the entire rack of animals was illuminated during each exposure to the masking stimulus with a theoretical yield of 24 animals per test. However, not all animals generated running records of equivalent quality and, to be included in any analysis involving wheelrunning, the animal’s data on the day of the test was required to meet three criteria: (1) during the 30 min prior to light stimulation, there could be no more than 10 min with zero counts; (2) during the 5 min prior to stimulation, there could be no more than 3 min with zero counts; and (3) the last 2 min prior to stimulation could not both have zero counts. As a result, N=18-24 animals contributed to the data for each group in experiments 1-5 below.
Light Stimulation
Light stimuli used for masking were, unless otherwise indicated, initiated 1 hr after lights off (ZT13 ). All stimuli were white light with unknown spectra. The “flash” stimuli were generated by a DynaLite Flash Head (model 2040) mounted in an animal colony room and directed at the center of animal cage rack, approximately 2 m across the room. The Dynalite Flash Head was powered by a DynaLite M1000er power supply (DynaLite, Union, NJ), the same combination employed previously(van den Pol et al., 1998;Vidal and Morin, 2007). The duration of each flash was 2 msec, as indicated by the manufacturer’s specifications. Unless otherwise stated, the irradiance of each flash was approximately 0.36 J/m2 (6.5 ×1011 lux) and the animals were exposed directly to the flashes from the flash head without intervening filters, in accordance with previous procedures. When variation in flash irradiance was necessary, neutral density filters (ND; Filmtools, Burbank, CA) and a leaf diaphragm were placed in the light path. Irradiance levels for all experiments were verified with a Gigahertz-Optik P-9710 photometer (Newburyport, MA) that has the capability of measuring millisecond light stimuli. When light “pulses” (5 min, in these studies) were employed, the source was a 100 watt incandescent reflector bulb (GE type 6E) with an irradiance of about 40 μW/cm2 (78 lux) in each cage. Light levels, measured immediately in front of each cage with the photodetector facing the source, did not vary by more than 2% of the value measured in front of the cage most directly in line with the light source. The animal housing room was painted white.
Timing and number of light stimuli were computer-controlled using custom software. In addition, no attempt was made to insure that each animal was exposed to the photic stimuli in an identical manner. Thus, if an animal faced away from the light source or had its eyes closed, the actual stimulus reaching the retina would differ from the measured value and contribute to the experimental variability. In a typical experiment, the flash-control computer would be set to initiate flashes at ZT13 on the current or next day. Pin photodiodes monitored the actual occurrence of each flash or other light stimulus and, when activated, sent a signal to the data collection computer.
Locomotor Data Collection and Reduction
Each rotation of the running wheel or movement within the field of PIR device closed a switch. Closure was detected by the data collection computer via custom software (WinCollectRT written by Glenn Hudson, Stony Brook University) which was also used for data reduction and export. Running records were plotted in typical raster format for visual evaluation after reducing the daily 1440 min of data to 288 bins of 5 min, with activity indicated if the number of wheel revolutions exceeded zero. PIR-detected activity was similarly plotted. However, the activity counts only registered about 1-2% of the activity detected by the running wheel, a difference probably attributable to the small size of the mice. PIR-detected counts provide a good index of general activity and departure from that level, but unlike the running wheel data, do not accurately reflect total distance moved in the cage.
For analysis of masking, the 1440 min data day of interest was exported to a spreadsheet where the data set for each animal per test group was reduced to 150 min (30 min before stimulus onset (the “baseline”) and 120 min after, with the time of light indicated by the pin photodiode signal). This was further modified for analysis as needed. The computer clock controlling stimulus timing was not electronically synchronized with the data collection computer clock. This, plus the use of 1 min bins for data collection, limited wheelrunning detection relative to stimulus onset to about 1 min resolution.
Assessment of masking
Two methods were most useful. The first consisted of a pictorial representation of relative locomotor activity level before, during and after various stimulus presentations. To obtain the pattern of masking for a particular test group, the median number of wheel revolutions per minute of the baseline was calculated for each animal. For each 5 min interval thereafter, the median wheel revolutions per minute were obtained, with this value expressed as a percentage of the baseline. This method provided a clear indication of both individual and group performance across time, but was not particularly conducive to statistical analysis because of the repeated measure variable and the frequent need for non-parametric statistical analysis. The second method combined both the effect of the stimulus on the amount of locomotion with the temporal characteristic of such an effect. This method simply summed the number of minutes, for each animal in a particular group, during which the wheel revolutions were equal to zero, referred to as “zero minutes.” Unless stated otherwise, the summation of zero minutes began with the onset of the stimulus and continued for 30 min. If the data violated either a normality or equal variance test, non-parametric statistics were used (Kruskall-Wallis one-way analysis of variance by ranks and Dunn’s test for post-hoc analysis). Otherwise, parametric analysis of variance was followed by Bonferroni tests. Statistics were calculated with SigmaStat v. 3.0.1 (Systat Software, San Jose, CA).
Preliminary Study: Flashes induce phase shifts and masking in mice
Mice entrained to LD12:12 were moved into constant dark (DD). After 7 days of DD, they were exposed to 10 flashes equally spaced over 5 min beginning at approximately circadian time 13 (CT13). This was followed by an additional 2 wks in DD. Phase shift magnitude and masking were both assessed as responses to the identical stimulus. Phase shift magnitude was measured using documented methods (Muscat and Morin, 2005). Briefly, a line was eye fitted through activity onsets for least 5 days prior to the stimulus and through onsets for at least 5 days afterward. Phase shift magnitude was calculated as the time difference between the two lines, measured on the day the stimulus was presented.
Experiment 1: Flash number and inter-flash interval vary
Light flashes (0, 1, 2, 3, 4, 5, 10, 20 or 100 per group) were delivered over 300 sec with the inter-flash interval (IFI) for more than 1 flash calculated as 300 sec/(N-1 flashes). Masking was assessed with respect to the number of flashes in the series.
Experiment 2: Flash number varies; inter-flash interval is constant
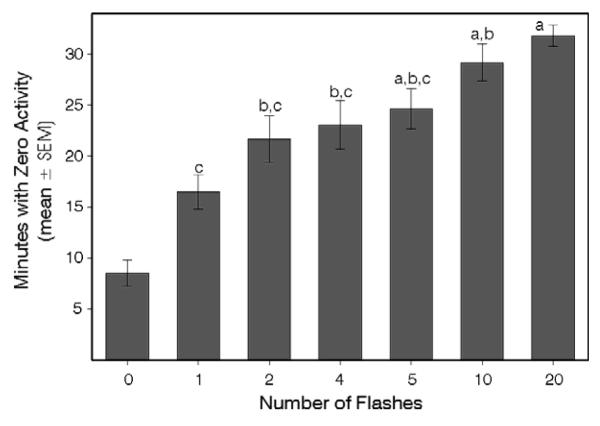
Mice were exposed to 0, 1, 2, 4, 5, 10 or 20 flashes beginning at ZT13. The IFI was fixed at 16 sec for all groups receiving 2 or more flashes and the effect of flash number on masking was assessed.
Experiment 3: Flash number is constant; inter-flash interval is short
Mice were exposed to 10 flashes with IFI set to 0.5, 1, 4, 8 or 16 sec; a 5 min light pulse group was also included. The IFI of 0.5 sec was the shortest possible with this device. The effect of short IFIs on masking was determined.
Experiment 4: Flash number is constant; inter-flash interval is long
Mice were exposed to 10 flashes with IFI set to 30, 60, 120, 240, or 480 sec (corresponding to total stimulus series delivery times of 4.5, 9, 18, 36 and 72 min, respectively). The effects of these long IFIs on masking were measured.
Experiment 5: Flash number and prolongation of masking
Mice were exposed to a 5 min light pulse beginning at ZT13. Based on data from Experiment 2 showing the time course of wheelrunning recovery after a 5 min pulse, mice were exposed to 0, 1, 2, 4, 5, 10 or 20 flashes beginning at ZT1325 (IFI = 16 sec), while masking magnitude was still maximal.
Experiment 6: Change in behavior associated with masking
Despite broad knowledge about the effects of acute nocturnal light on phase shifting or masking, there is little explicit information concerning light-induced change in other behavior. In this experiment, mice (N=9) were exposed to 10 flashes delivered over 5 min while having access to a running wheel, with locomotion simultaneously monitored by PIR. A second test included measurement of locomotion only by PIR (the running wheel was locked).
Mice (N=6) with access to running wheels and exposed to the same stimulus series were also videotaped under infrared illumination. The video was subsequently viewed and the behavior transcribed using EthoLog software (Ottoni, 2000; http://www.ip.usp.br/ebottoni/EthoLog/ethohome.html). The behaviors monitored were sleep posture, holding still, grooming, eating, drinking, scratching/digging, rearing, climbing, walking around the cage, running around the cage, or running in the wheel. The sleep posture consists of no gross movements, few fine muscle twitches, hunched or curled posture with no limb or head resisting gravity(Bergmann et al., 1987).
RESULTS
Preliminary Study
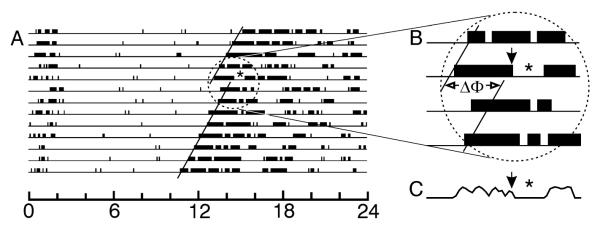
The stimulus series of 10 flashes administered to mice (N=28) at equal intervals across 5 min beginning at CT13 induced immediate suppression of wheelrunning and robust phase delays of the circadian locomotor rhythm (Fig. 1). Mean phase delay was 2.2 ± 0.2 hr (median = 2.4 hr, 25%ile=1.5 and 75%ile=2.9 hr). Masking, as indicated by the number of “zero minutes” during the 60 min after the initial flash averaged (± SEM) 45.4 ± 2.1 min (median = 45.0 min, 25%ile=39.0 and 75%ile=55.5 min). This study also demonstrated that masking studies of freerunning animals are much more difficult to conduct than when animals are entrained. There is a substantially greater likelihood of various types of experimental error and studies take much longer to complete. Therefore, all experiments proper were performed on mice held in LD conditions.
Figure 1.
Interval of wheelrunning suppression and phase shift are simultaneously induced by millisecond light flash stimulation in a mouse. (A) Running record of a mouse in constant dark. Solid lines indicate activity onsets before and after the light stimulus which consisted of 10 flashes, each 2 msec, delivered equally spaced across 5 min. (B) Enlarged portion of the running record showing the time of light stimulation, consequent interval of locomotor suppression and the associated phase shift (ΔΦ). (C) Plot of the wheel revolutions per 5 min bin during the interval corresponding to the running record in (B) on the day of light stimulation. Flashes are associated with a rapid drop in wheelrunning. The asterisk (*) in (A-C) indicates the interval of wheelrunning suppression. The black arrows in (B) and (C) indicate the time of the stimulus.
Experiment 1: Flash number and inter-flash interval vary
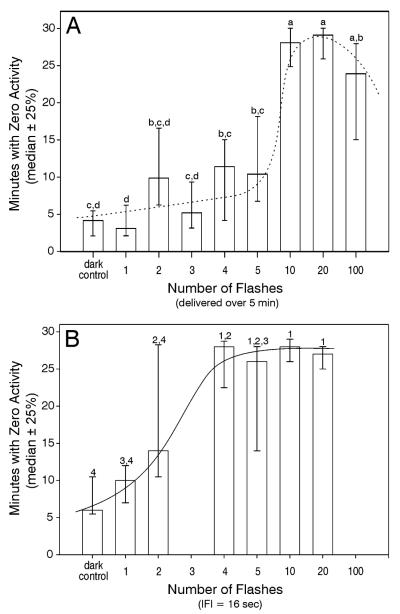
There was a strong effect of flashes on wheelrunning suppression when both flash number and inter-flash interval were allowed to vary (Fig. 2A; p<.001, Kruskall-Wallis one way ANOVA on ranks), as indicated by the number of zero minutes. Suppression was maximal in mice exposed to 10 or more flashes. The large variability in response to 2-5 flashes suggests an effect of those flashes that may be related to individual sensory or response differences. Dark control mice were exposed to the identical conditions as the other groups, except that the flash head was covered with opaque material. Control mice in this and other experiments typically had about 6-8 minutes with no wheel running. These minutes were scattered and not in a single block beginning in association with stimulus presentation. The results show that only a few flashes are necessary to suppress wheelrunning for a prolonged period (implicit in the measure of total zero minutes).
Figure 2.
Wheelrunning suppression varies with the number of flashes and also depends on the IFI. (A) Expt. 1: As the number of flashes delivered over a 5 min interval increases from 1 to 5, there is little change in the extent of wheelrunning suppression. Response jumps to maximal following 10 flashes per 5 min, suggesting a step function. All individuals responded to 10 flashes. (B) Expt. 2: Wheelrunning suppresion was more effectively achieved with 4-5 flashes presented with the IFI fixed at 16 sec. The difference between the Expts. 1 and 2 was that the IFI varied inversely with the number of flashes in the first, but remained fixed at 16 sec for the second. The solid lines indicate eye-estimated curves fitted to the data. The two experiments were conducted and analyzed independently. Groups sharing letter (Expt. 1) or number (Expt. 2) identifiers did not differ (p>.05). The groups receiving zero flashes experienced all sounds associated with delivery of 10 flashes, but no light.
Experiment 2: Flash number varies; inter-flash interval is fixed
In Expt. 1, flash number and IFI co-varied. The present experiment showed that the extent of light-induced wheelrunning suppression, as indicated by the zero minutes measure, varied significantly with the number of flashes when the IFI was fixed at 16 sec (Fig. 2B; p<.001, Kruskall-Wallis one way ANOVA on ranks) and reached its maximal level after only 4 flashes. This experiment was not conducted simultaneously with Expt.1, precluding a direct statistical comparison. However, the results appear to indicate that small numbers of flashes effectively suppress wheelrunning if the interval between the flashes is optimal.
Experiment 3: Flash number is fixed; inter-flash interval is short
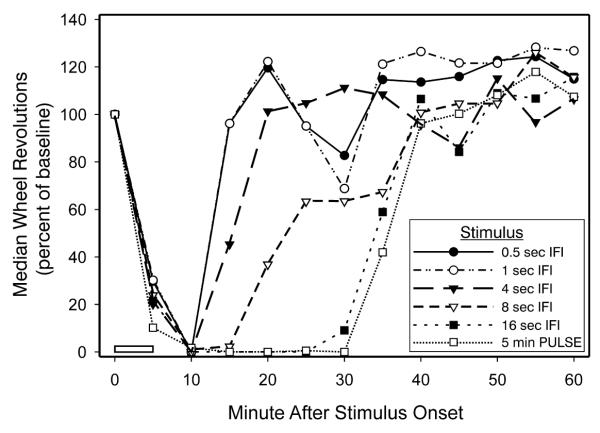
Expts. 1 and 2 addressed the issue of how nocturnal wheelrunning is affected by flash number. Here, the number of flashes was held constant (10) in order to determine the effects of short IFIs. The results are plotted (Fig. 3) to show the onset, duration and recovery of wheelrunning suppression consequent to the flash stimuli. All stimulus patterns rapidly induced suppression which was maximal about 10 min after stimulus onset. Time to recovery varied with the IFI, as longer IFIs yielded more prolonged suppression of wheelrunning. Ten flashes delivered with a 16 sec IFI elicited a response of maximal magnitude and with a duration that was virtually identical to that caused by a 5 min light pulse.
Figure 3.
Wheelrunning suppression is impaired when IFIs are short. Magnitude, indicated by the maximal percent change from baseline, is equivalent for all groups, but duration varies with the IFI of the stimulus pattern. The effects of flashes are shown in comparison with that elicited by a 5 min light pulse (□) administered beginning at time zero, as indicated by the open bar. All other groups received 10 flashes delivered with the IFIs indicated, the first flash delivered at time zero.
Experiment 4: Flash number is fixed; inter-flash interval is long
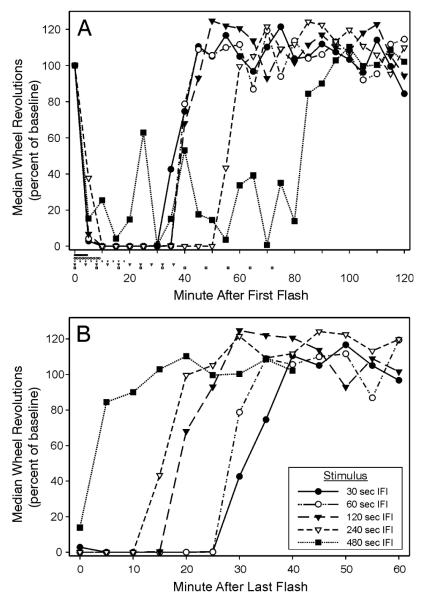
Whereas Expt. 3 determined that extent of wheelrunning suppression diminished as the IFI dropped from 16 sec to 0.5 sec, the present study was conducted to study whether wheelrunning suppression also declined as the IFI increased beyond 16 sec. The results show (Fig. 4A) that, although there was an overall effect of longer IFIs on magnitude, duration or both magnitude and duration of wheelrunning (p<.001, Kruskall-Wallis ANOVAs), the effect was not evident until IFI exceeded 120 sec. All flash series, except the 480 sec IFI group, yielded maximal wheelrunning suppression by 10 min. The 480 sec IFI group ran significantly more during the 30 min period following the first flash (p<.05 vs each of the other groups). With IFI = 240 sec, flashes were presented over a 36 min interval, but suppressed wheelrunning lasted to about 50 min. It was present in diminished form when IFI was extended to 480 sec (flashes distributed over 72 min), with running averaging about 22% of baseline with large variability.
Figure 4.
Wheelrunning suppression is impaired when the IFIs are long. (A) Ten flashes with a 30, 60, 120 or 240 sec IFI rapidly reduce wheelrunning to zero, with duration of locomotor suppression greater with a 240 sec IFI. The extent of suppression when IFI was 480 sec was prolonged but highly variable. Each group received its initial flash at time 0 and the timing all flashes administered to each group is indicated by the symbols below the abscissa. (B) The data from (A) are re-plotted relative to the time of the last flash to show the time to recovery from that flash. Recovery latency is inversely related to the IFI. The legend in (B) applies to both graphs. The last flash was 4.5, 9, 18, 36 and 72 min after the first for 30, 60, 120, 240 and 480 sec IFI groups, respectively.
When the data from mice exposed to long IFIs were plotted relative to the time of the last flash in the series (Fig. 4B), an orderly recovery progression was evident. Recovery latency was longest for the stimulus patterns that most effectively induced suppression of wheelrunning.
Experiment 5: Flash number and prolongation of wheelrunning suppression
The present study sought to determine whether the effect of stimulus that elicited a large and prolonged decrease in wheelrunning could be augmented by subsequent presentation of another light stimulus. A 5 min light pulse suppressed wheelrunning and additional light flashes occurring 25 min after onset of the light pulse increased the duration of suppression (Fig. 5). As more light flashes were added, the suppression duration (as indicated by the number of zero minutes during the 40 min interval from the first flash; 40 min was selected because this encompassed nearly all the minutes with zero activity of all groups) correspondingly increased (ANOVA, p<.001). Two additional flashes significantly increased the number of zero minutes (p<.05, Bonferroni test). As is evident in Fig. 5, 20 added flashes did not significantly augment the extent of wheelrunning suppression relative to that induced by 10 added flashes. The results are consistent with the view that during the interval of light-induced wheelrunning suppression, mice are not refractory to additional locomotor-suppressing effects of light.
Figure 5.
The duration of wheelrunning suppression increases in relation to the number of supplementary light flashes. A 5 min light pulse administered at time zero induced typical robust suppression of wheelrunning. One or more flashes (IFI fixed at 16 sec) administered 25 min after the onset of the 5 min pulse and during the interval of locomotor suppression induced by the pulse, significantly increased the zero counts during the next 40 min. The group receiving 0 flashes was exposed only to the initial 5 min light pulse. Groups with shared letter identifiers do not statistically differ; otherwise, p<.05.
Experiment 6: Change in behavior associated with masking
“Masking” has typically been evaluated in nocturnal rodents by evaluating change in the amount of wheelrunning in response to a stimulus. In this experiment, more general change in locomotion was examined using PIR detection of locomotion. In response to 10 flashes delivered over 5 min, both general locomotor activity, and simultaneously monitored wheelrunning, abruptly decreased and showed the expected prolonged reduction (data not shown). General locomotor activity assessed in mice lacking a running wheel also showed an expected abrupt decrease in locomotion with a prolonged duration. The data are not shown, but are very similar to the results obtained with running wheels from the 16 sec IFI group (as in Fig. 3).
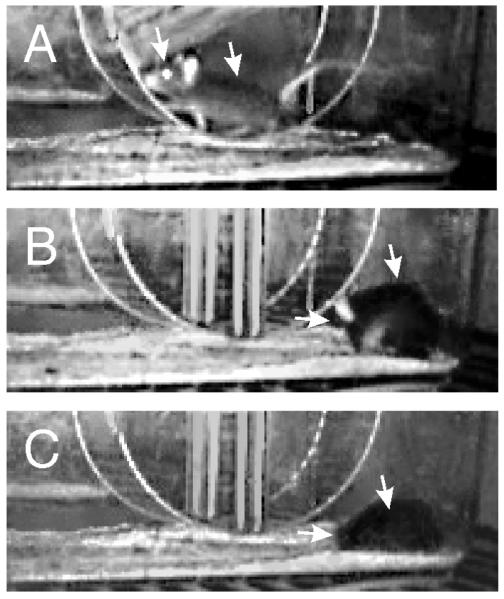
Video analysis verified that light flashes do not induce freezing, seizures or other unexpected behaviors. Rather, light onset during wheelrunning or non-wheel locomotion (Fig. 6A) was almost immediately followed by reduced activity, especially horizontal movement, which initiated an interval of quiescence. During this interval, mice either sat quietly, groomed (Fig. 6B) or engaged in an alternating mix of both behaviors. Either behavior, or the mix, had a median duration of 181 sec (25%ile=51.2 and 75%ile=203.7 sec). The mice spent an average 6.3 ± 3.0 % of the time engaged in wheelrunning during the interval. The quiescent phase ended with each mouse assuming a posture consistent with behavioral sleep (Fig. 6C). Assumption of the sleep posture had a mean latency of 10.14 ± 1.09 min (from the initial flash) and was maintained for an average 25.15 ± 3.82 min. Thus, the total “masking” time of quiescence plus apparent sleep time averaged about 35 min. An edited movie illustrating the transition from highly active to adoption of the sleep posture is available as Online Supplementary Material SOM2.
Figure 6.
Still images extracted from a video stream captured under infrared illumination at 640×480 pixel resolution show characteristic events during the three behavioral states. (A) While wheelrunning; (B) Grooming, about 6.5 min after the first flash, during which the left ear was noticeable as the animal’s head was bobbing up and down; (C) Behavioral sleep, which began (for this animal) 8.3 min after the first flash, during which the left ear is now downward, the mouse having assumed a reclining position on its left side with snout facing away from the camera. Behavioral sleep lasted 18.7 min. A video clip summarizing the behavior of this animal is shown in Supplementary Online Material SOM2. Left arrow points to the top head just rostral to the ears; right arrow points to the spine.
DISCUSSION
The present data support the view that “masking” consists of a change in behavioral state that is accomplished over relatively few minutes as animals transition from being highly active to showing behavioral sleep. This transition, while initiated in response to light, is not contingent upon the continued presence of light. A prime index of masking, light-induced suppression of locomotion, shows that only a few milliseconds of light exposure produces a drop in locomotion equivalent in magnitude and duration to that induced by a much longer light pulse. Moreover, the results indicate that the duration of locomotor suppression can be substantially prolonged by lengthening the interval between flashes. The neural processes governing “masking” may also underlie the phenomenon of light-induced sleep (Altimus et al., 2008;Lupi et al., 2008). Patterns of flash stimuli that effectively induce suppression of mouse locomotion are similar to those that effectively elicit large hamster phase shift responses (Vidal and Morin, 2007), raising the possibility that these two phenomena also share common control mechanisms.
Masking
Redlin and Mrosovsky (1999b) noted that, in some masking studies, locomotion often remains suppressed well after the light is turned off. That observation is consistent with the present results which emphasize the fact that locomotor suppression, whether induced by a few flashes or a 5 min light pulse, remains functional for about 25-30 min in the absence of further light.
The current experiments also reveal that light-induced locomotor suppression has several properties. “Magnitude” reflects the maximal extent to which locomotion is inhibited. For example, Fig. 2 shows that the magnitude varies greatly according to the number of flashes delivered. Magnitude effects have been studied using 1 hr light pulses (Mrosovsky et al., 1999;Redlin et al., 1999), but the pulses in such experiments obscure any difference between acute and longer term effects of photic stimulation. Fig. 3 illustrates the property of “duration” which is defined as the time from first light exposure to the return to 100% of baseline. The variable, “zero minutes,” which is the number of minutes with zero wheel revolutions, is correlated with both duration and magnitude. It is readily quantifiable and proved to be quite useful for the analysis in several of the present studies. A third property, “latency,” could not be satisfactorily measured in the present studies because wheel running does not provide sufficient resolution. However, it can be inferred that latency for most animals is likely to be 5 min or less (e.g., Fig. 3).
There is no information regarding the extent to which the three properties of light-induced locomotor suppression are correlated with phase shift responses to the same stimuli or whether the two types of responses co-vary with stimulus intensity, wavelength or circadian rhythm phase. Such information might allow more complete understanding of the mechanistic relationship between light-induced locomotor suppression and phase shifts. At the present time, it is impossible to distinguish effects of light on the SCN circadian clock cells and SCN cells controlling light-induced suppression of locomotion. The phase-dependent effect of light is one of the defining characteristics of circadian rhythm phase control. Light suppression of locomotion is also phase dependent (Redlin and Mrosovsky, 1999b; note that the methods necessary to demonstrate this phase dependence are substantially different from usual procedures for detection of masking), but only to the extent that magnitude of response suppression varies according to circadian time. Unlike the effects of light on circadian rhythms, there is nothing equivalent to a light-type phase response curve with a masking “dead zone” or a cross-over point at which a response to light abruptly changes from negative to positive masking.
Masking and Sleep
The present video analysis of behavior shows that flash-induced masking is a two stage process. The initial stage, lasting for about 10 min, starts with a rapid change from a highly active state into a state of behavioral quiescence. The second stage (about 25 min) is spent immobile, as if asleep. The latency to behavioral sleep is consistent with the time to sleep onset during prolonged light exposure, as inferred from EEG records (Lupi et al., 2008). Light-induced behavioral quiescence may represent a required step that causally progresses to the sleep state (Altimus et al., 2008;Lupi et al., 2008). Alternatively, light might simply induce a state of relative inactivity that is conducive to sleep, but not causal of it.
In previous investigations, light-induced sleep was studied in mice exposed to light for several hours without simultaneous measurement of sleep and locomotion. The authors of one study observed that, unlike masking, sleep cannot be stimulated by rod/cone photoreception (Lupi et al., 2008). They concluded that masking and light-induced sleep must be separate processes because of the apparent difference in the photoreceptors mediating the two responses. On the other hand, light-induced sleep may very well be mediated by both rod/cone and ipRGC photoreception (Altimus et al., 2008), leaving open the possibility that a common substrate mediates both light-induced sleep and masking. Regardless of which photoreceptors contribute to the two responses, under some lighting conditions, sleep and masking may be differentially regulated (Altimus et al., 2008).
Given the present video observations revealing the sequence of events during the interval of light-induced locomotor suppression and the published data on light-induced sleep, it may be that “masking” is an outmoded term. It implies that something has been “concealed” by light, when the opposite appears to be the case (light during the night appears to induce a state change). “Masking” may have a more limited application strictly in reference to the extent to which locomotion is light-suppressed. On the other hand, the present video and published EEG (Altimus et al., 2008;Lupi et al., 2008) evidence demonstrating light-induced sleep is a subject that requires further study in order to determine precisely which photic input pathways mediate behavioral change and how millisecond light exposure converts animals from aroused/active to quiescent/sleepy/asleep. Because even brief light exposure induces a sequence of behavioral quiescence followed by sleep, the term “photosomnolence” more accurately reflects the state change that occurs and endures across the entire interval of locomotor suppression.
Light input pathways for masking and phase shift responses are similar
The anatomical photic input pathways which mediate masking and circadian rhythm phase shifts are very similar, if not identical. Both masking and phase shift responses can be induced via rod/cone or ipRGC photoreception (Hattar et al., 2003;Mrosovsky et al., 2001;Mrosovsky and Hattar, 2003;Thompson et al., 2008). Rod/cone effects on both responses requires photic information to be transmitted through ipRGCs (Gõz et al., 2008;Guler et al., 2008;Hatori et al., 2008); with or without melanopsin photopigment present) and to the SCN via the retinohypothalamic tract (RHT) (Baver et al., 2008;Gooley and Saper, 2003;Hattar et al., 2006;Morin et al., 2003;Sollars et al., 2003). It is parsimonious to conclude that, on basic neuroanatomical grounds, the identical input pathway mediates both phase shift and masking responses to light.
Functionally, RHT transection eliminates rhythm entrainment and may also block masking (Johnson et al., 1988). SCN lesions abolish both rhythmicity and entrainment (Rusak, 1977), while rhythm restoration via embryonic SCN transplant restores neither entrainment or apparent masking effects (Lehman et al., 1987). Published studies of SCN lesion effects on masking are contradictory (masking retained - Redlin and Mrosovsky, 1999a; masking lost - Li et al., 2005). Destruction of other parts of the visual system (visual cortex, superior colliculus and pretectal nuclei (Redlin et al., 2003), dorsal lateral geniculate (Edelstein and Mrosovsky, 2001;Redlin et al., 1999), intergeniculate leaflet and ventral lateral geniculate (Redlin et al., 2003;Redlin et al., 1999) do not eliminate negative masking (all but the visual cortex receive input from ipRGCs; Hattar et al., 2006). Collectively, the functional anatomy investigations support the premise that the RHT and SCN mediate both light-induced circadian rhythm phase shifts and masking.
Masking and phase shifting also share substantial similarities with respect to their responsiveness to light. Their amplitudes have similar sensitivities to the intensity of the stimulus (Dkhissi-Benyahya et al., 2000;Mrosovsky et al., 1999;Muscat and Morin, 2005;Nelson and Takahashi, 1991;Redlin and Mrosovsky, 1999b;Thompson et al., 2008). They are readily elicited by millisecond light stimuli (present data and van den Pol et al., 1998;Vidal and Morin, 2007). Specific flash patterns elicit masking and phase shifting responses which are remarkably similar (e.g., responses to flash number or IFI, plus a shared failure to show normal light energy integration (present data and Vidal and Morin, 2007). Therefore, the basic and functional neuroanatomical perspectives support the view derived from the behavioral analysis that the underlying photic input pathway to, and probably including, the SCN is likely to be the same for photic regulation of masking and phase shift behavior. Similar logic would also argue for the inclusion of melatonin regulation by light as being under control by the same photic input pathway (see Redlin, 2001).
It should be noted that the ability of rodents to integrate light pulse energy over time (3 sec to 1 hr) is well established for phase shift response. The present data demonstrate that the continued presence of light is not required for a robust suppression of locomotion. Therefore, a test of light energy integration for masking should be possible and, if the principle of light energy integration applies, should yield reciprocity data similar to what has previously been observed for phase shifting (Dkhissi-Benyahya et al., 2000;Muscat and Morin, 2005;Nelson and Takahashi, 1991). An absence of such reciprocity might provide a clue as to the point of divergence between the systems governing photosomnolence and phase shifting.
Summary
The present studies use patterns of millisecond light flashes or short light pulses to show that locomotor suppression is rapidly induced, then maintained in a prolonged fashion absent additional light. Evaluation of mouse behavior recorded with infrared video methods shows that the effects of light are twofold. The initial light exposure rapidly induces an interval of behavioral quiescence which gives way to a more prolonged period during which animals maintain a posture typical of sleep. The results are consistent with prior data showing that EEG-detected sleep is induced in mice exposed to prolonged light. Use of brief light stimuli may simplify sleep studies by giving investigators greater control over the sequence of events. With respect to the phenomenon of light-induced locomotor suppression known as “masking,” the data suggest that it may be more profitably considered to reflect a sequence of events involving light-induced activity reduction which is followed by sleep onset and maintenance. The extent to which the steps in this sequence are causally linked remains to be determined. In addition, light controls locomotor suppression and phase shifts through very similar, if not identical, anatomical input pathways and the two responses appear to be similarly regulated by particular patterns of flash stimuli. The point in the regulatory pathway at which there is divergence between the mechanisms controlling phase shifts and those yielding locomotor suppression also remains to be determined.
Supplementary Material
Acknowledgments
Supported by NIH grants R01 NS22168 and NS061804 to LPM. Mr. Pablo Lituma and Ms. Annie Xu provided excellent technical assistance. Exemplary assistance from the editor contributed greatly to the manuscript quality.
REFERENCES
- Albers HE, Lydic R, Moore-Ede MC. Entrainment and masking of circadian drinking rhythms in primates: influence of light intensity. Physiol Behav. 1982;28:205–211. doi: 10.1016/0031-9384(82)90063-4. [DOI] [PubMed] [Google Scholar]
- Altimus CM, Guler AD, Villa KL, McNeill DS, LeGates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci USA. 2008;105:19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harbor Symp Quant Biol. 1960;25:11–27. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- Bergmann BM, Winter JB, Rosenberg RS, Rechtschaffen A. NREM sleep with low-voltage EEG in the rat. Sleep. 1987;10:1–11. doi: 10.1093/sleep/10.1.1. [DOI] [PubMed] [Google Scholar]
- Dkhissi-Benyahya O, Sicard B, Cooper HM. Effects of irradiance and stimulus duration on early gene expression (Fos) in the suprachiasmatic nucleus: Temporal summation and reciprocity. J Neurosci. 2000;20:7790–7797. doi: 10.1523/JNEUROSCI.20-20-07790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein K, Mrosovsky N. Behavioral responses to light in mice with dorsal lateral geniculate lesions. Brain Res. 2001;918:107–112. doi: 10.1016/s0006-8993(01)02966-3. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Saper CB. A broad role for melanopsin in non-visual photoreception based on neuroanatomical evidence in rats. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gõz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE. 2008;3:e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau K-W, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gilbert J, Davis FC. Disruption of masking by hypothalamic lesions in Syrian hamsters. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:23–30. doi: 10.1007/s00359-004-0569-5. [DOI] [PubMed] [Google Scholar]
- Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11:1068–1073. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- Minors DS, Waterhouse J. Masking in humans: the problem and some attempts to solve it. Chronobiol Int. 1989;6:29–53. doi: 10.3109/07420528909059140. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet and visual midbrain: bifurcation and melanopsin immunoreactivity. J Comp Neurol. 2003;465:401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Masking: history, definitions, and measurement. Chronobiol Int. 1999;16:415–429. doi: 10.3109/07420529908998717. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Nonphotic enhancement of adjustment to new light-dark cycles: masking interpretation discounted. J Biol Rhythms. 1989;4:365–370. doi: 10.1177/074873048900400305. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Foster RG, Salmon PA. Thresholds for masking responses to light in three strains of retinally degenerate mice. J Comp Physiol [A] 1999;184:423–428. doi: 10.1007/s003590050341. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Lucas RJ, Foster RG. Persistence of masking responses to light in mice lacking rods and cones. J Biol Rhythms. 2001;16:585–588. doi: 10.1177/074873001129002277. [DOI] [PubMed] [Google Scholar]
- Muscat L, Morin LP. Binocular contributions to the responsiveness and integrative capacity of the circadian rhythm system to light. J Biol Rhythms. 2005;20:513–525. doi: 10.1177/0748730405280458. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) J Physiol (Lond ) 1991;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoni EB. EthoLog 2.2: A tool for the transcription and timing of behavior observation sessions. Behav Res Meth Instrum Comput. 2000;32:446–449. doi: 10.3758/bf03200814. [DOI] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Redfern P, Minors D, Waterhouse J. Circadian rhythms, jet lag, and chronobiotics: an overview. Chronobiol Int. 1994;11:253–265. doi: 10.3109/07420529409067793. [DOI] [PubMed] [Google Scholar]
- Redlin U. Neural basis and biological function of masking by light in mammals: suppression of melatonin and locomotor activity. Chronobiol Int. 2001;18:737–758. doi: 10.1081/cbi-100107511. [DOI] [PubMed] [Google Scholar]
- Redlin U, Cooper HM, Mrosovsky N. Increased masking response to light after ablation of the visual cortex in mice. Brain Res. 2003;965:1–8. doi: 10.1016/s0006-8993(02)03844-1. [DOI] [PubMed] [Google Scholar]
- Redlin U, Mrosovsky N. Masking by light in hamsters with SCN lesions. J Comp Physiol [A] 1999a;184:439–448. doi: 10.1007/s003590050343. [DOI] [PubMed] [Google Scholar]
- Redlin U, Mrosovsky N. Masking of locomotor activity in hamsters. J Comp Physiol [A] 1999b;184:429–437. doi: 10.1007/s003590050342. [DOI] [PubMed] [Google Scholar]
- Redlin U, Vrang N, Mrosovsky N. Enhanced masking response to light in hamsters with IGL lesions. J Comp Physiol [A] 1999;184:449–456. doi: 10.1007/s003590050344. [DOI] [PubMed] [Google Scholar]
- Rensing L. Is “masking” an appropriate term? Chronobiol Int. 1989;6:297–300. doi: 10.3109/07420528909056933. [DOI] [PubMed] [Google Scholar]
- Rusak B. The role of the suprachiasmatic nuclei in the generation of circadian rhythms in the golden hamster, Mesocricetus auratus. J Comp Physiol. 1977;118:145–164. [Google Scholar]
- Sollars PJ, Smeraski CA, Kaufman JD, Ogilvie MD, Provencio I, Pickard GE. Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the hypothalamic suprachiasmatic nucleus. Vis Neurosci. 2003;20:601–610. doi: 10.1017/s0952523803206027. [DOI] [PubMed] [Google Scholar]
- Thompson S, Foster RG, Stone EM, Sheffield VC, Mrosovsky N. Classical and melanopsin photoreception in irradiance detection: negative masking of locomotor activity by light. Eur J Neurosci. 2008;27:1973–1979. doi: 10.1111/j.1460-9568.2008.06168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Cao V, Heller HC. Circadian system of mice integrates brief light stimuli. Am J Physiol Regul Integr Comp Physiol. 1998;275:R654–R657. doi: 10.1152/ajpregu.1998.275.2.R654. [DOI] [PubMed] [Google Scholar]
- Van der Horst GTJ, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, De Wit J, Verkerk A, Eker APM, Van Leenen D, Buijs R, Bootsma D, Hoeijmakers JHJ, Yasui A. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- Vidal L, Morin LP. Absence of normal photic integration in the circadian visual system: response to millisecond light flashes. J Neurosci. 2007;27:3375–3382. doi: 10.1523/JNEUROSCI.5496-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.