Abstract
Background
Natural killer (NK) cell dysfunction is associated with hyperresponse of corticotropin releasing hormone (CRH) to immune challenge and with a loss of β-endorphin (BEP) neurons in fetal alcohol exposed animals. Recently, we established a method to differentiate neural stem cells into BEP neurons using cyclic adenosine monophosphate (cAMP)-elevating agents in cultures. Hence, we determined whether in vitro differentiated BEP neurons could be used for reversing the compromised stress response and immune function in fetal alcohol exposed rats.
Methods
To determine the effect of BEP neuron transplants on NK cell function, we implanted in vitro differentiated BEP neurons into the paraventricular nucleus of pubertal and adult male rats exposed to ethanol or control in utero. The functionality of transplanted BEP neurons was determined by measuring proopiomelanocortin (POMC) gene expression in these cells and their effects on CRH gene expression under basal and after lipopolysaccaride (LPS) challenge. In addition, the effectiveness of BEP neurons in activating NK cell functions is determined by measuring NK cell cytolytic activity and interferon-γ (IFN-γ) production in the spleen and in the peripheral blood mononuclear cell (PBMC) following cell transplantation.
Results
We showed here that when these in vitro differentiated BEP neurons were transplanted into the hypothalamus, they maintain biological functions by producing POMC and reducing the CRH neuronal response to the LPS challenge. BEP neuronal transplants significantly increased NK cell cytolytic activity in the spleen and in the PBMC and increased plasma levels of IFN-γ in control and fetal alcohol exposed rats.
Conclusions
These data further establish the BEP neuronal regulatory role in the control of CRH and NK cell cytolytic function and identify a possible novel therapy to treat stress hyper-response and immune deficiency in fetal alcohol exposed subjects.
Keywords: Fetal Alcohol, β-Endorphin, Stress Axis, Stem Cell
CHILDREN PRENATALLY EXPOSED to alcohol often have an increased incidence of bacterial infections such as urinary tract and upper respiratory tract infections. Fetal alcohol exposed children also had lower cell counts of eosinophils and neutrophils, decreased circulating E-rosette-forming lymphocytes, reduced mitogen-stimulated proliferative responses by peripheral blood leukocytes, and hypo-γ-globulinemia (Johnson et al., 1981). One of the immune components suppressed by prenatal ethanol exposure is the natural killer (NK) cell, which is critical for killing tumor and virally infected cells (Albertsson et al., 2003; Arjona et al., 2006; Colucci et al., 2003; Dewan et al., 2005). NK cells are tightly regulated by the neuroendocrine system involving hypothalamic β-endorphin (BEP) neurons (Boyadjieva et al., 2002, 2006; Dokur et al., 2004a,b, 2005), which are reduced in prenatal ethanol exposed offspring (Arjona et al., 2006). BEP neurons are believed to reduce corticotrophin releasing hormone (CRH) neuronal mediate sympathetic influence on NK cell activity in the spleen (Boyadjieva et al., 2006).
We have previously determined the effects of ethanol on BEP-regulated NK cell function. We found that chronic ethanol administration suppresses the response of the proopiomelanocortin (POMC) gene and the NK cells to the immune challenge. Furthermore, we found that ethanol inhibits the response of proinflammatory cytokines to the immune challenge, but not the response of anti-inflammatory cytokines (Chen et al., 2006). We found that adult rats exposed to ethanol during their fetal life had reduced expression of granzyme B and interferon γ (IFN-γ) which is associated with decreased NK cell cytotoxic activity (Arjona et al., 2006). Hence, the question arises whether the BEP neuronal deficiency in fetal alcohol exposed animals affects the NK cell function.
We have recently developed a method to isolate neural stem cells from the fetal rat hypothalamus and established a hormone treatment paradigm that differentiates these stem cells into BEP neurons in culture (Sarkar et al., 2008). As they do in vivo, these cells in cultures showed immunostaining for BEP, expressed the gene POMC, and produced an increased amount of the gene and the peptide in response to a regulatory hormone, prostaglandin E. When these neurons were transplanted into the paraventricular nucleus (PVN) of the hypothalamus, they developed many BEP-immunoreactive neurofibers. The transplanted neurons produced POMC, mRNA and BEP peptide. In this study, we determined the effectiveness of these neurons in controlling CRH neuronal hyperresponse to an immune challenge and in enhancing NK cell cytolytic functions in fetal alcohol-exposed rats.
MATERIALS AND METHODS
Preparation of BEP Cells From Neural Stem Cells
Using cells from the hypothalamus of rat embryos, it has been previously shown that cyclic adenosine monophosphate (cAMP)-elevating agents protect against ethanol-induced death of BEP neurons (De et al., 1994). These findings have raised the question of whether this neurotrophic factor can be used to direct heterologous sets of neural stem cells into specific neuronal phenotype. We used cAMP and pituitary adenylate cyclase activating peptide (PACAP) to differentiate BEP neurons from rat fetal neural stem cells as we recently described (Sarkar et al., 2008). Briefly, fetal brains were obtained from 17-day-old pregnant rats (Charles River Laboratory, Gilroy, MA), mediobasal hypothalamic tissues from these rats were dissociated, and mixed hypothalamic cell cultures were prepared as previously described (De et al., 1994). Neurons were separated from glial cells by filtering mixed hypothalamic cells through a 48-μM nylon mesh. Hypothalamic cells were then sedimented at 400×g for 10 minutes, pellets were resuspended in HEPES-buffered Dulbecco's Modified Eagle's Medium (HDMEM, 4.5 g/l glucose) and cells were cultured into polyornithine coated 25 cm2 tissue culture flasks (2.5 million cells/flask) in HDMEM-containing 10% FBS and 1% penicillin/streptomycin. On day 2, the culture medium was replaced with HDMEM containing 10% FBS, 33.6 μg/ml of uridine, and 13.6 μg/ml of 5-fluorodeoxyuridine to prevent the overgrowth of astroglial cells. All of these chemicals were obtained from Sigma (Milwaukee, WI). On day 3, the culture medium was replaced with HDMEM-containing serum supplement (30 nM selenium, 20 nM progesterone, 1 μM iron-free human transferrin, 5 μM insulin, and 100 μM putrescin) and 1% penicillin/streptomycin. Cells were maintained for the next 2 days with this medium. By this time, these cultures were approximately 85 to 90% neurons, as determined by MAP-2 positivity.
Enriched hypothalamic neurons were maintained in HDMEM containing 10% FBS for 3 weeks. Cells were trypsinized and cultured weekly. By the beginning of the third week, many neurospheres started to develop. These spheres were separated and dissociated into single cells using trypsin/ethylene diamine tetraacetic acid (EDTA) (Sigma) solution and cultured in suspension or in poly-l-ornithine-coated 24-well plates (20,000 cells/well) in stem cell medium [DMEM-F-12, lymphokine inhitory factor (LIF), 0.1 mg/ml; l-glutamine, 10 mM; rat basic fibroblast growth factor (bFGF), 20 ng/ml; minimal essential medium (MEM) amino acid solution, MEM amino acids solution, 0.5%; all of the chemicals were from Sigma except bFGF, which was obtained from R&D Systems, Minneapolis, MN]. Cells were cultured for a period of 2 weeks. These cells grew and developed secondary neurospheres. These neurospheres were then differentiated by treating for 1 week with PACAP (10 μM; SynPep, Dublin, CA) and dbcAMP (10 μM; Sigma) and then placed in defined cell culture medium without the drugs for 1 week. Cells were used for grafting or immunocytochemical characterization of differentiated cells at 14 days after the initiation of differentiation. Prior to transplantation, secondary neurospheres were dissociated and resuspended at a concentration of 20,000 viable cells/μl in HDMEM-containing serum supplement and used for the transplantation. Primary cortical cells were prepared from 17-day-old fetal brains as previously described (Noh and Gwag, 1997). The dissociated cells were plated in T25 cm2 flasks in Eagle's MEM supplemented with 5% horse serum, 5% FBS, 21 mM glucose, 26.5 mM bicarbonate, and 2 mM l-glutamine. These cortical cells were cultured for 2 to 3 days in 2.5 μM arabinoside C for 2 days. These cells were then maintained in defined cell culture medium without drugs prior to use for transplantation.
Animal Surgery and Transplantation
Rats were exposed to ethanol in utero by feeding pregnant rats a liquid diet containing ethanol, as we previously described (Arjona et al., 2006). Pregnant Sprague–Dawley rats were individually housed in 12-hour light/12-hour dark cycles (lights on at 7:00 am) at a constant temperature (22°C) and provided with free access to water throughout the study. On gestational days 7 to 21, pregnant rats were fed chow ad libitum (ad lib-fed), a liquid diet containing ethanol (BioServe Inc., Frenchtown, NJ), or pair-fed an isocaloric liquid control diet (with the ethanol calories replaced by maltose–dextrin). The concentration of ethanol varied (1.7% to 5.0% v/v) in the diet for the first 4 days to habituate the animals with the alcohol diet. After this habituation period, animals were fed the liquid diet containing ethanol at a concentration of 6.7% v/v, which provided about 35% of the total dietary calories. Alcohol fed pups were removed from their original litter and placed with fostering dams (8 pups/litter). Pair-fed pups and ad lib-fed pups were culled and kept in their original litters with their mothers (8 pups/litter). All the nursing dams were fed rodent chow meal and water ad libitum. Pups were kept with their mother until postnatal day 22 and then weaned, housed by sex, and provided rodent chow meal and water ad libitum. A maximum of 1 rat from a litter was used in 1 experimental group.
In vitro differentiated BEP cells were dissociated using 0.05% trypsin/EDTA, washed and resuspended at a concentration of 20,000 cells/μl in HDMEM containing serum supplement for transplantation. Cortical cells were prepared and maintained in cultures for 4 days, trypsinized, and resuspended at a concentration of 20,000 cells/μl in HDMEM containing serum supplement for transplantation. Cells were placed on ice throughout the grafting session. Cell viability, assessed by the Trypan blue exclusion assay, was routinely >90%. The composition of the differentiated cultures, with respect to the absence of undifferentiated neural stem cells and the presence of mature BEP-producing cells, was verified before grafting by staining for the immature neural marker nestin and/or vimentin, and for BEP using immunohistochemistry. In some experiments, BEP cells were frozen and thawed 3 cycles and used as a nonviable BEP cells control at a concentration of 20,000 cells/μl of media.
Male rats between 35 and 90 days of age were anesthetized with sodium pentobarbital (50 to 70 mg/kg, i.p.; Henry Schein, Indianapolis, IN) and injected with 1.0 μl of stem cell suspension into either 1 or both PVN lobes; the coordinates were set 0.5 mm from the midline, 1.8 mm behind bregma, 0.5 mm lateral of bregma, and 7.5 mm below the cortex using a 5-μl Hamilton syringe. Each injection was over 5-minutes in duration. Following the injection, the cannula was left in place for 20 minutes to prevent cells being sucked out upon removal of the cannula. The cannula was then slowly removed over a 10-minute period. The dura was closed with 9-0 suture, muscle was reapposed and the skin was closed with wound clips. Rats were injected with 30,000 IU of penicillin (Henry Schein) and placed on a heating pad for recovery. No immune suppression was used. Animal surgery and care were performed in accordance with institutional guidelines and complied with NIH policy. The animal protocol was approved by the Rutgers Animal Care and Facilities Committee.
Immunohistochemical Characterization of Cells
Cell cultures were fixed in 4% paraformaldehyde for 30 minutes and then in 70% ethanol for an additional 30 minutes. Cells were incubated with primary antibodies overnight at 4°C. Primary antibodies used were monoclonal antibodies for nestin (BD Biosciences, San Jose, CA; 1 μg/ml), vimentin (clone V9, mouse ascites fluid, 0.22 μg/ml; Sigma; 1:40), and polyclonal primary rabbit antibody for BEP (1:1,000; Peninsula Laboratories, San Carlos, CA). The secondary antibody used to react with mouse primary antibodies was Alexa Fluor 488 donkey antimouse IgG (4 μg/ml; Molecular Probes, Eugene, OR) and the secondary antibody used to react with the rabbit primary antibody was Alexa Fluor 594 donkey anti-rabbit IgG (H + L) (4 μg/ml; Molecular Probes). Both of these secondary antibodies as well as IgG from normal rabbit and from normal horse failed to stain cells in the absence of a primary antibody.
The Functionality of BEP Cells
The functionality of cell transplants was studied by comparing the changes in the expression of POMC and CRH mRNA in the PVN 2 weeks after the transplantation between the animals with BEP cell or control (nonviable BEP cell) transplants. In addition, the lipopolysaccharide (LPS)-induced changes in the expression of POMC and CRH mRNA in the PVN were also determined in these rats. AF and PF rats were transplanted with BEP (20,000 cells/1 μl) into the left PVN and nonviable BEP cells (CONT; 20,000 cells/1 μl) into the right PVN. After 2 weeks of surgery, animals were injected i.p. with 100 μg/kg of LPS or saline. We used a 100 μg/kg dose of LPS for a period of 3 hours (which was found to be an effective dose; Sarkar et al., 2007) to determine the changes in the hypothalamic CRH and BEP responses. From the brains of these animals, we collected PVN by tissue punch to determine POMC mRNA and CRH mRNA levels by real-time RT-PCR methods as previously described (Chen et al., 2004).
Innate Immune System Response to BEP Cell Transplants
The immune system response to BEP cell transplants was determined by measuring the NK cell cytolytic activity in the spleen and peripheral blood mononuclear cell (PBMC) as well as cytokine (IFN-γ) levels in the plasma following 4 weeks of transplantation. Since the study was conducted for a long time period, we used cortical cells as the control. At the end of the experiment, rats were decapitated and the spleens and peripheral blood were obtained and used for isolation of splenocytes and PBMC to measure the NK cell cytolytic activity as described previously (Boyadjieva et al., 2002; Sarkar et al., 2008). The percentages of cytolytic activity at effector:target ratios of 200:1, 100:1, 50:1, and 25:1 were converted to lytic units per 106 effector cells according to Pross and colleagues (1981). Each assay was conducted in quadruplicate. Plasma levels of INF-γ were measured by enzyme-linked immuno sorbent assay (Amersham Biosciences, Piscataway, NJ).
Statistics
We determined the mean ± SEM of the data. The significance of the differences between 2 experimental groups was analyzed by the t-test. Multiple groups of data were analyzed using the 2-way analysis of variance followed by Bonferroni post-hoc test. Graph Pad Prism 4.0 (Graph Pad Software, Inc., San Diego, CA) was used for statistical analyses and graph preparation. A value of p < 0.05 was considered significant.
RESULTS
Determination of Functionality of Neural Stem Cell-Derived BEP Cells Transplanted in the PVN
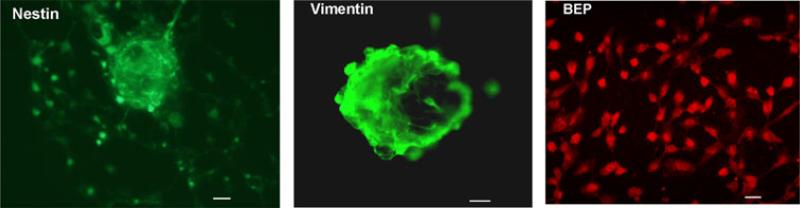
Neural stem cells prepared from fetal rat hypothalamus were stained with stem cell markers nestin and vimentin (Fig. 1A and B). Treatment of these cells with cAMP and PACAP differentiated the neural stem cells into BEP neurons (Fig. 1C). No staining was observed when undifferentiated cells were stained with anti-BEP antibody and differentiated BEP neurons were stained with vimentin and nestin (data not shown).
Fig. 1.
Immunocytochemical characterization of neural stem cells before and after differentiation. Neuronal stem cells were stained for Nestin (left) and Vimentin (middle) prior to differentiation and stained for BEP (right) after differentiation. “-”, 10 mm.
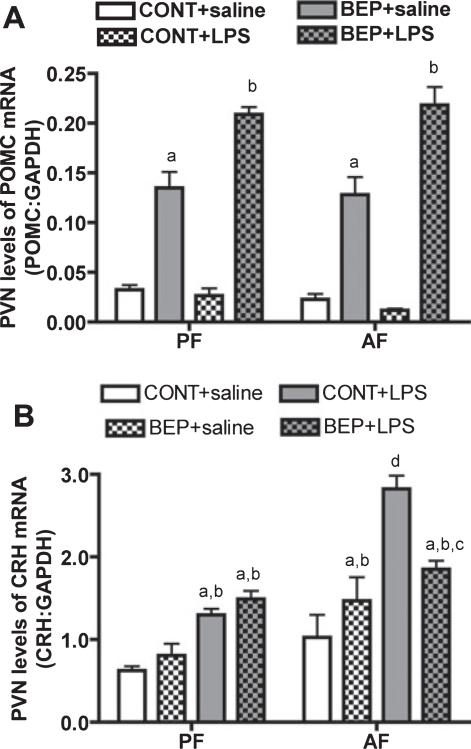
To determine the effect of BEP neuron transplants on NK cell function, we implanted in vitro differentiated BEP neurons in 1 PVN lobe and control cells into the other lobe of male rats exposed to an ethanol or control diet (pair-fed) during the prenatal period. The transplanted cells were found primarily in the medial parvocellular area of PVN but their terminals were also distributed to the lateral and posterolateral magnocellular area of PVN (data are not shown). We have previously shown that in vitro differentiated BEP neurons remain viable for a minimum period of 3 months when transplanted in the PVN, as these neurons produce elevated amounts of POMC gene and BEP (Sarkar et al., 2008). In agreement with these findings, we show here that the PVN side with the BEP cell transplants showed higher levels of POMC mRNA than the contralateral side of the PVN where control cells were transplanted (Fig. 2). The POMC level in the PVN containing control cells was low (Fig. 2A)and was similar to that found in untreated ad lib-fed rats (data not shown). Determination of POMC responses to LPS revealed that LPS moderately increased the POMC levels in the PVN containing the BEP neurons but did not affect PVN levels of POMC in the contralateral side in control cell transplants in alcohol-fed animals. LPS was also ineffective in altering POMC levels in the PVN of control-fed rats (Fig. 2A). These results suggest that that BEP neuron transplants were able to synthesize its gene product following transplantation and were able to respond to an immune challenge.
Fig. 2.
Physiological responses of transplanted cells in the PVN. Male rats (35- to 40-day old) fed during embryonic days 11 through 21 via dams; with alcohol (alcohol-fed rats; AF) or isocaloric liquid diet (pair-fed rats; PF) were transplanted with BEP cells (20,000 cells/1 μl) into the left PVN or nonviable BEP cells (CONT; 20,000 cells/1 μl) into the right PVN. After 2 weeks, rats underwent LPS or saline treatment and then 3 hours after they were sacrificed and the PVN tissues of these rats were collected and used for POMC or CRH measurements. POMC mRNA (A) and CRH mRNA (B) levels in the PVN lobe with BEP cells and in the contralateral lobe of the PVN with CONT after i.p. administration of LPS or of saline. n =6. POMC mRNA data identified no interaction between in utero feedings and cell treatments (F = 0.5116, df-3, p < 0.6781), while CRH mRNA data showed significant interaction between in utero feedings and cell treatments (F = 161.1, df-3, p < 0.001), ap < 0.001 versus PF + CONT + saline or AF + CONT + saline. bp <0.01 versus PF + CONT + LPS or AF + CONT + LPS. cp < 0.01 versus PF + BEP + saline. dp < 0.001 versus rest of the groups.
Previously we have shown that administration of BEP peptide into the PVN increases the cytolytic function of splenic NK cells (Boyadjieva et al., 2001) by suppressing the inhibitory action of CRH on the spleen via sympathetic neurons (Boyadjieva et al., 2006). Whether or not transplanted BEP cells inhibit CRH neuronal function was tested. Rats exposed to ethanol during the prenatal period are known to demonstrate CRH hyperresponsiveness to an immune challenge (Lee et al., 2000; Taylor et al., 1988). Similarly, in this study we show that LPS increased CRH mRNA levels in the PVN of both alcohol-fed and control-fed rats, but the magnitude of the CRH response to LPS was higher in alcohol-fed rats (Fig. 2B). LPS increased the level of CRH mRNA in the PVN infused with viable BEP neurons or with control cells in alcohol-fed rats. However, the response of the PVN lobe containing BEP neurons was much lower than the response of the PVN lobe treated with the control cell transplants. The levels of CRH mRNA in control transplanted rats were similar to those found in untreated alcohol-fed and control-fed rats (Boyadjieva et al., 2006; Sarkar et al., 2007). These results suggest that BEP neuron transplants are able to suppress CRH neuronal function in fetal alcohol exposed animals.
BEP Neuronal Transplants Increased NK Cell Cytolytic Function and Altered Production of IFN-γ
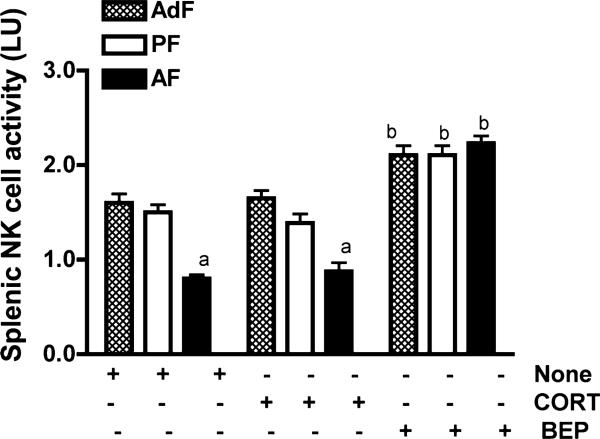
In this experiment, we determined whether BEP cell transplants alter NK cell cytolytic function and correct immune deficiencies in fetal alcohol exposed rats. For controls we used pair-fed rats and/or ad lib-fed rats. As expected, fetal alcohol exposed rats showed reduced splenic NK cell cytolytic function as compared to ad lib-fed and pair-fed controls (Fig. 3). Transplantation of BEP cells, but not cortical cells (CORT), in 1 lobe of the PVN increased splenic NK cell cytolytic activity in both alcohol-fed and control-fed rats in such a way that the cytolytic levels in these rats were indistinguishable.
Fig. 3.
Determination of the effect of BEP transplants in the PVN on NK cell cytolytic function. Adult male rats (90-day old) fed during embryonic days 7 through 21 via dams; with alcohol (alcohol-fed rats; AF), an isocaloric liquid diet (pair-fed rats; PF) or a regular diet (ad lib-fed rats; AdF) were transplanted with BEP (20,000 cells/1 μl) or cortical cells (CORT; 20,000 cells/1 μl) into the left PVN. After 4 weeks, rats were sacrificed and the spleens were collected. Splenocytes were prepared and assayed for NK cell cytolytic activity against YAC-lymphoma cells by a standard 4 hours 51Cr release cytolytic assay. The histograms represent mean ± SEM of NK activity in lytic units (LU). n = 5 to 7 rats. NK cytolytic activity data identified significant interaction between in utero feedings and cell treatments (F = 10.66, df-4, p < 0.001). ap < 0.001 versus AdF and PF rats. bp < 0.001 versus CORT cell-treated or untreated animals fed similarly during embryonic life.
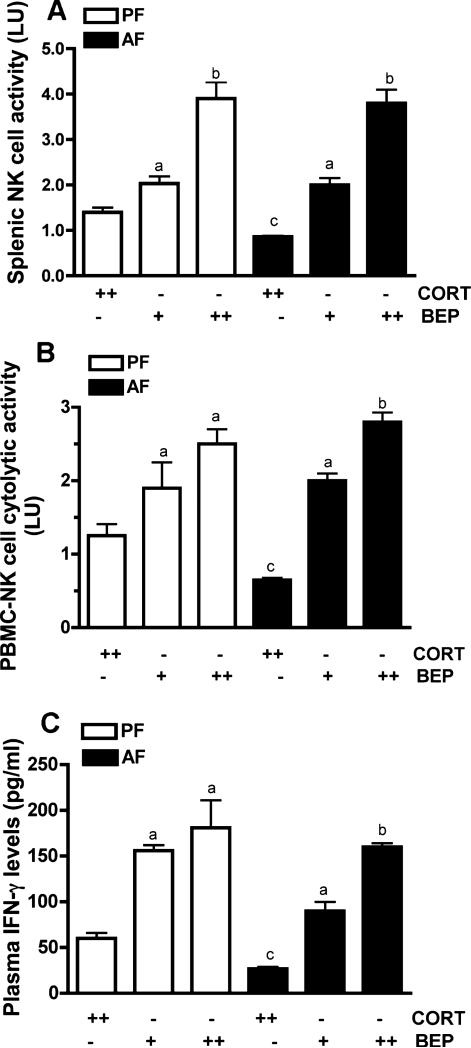
To further characterize the BEP cell transplants’ influence on NK cells, we studied the effects of these transplants either in 1 PVN lobe or in both PVN lobes of alcohol-fed rats and pair-fed rats (because ad lib-fed rats and pair-fed rats showed similar responses to BEP transplants, we only used pair-fed rats as controls) to determine whether bilateral transplants are more effective than unilateral transplants. Our data shows that BEP neuron-induced activation of splenic NK cells was greater when these cells were transplanted in both PVNs as compared to only 1 PVN (Fig. 4A). BEP transplants produced similar response effects on NK cell cytolytic activity in PBMC (Fig. 4B) and on IFN-γ levels in plasma of control and/or alcohol-fed rats (Fig. 4C). These results suggest that BEP cell transplants are effective in activating NK cell cytolytic functions.
Fig. 4.
Comparison of the effect of unilateral or bilateral BEP transplants in the PVN on NK cell functions. Adult male rats (90-day old) alcohol-fed (AF) or pair-fed (PF) during the prenatal period were transplanted with BEP or cortical cells (CORT) into 1 PVN (+) or 2 PVN (++). After 3 weeks, the spleen and approximately 1 ml of blood was collected from the orbital sinus of each rat and used for peripheral blood mononuclear cell (PBMC) preparation and plasma separation. (A, B) PBMC and splenic samples were used to determine the NK cytolytic activity by a standard 4 hours 51Cr release cytolytic assay. (C) Plasma samples were used to determine IFN-γ. The histograms represent mean ± SEM values from 5 to 7 rats. NK cytolytic activity of PBMC and spleen data identified no significant interaction between in utero feedings and cell treatments (PBMC, F = 2.781, df-2, p < 0.083; Spleen, F = 0.8398, df-2, p < 0.4436). There was also no significant interaction between in utero feeding and cell treatment of the IFN-γ response to cell transplants (F = 0.1553; df-2; p < 2.029). ap < 0.05 versus CORT-cell transplant in rats that were similarly treated prenatally. bp < 0.01 versus +BEP-cell transplant in rats that were similarly treated prenatally. cp < 0.001, compared to CORT-cell transplants and PF-treated groups.
DISCUSSION
In this study, we found that ex vivo produced BEP neuronal transplants significantly increased NK cell cytolytic activity in the spleen and in the PBMC in control and eliminated the NK cell functional deficiency in fetal alcohol exposed rats. In addition, the transplants increased plasma IFN-γ levels in these rats. These data are in agreement with the finding that administration of BEP peptide into the PVN increases NK cell functions (Boyadjieva et al., 2002). In situ BEP neurons are known to produce other POMC-derived peptides. Hence, neural stem cell derived BEP cells may have also produced other POMC derived peptides. However, the role of these peptides on NK cells are not clearly understood at present. Because the transplants increased BEP and inhibited CRH levels in the hypothalamus, it is possible that the peptide may have inhibited sympathetic input to the spleen via CRH to increase NK cell cytolytic function (Boyadjieva et al., 2006). These data provide strong evidence that hypothalamic BEP neurons play a critical role in controlling NK cell functions. These data support the hypotheses that BEP neuronal deficiency may, at least partly, be responsible for the NK cell functional deficiency in fetal alcohol exposed animals.
Natural killer cell function is a critical component of the innate immune response, which provides the first line of defense against pathogens and malignancy. A decreased NK cell cytotoxic activity may account, at least in part, for the increased susceptibility to infection that has been associated to fetal ethanol exposure (Church and Gerkin, 1988; Gauthier et al., 2005; Johnson et al., 1981). Moreover, abnormal IFN-γ levels together with decreased levels of cytotoxic function would hamper per se the initiation and maintenance of both innate and adaptive immune responses against infection.
Emerging evidence suggests that breast cancer may originate during early life. In particular, offspring of mothers who, during pregnancy, exhibited behaviors that are associated with increased incidence of breast cancer may be at risk. These behaviors include intake of alcohol or a stressful life style (Hilakivi-Clarke et al., 2004; Potischman and Troisi, 1999). It has been shown previously that intense exercise-induced stress enhances mammary carcinogenesis in the rat model (Saez et al., 2007). Higher levels of stress have been shown to be associated with increased sympathetic activity and decrements in NK cell cytolytic activity and IFN-γ levels (Varker et al., 2007). Furthermore, interventions aimed at reducing stress and enhancing optimism in women with breast cancers have been documented to promote optimal immune response (Ah et al., 2007). These studies identify the importance of stress maintenance in promoting NK cell function in breast cancer patients. As hypothalamic BEP is a suppressor of the CRH neuronal function and sympathetic neuronal influence on NK cells (Boyadjieva et al., 2006), BEP cell therapy may have a significant impact on activating NK cell function in cancer patients. Our data show that BEP neuron transplants in the hypothalamus significantly improve NK cell cytolytic activity and IFN-γ levels in rats. Our data also show that bilateral BEP transplants are more effective than unilateral transplants identifying a relationship between the BEP cell number and the amount of NK cell cytolytic activity as well as demonstrating the communication between both lobes of the PVN and the spleen. Although NK cells are highly efficient in the cellular immune response against malignant tumors, clinical studies using autologous NK cells have been reported in only a very limited number of cases due to the fact that selective NK expansion is difficult to achieve in this patient population (Ishikawa et al., 2004). The stem cell derived BEP cell transplant procedures that we have developed may be a major breakthrough in activating the ability of NK cells to prevent tumor growth and metastasis. Because neuronal differentiation from neural stem cells persists into adulthood (Lagace et al., 2007), the BEP-inducing therapies by cAMP-activating agents may hold promise as an adjuvant treatment for immune diseases and cancer not only in fetal alcohol exposed patients but also to nonfetal alcohol exposed cancer patients.
ACKNOWLEDGMENTS
The authors thank Cuiping Chen and Peter Kuhn for technical assistance in the RT-PCR and histochemistry.
This work was supported by National Institute of Health Grant AA15718.
REFERENCES
- Ah DV, Kang DH, Carpenter JS. Stress, optimism, and social support: impact on immune responses in breast cancer. Res Nurs Health. 2007;30:72–83. doi: 10.1002/nur.20164. [DOI] [PubMed] [Google Scholar]
- Albertsson PA, Basse PH, Hokland M, Goldfarb RH, Nagelkerke JF, Nannmark U, Kuppen PJ. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol. 2003;24:603–609. doi: 10.1016/j.it.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Arjona A, Boyadjieva N, Kuhn P, Sarkar DK. Fetal ethanol exposure disrupts the daily rhythms of splenic granzyme B, IFN-γ, and NK cell cytotoxicity in adulthood. Alcohol Clin Exp Res. 2006;30:1039–1044. doi: 10.1111/j.1530-0277.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- Boyadjieva N, Advis JP, Sarkar DK. Role of BEP, corticotropin-releasing hormone and autonomic nervous system in mediation of the effect of chronic ethanol on natural killer cell cytolytic activity. Alcohol Clin Exp Res. 2006;30:1761–1767. doi: 10.1111/j.1530-0277.2006.00209.x. [DOI] [PubMed] [Google Scholar]
- Boyadjieva NI, Dokur M, Advis JP, Meadows GG, Sarkar DK. Beta-endorphin modulation of lymphocyte proliferation: effects of ethanol. Alcohol Clin Exp Res. 2002;26:1719–1727. doi: 10.1097/01.ALC.0000036925.42090.9D. [DOI] [PubMed] [Google Scholar]
- Boyadjieva N, Meadows GG, Sarkar DK. Chronic ethanol inhibits natural killer cell cytolytic activity: role of opioid peptide beta-endorphin. J Immunol. 2001;167:5645–5652. doi: 10.4049/jimmunol.167.10.5645. [DOI] [PubMed] [Google Scholar]
- Chen CP, Boyadjieva N, Advis JP, Sarkar DK. Ethanol suppression of the hypothalamic POMC level and the splenic NK cell cytolytic activity is associated with a reduction in the expression of proinflammatory cytokines but not antiinflammatory cytokines in neuroendocrine and immune cells. Alcohol Clin Exp Res. 2006;30:1925–1932. doi: 10.1111/j.1530-0277.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of proopiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem. 2004;88:1547–1554. doi: 10.1046/j.1471-4159.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- Church MW, Gerkin KP. Hearing disorders in children with fetal alcohol syndrome: findings from case reports. Pediatrics. 1988;82:147–154. [PubMed] [Google Scholar]
- Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413–425. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- De A, Boyadjieva N, Pastorcic M, Reddy BV, Sarkar DK. cAMP and ethanol interact to control apoptosis and differentiation in hypothalamic β-endorphin neurons. J Biol Chem. 1994;269:26697–26705. [PubMed] [Google Scholar]
- Dewan MZ, Terunuma H, Ahmed S, Ohba K, Takada M, Tanaka Y, Toi M, Yamamoto N. Natural killer cells in breast cancer cell growth and metastasis in SCID mice. Biomed Pharmacother. 2005;59(Suppl. 2)):S375–S379. doi: 10.1016/s0753-3322(05)80082-4. [DOI] [PubMed] [Google Scholar]
- Dokur M, Boyadjieva N, Advis JP, Sarkar DK. Modulation of hypothalamic β-endorphin-regulated expression of natural killer cell cytolytic activity regulatory factors by ethanol in male Fisher-344 rats. Alcohol Clin Exp Res. 2004b;28:1180–1186. doi: 10.1097/01.alc.0000134222.20309.71. [DOI] [PubMed] [Google Scholar]
- Dokur M, Boyadjieva N, Sarkar DK. Catecholaminergic control of NK cell cytolytic activity regulatory factors in the spleen. J Neuroimmunol. 2004a;151:148–157. doi: 10.1016/j.jneuroim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Dokur M, Chen CP, Advis JP, Sarkar DK. β-endorphin modulation of interferon-γ, perforin and granzyme B levels in splenic NK cells: effects of ethanol. J Neuroimmunol. 2005;166:29–33. doi: 10.1016/j.jneuroim.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Gauthier TW, Drews-Botsch C, Falek A, Coles C, Brown LA. Maternal alcohol abuse and neonatal infection. Alcohol Clin Exp Res. 2005;29:1035–1043. doi: 10.1097/01.alc.0000167956.28160.5e. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Cabanes A, de Assis A, Wang M, Khan G, Shoemaker WJ, Stevens RG. In utero alcohol exposure increases mammary tumorigenesis in rats. Br J Cancer. 2004;90:2225–2231. doi: 10.1038/sj.bjc.6601793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa E, Tsuboi K, Saijo K, Harada H, Takano S, Nose T, Ohno T. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24:1861–1871. [PubMed] [Google Scholar]
- Johnson S, Knight R, Marmer DJ, Steele RW. Immune deficiency in fetal alcohol syndrome. Pediatr Res. 1981a;15:908–911. doi: 10.1203/00006450-198106000-00005. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, DiLeone RJ, Greer CA, Mandyam CD, Eisch AJ. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Rivier C. Increased activity of the hypothalamic-pituitary-adrenal axis of rats exposed to alcohol in utero: role of altered pituitary and hypothalamic function. Mol Cell Neurosci. 2000;16:515–528. doi: 10.1006/mcne.2000.0890. [DOI] [PubMed] [Google Scholar]
- Noh JS, Gwag BJ. Attenuation of oxidative neuronal necrosis by a dopamine D1 agonist in mouse cortical cell cultures. Exp Neurol. 1997;146:604–608. doi: 10.1006/exnr.1997.6569. [DOI] [PubMed] [Google Scholar]
- Potischman N, Troisi R. In-utero and early life exposures in relation to risk of breast cancer. Cancer Causes Control. 1999;10:561–573. doi: 10.1023/a:1008955110868. [DOI] [PubMed] [Google Scholar]
- Pross HF, Baines MG, Rubin P, Sharagge P, Patterson MS. Spontaneous human lymphocyte-mediated cytotoxicity against tumor cells: the quantitation of natural killer cell activity. J Clin Immunol. 1981;1:51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- Saez MD, Barriga C, Garcia JJ, Rodriguez AB, Ortega E. Exercise-induced stress enhances mammary tumor growth in rats: beneficial effect of the hormone melatonin. Mol Cell Biochem. 2007;294:19–24. doi: 10.1007/s11010-005-9067-5. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Boyadjieva NI, Chen CP, Ortigüela M, Reuhl K, Clement KM, Kuhn P, Marano J. Cyclic adenosine monophosphate differentiated beta-endorphin neurons promote immune function and prevent prostate cancer growth. PNAS. 2008;105:9105–9110. doi: 10.1073/pnas.0800289105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar DK, Kuhn P, Marano J, Chen CP. Alcohol exposure during the developmental period induces beta-endorphin neuronal death and causes alteration in the opioid control of stress axis function. Endocrinology. 2007;148:2828–2834. doi: 10.1210/en.2006-1606. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Van Zuylen JE, Redei E. Maternal alcoholism and stress responsiveness in offspring. Adv Exp Med Biol. 1988;245:311–317. doi: 10.1007/978-1-4899-2064-5_25. [DOI] [PubMed] [Google Scholar]
- Varker KA, Terrell CE, Welt M, Suleiman S, Thornton L, Andersen BL, Carson WE., III Impaired natural killer cell lysis in breast cancer patients with high levels of psychological stress is associated with altered expression of killer immunoglobin-like receptors. J Surg Res. 2007;139:36–44. doi: 10.1016/j.jss.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]