Abstract
OBJECTIVE
The study objective was to determine the independent and joint associations of prenatal tobacco and childhood lead exposures with attention-deficit/hyperactivity disorder (ADHD), as defined by current diagnostic criteria, in a national sample of US children.
METHODS
Data are from the 2001–2004 National Health and Nutrition Examination Survey, a cross-sectional, nationally representative sample of the US population. Participants were 8 to 15 years of age (N = 2588). Prenatal tobacco exposure was measured by report of maternal cigarette use during pregnancy. Lead exposure was assessed by using current blood lead levels. The Diagnostic Interview Schedule for Children was used to ascertain the presence of ADHD in the past year, on the basis of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria.
RESULTS
A total of 8.7% (95% confidence interval [CI]: 7.3%–10.1%) of children met criteria for ADHD. Prenatal tobacco exposure (adjusted odds ratio [aOR]: 2.4 [95% CI: 1.5–3.7]) and higher current blood lead concentrations (aOR for third versus first tertile: 2.3 [95% CI: 1.5–3.8]) were independently associated with ADHD. Compared with children with neither exposure, children with both exposures (prenatal tobacco exposure and third-tertile lead levels) had an even greater risk of ADHD (aOR: 8.1 [95% CI: 3.5–18.7]) than would be expected if the independent risks were multiplied (tobacco-lead exposure interaction term, P < .001).
CONCLUSIONS
Prenatal tobacco and childhood lead exposures are associated with ADHD in US children, especially among those with both exposures. Reduction of these common toxicant exposures may be an important avenue for ADHD prevention. Pediatrics 2009;124: e1054–e1063
Keywords: attention-deficit/hyperactivity disorder, lead exposure, tobacco exposure, toxicant interactions, joint effects
Attention-deficit/hyperactivity disorder (ADHD), one of the most common childhood neurobehavioral conditions, is characterized by developmentally in-appropriate difficulties sustaining attention, controlling impulses, and modulating activity levels. It is associated with substantial health care costs1 and impaired social, academic, and occupational functioning.2,3 Although ADHD is thought to be a highly familial disorder,4 environmental factors also have been implicated.5,6 Many, but not all, previous studies documented an association between prenatal tobacco exposure and attention problems.5,7–10 Lead exposure also has been linked to inattention and impulsivity,11,12 although most previous studies enrolled children with lead levels higher than those that are typical today.11–15 Few previous studies documented the relationship between ADHD, defined on the basis of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), criteria, and prenatal tobacco exposure10,16–18 or childhood lead exposure,19,20 and none used nationally representative samples. Furthermore, studies examining joint effects of lead and tobacco are lacking, despite calls to evaluate the combined effects of environmental exposures21–23 on ADHD.24 Because both lead and prenatal tobacco exposures alter dopamine systems,25–28 the combination of these toxicants may be especially relevant to ADHD pathogenesis. The purpose of this study was to determine the independent and joint effects of tobacco and lead exposures on DSM-IV–defined ADHD in a national sample of US children.
METHODS
Sample
The National Health and Nutrition Examination Survey (NHANES) is a multistage, probability sample survey of the US population. In 2001–2004, 3907 children 8 to 15 years of age participated in NHANES. Data regarding ADHD DSM-IV diagnostic status were available for 3077 children (78.8% of total), and 2588 children (66.2% of total) had complete data regarding lead exposure, smoke exposure, and additional predictors. Children with and without ADHD DSM-IV data did not differ in terms of gender, prenatal smoke exposure, birth weight, or NICU admission (all P> .26). However, children lacking DSM-IV data were significantly more likely to be younger (mean age: 9.9 vs 12.1 years), poorer (lowest income quintile: 24.9% vs 18.9%), and exposed to lead (third tertile of exposure: 40.9% vs 29.2%) and household tobacco smoke (third tertile of exposure: 49.7% vs 31.7%).
Outcomes
Our primary outcome was ADHD, defined on the basis of meeting DSM-IV criteria for any ADHD subtype. The National Institute of Mental Health Diagnostic Interview Schedule for Children (DISC) was used to assess DSM-IV–defined ADHD on the basis of standardized algorithms, as in previous studies.29 The DISC is a structured diagnostic interview instrument designed for use in epidemiological and clinical studies, with reliable versions available in English30 and Spanish.31,32 Caregivers completed the ADHD DISC module 2 to 4 weeks after the child’s NHANES Mobile Examination Center evaluation, providing information about the child’s ADHD symptoms, the age of onset, symptom pervasiveness, and related impairments in the previous 12 months.
To account for children who did not currently meet ADHD DSM-IV criteria because of appropriate medication treatment, we created a secondary outcome variable for which children were considered to have ADHD if they either demonstrated positive findings on the DISC for any ADHD subtype or had been treated with ADHD medications during the past year and had a caregiver report of a previous ADHD diagnosis. Questions on the DISC inquire about the use of “medicine for being overactive, being hyperactive, or having trouble paying attention” in the past 12 months. To determine whether a child had a previous ADHD diagnosis, caregivers were asked, during a separate NHANES interview module, “Has a doctor or health professional ever told you that [child’s name] had attention deficit disorder?”
Primary Predictors
Primary predictors included prenatal tobacco smoke exposure and current lead exposure. Prenatal tobacco exposure was assessed by asking caregivers, “Did the child’s biological mother smoke at any time while she was pregnant with him/her?” No information on the quantity of cigarettes smoked was collected. Current blood lead concentrations were determined through graphite furnace atomic absorption spectrophotometry.33,34 The limit of detection was 0.3 µg/dL, and 33 children had blood lead levels below this threshold. For primary analyses, lead levels were categorized in tertiles by using weighted percentages, with the first tertile representing the lowest level of exposure and the third tertile the highest. Secondary analyses evaluated the association between ADHD and natural logarithmically transformed lead levels.
Additional Predictors
We examined covariates and potential confounders for the association of prenatal tobacco and current lead exposures with ADHD. Predictors were chosen on the basis of their association with ADHD in previous studies and included child gender,35 household income/poverty line ratio,29 age,35 race/ethnicity,36 mother’s age at child’s birth,37 birth weight,38 NICU admission,39 postnatal secondhand tobacco smoke exposure,40,41 and preschool attendance.5 The household income/poverty line ratio, that is, the ratio of the reported household income to the poverty threshold appropriate for the household size, was categorized into quintiles. Child race/ethnicity was designated by caregivers and included the categories of non-Hispanic black, Mexican American, other Hispanic, non-Hispanic white, and other (including multiracial). Because of proportionately small numbers of subjects in the other Hispanic (n = 114) and other (including multiracial) (n = 107) groups, the groups were combined into a single other race/ethnicity category. Current secondhand tobacco exposure was assessed by using the child’s serum levels of cotinine, a metabolite of nicotine. Cotinine levels were measured with high performance liquid chromatography-tandem mass spectrometry.42 Cotinine levels of >10 ng/mL (n = 64) often are indicative of active smoking, which has been associated with ADHD in adolescent children43; therefore, children with values above this level were excluded from analyses, to prevent confounding of the effects of secondhand tobacco exposure.44 In models predicting either fulfillment of ADHD DSM-IV criteria or previous disorder recognition plus ADHD medication treatment, health insurance status was included to account for differing health care access.
Analyses
The Cincinnati Children’s Hospital Medical Center institutional review board determined this study to be exempt from review. Descriptive statistics on the national prevalence rates of ADHD (any subtype) are presented for the primary predictors and additional predictors. Logistic regression analyses were used to analyze the associations between the primary predictors and ADHD status. Additional predictors associated with ADHD (χ2 test, P < .2) in bivariate analyses were included in the logistic regression analyses. In addition, secondary analyses excluded children with lead levels of ≥5 µg/dL (n = 51), to determine whether they had excessive influence on the models.
After developing the multivariate main effects model, we tested for joint toxicant effects. We first modeled the potential prenatal tobacco-lead exposure interaction by using a variable with 6 categories, as recommended by Rothman,45 that is, no prenatal tobacco exposure and lead levels in the first tertile (reference category), no prenatal tobacco exposure and lead levels in the second tertile, no prenatal tobacco exposure and lead levels in the third tertile, prenatal tobacco exposure and lead levels in the first tertile, prenatal tobacco exposure and lead levels in the second tertile, and prenatal tobacco exposure and lead levels in the third tertile. We also tested whether the formal prenatal tobacco-lead exposure interaction term was statistically significant.
We calculated population attributable fractions (PAFs) of ADHD for lead and prenatal tobacco exposures by using the Miettinen formula.46 Because these independent risk factors are not mutually exclusive, we also estimated the PAFs for children with prenatal tobacco exposure, childhood lead exposure, or both.
Regression diagnostic analyses were conducted to identify colinearity and influential observations. No evidence of colinearity was identified (all condition indices were <12). Influential observations were excluded from analyses for assessment of whether their inclusion altered results (n = 3). Exclusion of outliers did not influence significantly the estimates for prenatal tobacco or current lead exposure. Therefore, findings for the full sample, including outliers, are reported.
Because of the complex differential probabilities of selection to achieve oversampling of selected groups in the NHANES cohort, sample weights were applied according to National Center for Health Statistics guidelines for generation of all estimates. Analyses were performed by using SUDAAN 9 (Research Triangle Institute, Research Triangle Park, NC) procedures for analysis of complex surveys.
RESULTS
Main Effects of Environmental and Sociodemographic Factors
Among 8-to 15-year-old participants, 8.7% (95% confidence interval [CI]: 7.3%–10.1%) met DSM-IV–defined ADHD criteria in the year before the survey, equivalent to 2.4 million children in the United States, as reported in our previous study.29 In bivariate analyses, ADHD rates were higher for children with prenatal tobacco exposure (P < .001), increasing lead exposure (P <.001), current household tobacco exposure (assessed through serum cotinine levels; P = .01), male gender (P < .001), and a history of attending preschool (P = .01) (Table 1).
TABLE 1.
Prevalence of ADHD According to Environmental Exposure, Sociodemographic Characteristics, and Medical Factors
| Characteristic | No. With ADHDa | Weighted Proportion With ADHD, Estimate (95% CI), % |
Pb |
|---|---|---|---|
| Total | 222 | 8.7 (7.3–10.1) | |
| Blood lead level | <.001 | ||
| First tertile (0.2–0.8 µg/dL) | 43 | 5.2 (3.4–7.0) | |
| Second tertile (0.9–1.3 µg/dL) | 63 | 9.1 (5.8–12.5) | |
| Third tertile (>1.3 µg/dL) | 107 | 13.6 (11.0–16.2) | |
| Prenatal tobacco exposure | <.001 | ||
| Yes | 63 | 16.8 (12.3–21.4) | |
| No | 152 | 6.6 (5.2–8.0) | |
| Household smoke exposurec | .01 | ||
| First tertile (0.2–0.027 ng/mL) | 47 | 7.0 (4.1–9.9) | |
| Second tertile (0.028–0.202 ng/mL) | 70 | 8.3 (5.5–11.2) | |
| Third tertile (0.203–10 ng/mL) | 88 | 12.4 (9.8–15.1) | |
| Gender | <.001 | ||
| Male | 141 | 11.8 (9.8–13.8) | |
| Female | 81 | 5.4 (4.2–6.6) | |
| Age | .08 | ||
| 8–11 y | 119 | 10.0 (7.9–12.1) | |
| 12–15 y | 103 | 7.5 (5.5–9.4) | |
| Race/ethnicity | .06 | ||
| Mexican American | 45 | 6.0 (4.3–7.8) | |
| Non-Hispanic black | 76 | 8.7 (6.4–10.9) | |
| Other race/ethnicity | 17 | 5.2 (1.8–8.7) | |
| Non-Hispanic white | 84 | 9.8 (7.4–12.1) | |
| Income/poverty ratio | |||
| First quintile (0–0.93) | 69 | 11.0 (7.9–14.0) | .19 |
| Second quintile (0.94–1.70) | 46 | 9.6 (4.7–14.5) | |
| Third quintile (1.71–2.75) | 40 | 8.5 (4.6–12.5) | |
| Fourth quintile (2.76–4.24) | 34 | 9.0 (5.5–12.6) | |
| Fifth quintile (>4.24) | 28 | 6.4 (3.6–9.1) | |
| Attended preschool | .01 | ||
| Yes | 171 | 9.5 (7.7–11.3) | |
| No | 51 | 6.4 (4.8–8.0) | |
| Mother’s age at child’s birth | .12 | ||
| ≤18 y | 28 | 13.1 (7.1–19.1) | |
| >18 y | 194 | 8.4 (7.0–9.7) | |
| NICU admission | .43 | ||
| Yes | 32 | 10.2 (6.1–14.2) | |
| No | 186 | 8.4 (6.9–9.9) | |
| Birth weight | .18 | ||
| <2.5 kg | 23 | 14.3 (6.6–21.9) | |
| ≥2.5 kg | 197 | 8.3 (6.6–9.9) |
Subjects with ADHD met DSM-IV criteria for any ADHD subtype.
For between-group comparison of prevalence rates in χ2 analyses.
Assessed through serum cotinine levels.
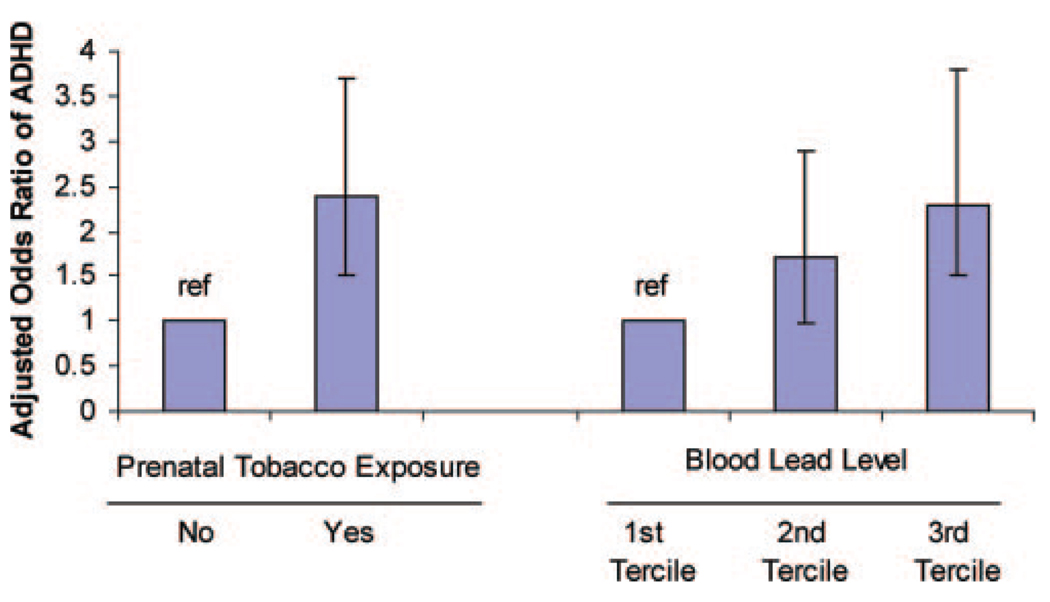
In multivariate models, prenatal tobacco exposure and current blood lead concentrations were significant predictors of DSM-IV–defined ADHD (Table 2 and Fig 1). Children who were exposed to tobacco prenatally were more than twice as likely to meet ADHD criteria, compared with children who were not exposed (adjusted odds ratio [aOR]:2.4 [95% CI: 1.5–3.7]). Compared with children in the lowest tertile of lead exposure, those with lead levels in the highest tertile were at significantly higher risk of ADHD (aOR: 2.3 [95% CI: 1.5–3.8]). Current household tobacco exposure was not significantly linked to ADHD in multivariate models (aOR for third versus first tertile of exposure: 0.8 [95% CI: 0.4–1.7]). Multivariate analyses confirmed that children who had attended preschool (aOR: 1.6 [95% CI: 1.1–2.1]) and boys (aOR versus girls: 1.9 [95% CI: 1.4–2.5]) had increased likelihoods of ADHD, whereas Mexican American (aOR: 0.5 [95% CI: 0.3–0.9]) and black (aOR: 0.5 [95% CI: 0.3–0.9]) children had lower likelihoods of ADHD, compared with non-Hispanic white children.
TABLE 2.
AORs for ADHD According to Environmental Exposure, Sociodemographic Characteristics, and Medical Factors (N= 2588)
| Characteristic | AOR (95% CI) | P |
|---|---|---|
| Blood lead level | ||
| First tertile (0.2–0.8 µg/dL) (reference) | 1.0 | |
| Second tertile (0.9–1.3 µg/dL) | 1.7 (0.97–2.9) | .06 |
| Third tertile (>1.3 µg/dL) | 2.3 (1.5–3.8) | .001 |
| Prenatal tobacco exposure | ||
| Yes | 2.4 (1.5–3.7) | .001 |
| No (reference) | 1.0 | |
| Household smoke exposurea | ||
| First tertile (0.2–0.027 ng/mL) (reference) |
1.0 | |
| Second tertile (0.028–0.202 ng/mL) | 0.9 (0.4–1.7) | .70 |
| Third tertile (0.203–10 ng/mL) | 0.8 (0.4–1.7) | .58 |
| Gender | ||
| Male | 1.9 (1.4–2.5) | <.001 |
| Female (reference) | 1.0 | |
| Age | ||
| 8–11 y | 1.1 (0.8–1.6) | .60 |
| 12–15 y (reference) | 1.0 | |
| Race/ethnicity | ||
| Mexican American | 0.5 (0.3–0.9) | .03 |
| Non-Hispanic black | 0.5 (0.3–0.9) | .02 |
| Other race/ethnicity | 0.4 (0.2–1.1) | .08 |
| Non-Hispanic white (reference) | 1.0 | |
| Income/poverty ratio | ||
| First quintile (0–0.93) | 1.9 (0.9–4.1) | .09 |
| Second quintile (0.94–1.70) | 1.5 (0.7–3.4) | .31 |
| Third quintile (1.71–2.75) | 1.5 (0.8–3.1) | .23 |
| Fourth quintile (2.76–4.24) | 1.4 (0.7–2.6) | .35 |
| Fifth quintile (>4.24) (reference) | 1.0 | |
| Attended preschool | ||
| Yes | 1.6 (1.1–2.1) | .01 |
| No (reference) | 1.0 | |
| Mother’s age at child’s birth | ||
| ≤18 y | 1.9 (0.96–3.6) | .06 |
| >18 y (reference) | 1.0 | |
| Birth weight | ||
| <2.5 kg | 1.5 (0.7–2.9) | .25 |
| ≥2.5 kg (reference) | 1.0 |
Subjects with ADHD met DSM-IV criteria for any ADHD subtype.
Assessed through serum cotinine levels.
FIGURE 1.
AORs for ADHD according to environmental factors. Subjects with ADHD met DSM-IV criteria for any ADHD subtype. ref indicates reference group.
We examined the stability of our results by (1) conducting sensitivity analyses to evaluate effects at lead levels of ≤5 µg/dL, (2) determining the relationship of the environmental exposures to DSM-IV–defined ADHD when lead and cotinine levels were modeled as natural logarithmically transformed continuous variables, and (3) expanding the definition of our ADHD outcome. When the sample was restricted to children with lead concentrations of ≤5 µg/dL, increasing lead levels were still significantly associated with DSM-IV–defined ADHD; compared with children in the lowest tertile (nondetectable to 0.8 µg/dL), those with lead levels in the highest tertile (>1.3–5 µg/dL) had a more than twofold increased risk of ADHD (aOR for third versus first tertile: 2.3[95% CI: 1.4–3.7]). In addition, the observed relationships with DSM-IV–defined ADHD did not change for either lead exposure (aOR: 1.8 [95% CI: 1.2–2.8]) or current household tobacco exposure (aOR: 1.0 [95% CI: 0.9–1.1]) when lead and cotinine levels were treated as natural logarithmically transformed continuous variables. When our outcome was broadened to include either children who currently met DSM-IV criteria for ADHD (8.7% of the sample [95% CI: 7.3%–10.1% of the sample]) or those who did not meet DSM-IV criteria for ADHD but had both a parent-reported previous diagnosis of ADHD and treatment with ADHD medications (3.3% of the sample [95% CI: 2.4%–4.1% of the sample]), both prenatal tobacco exposure (aOR: 2.0 [95% CI: 1.3–3.1]) and current lead exposure (aOR: 2.0 [95% CI: 1.3–3.0]) remained associated with ADHD.
Joint Toxicant Effects
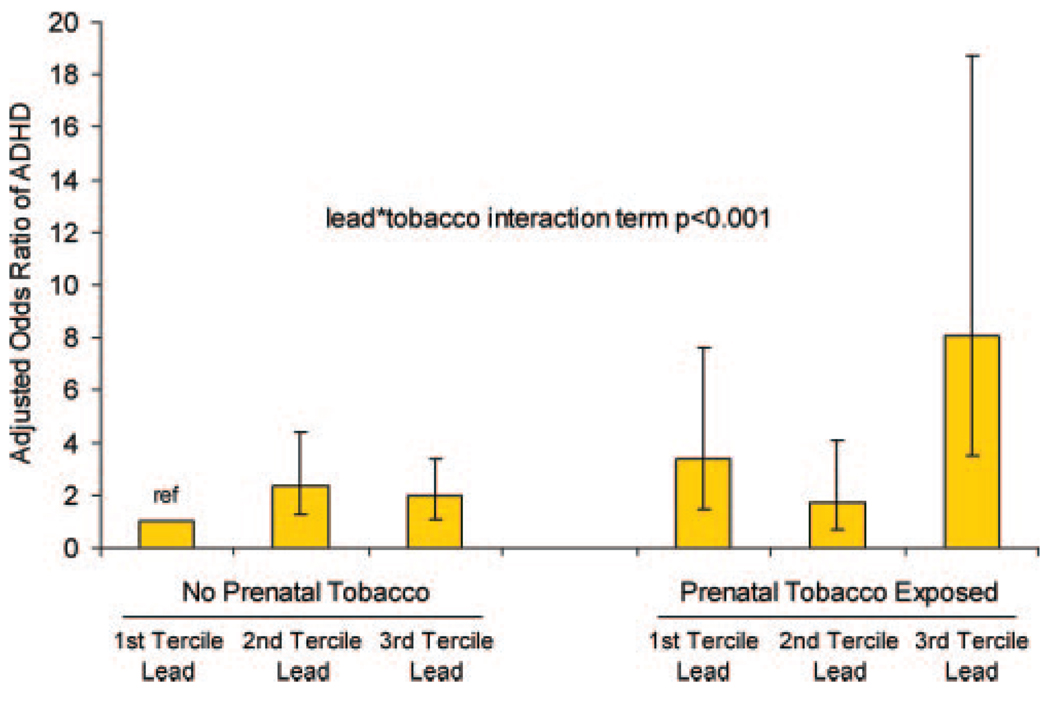
Compared with children with neither exposure, children with both prenatal tobacco exposure and lead levels in the highest tertile had a greater than eightfold increased likelihood of DSM-IV–defined ADHD (aOR: 8.1 [95% CI: 3.5–18.7]) (Fig 2). The formal lead-prenatal tobacco exposure interaction term was statistically significant (P <.001), which indicated that the joint effects of exposures operated in a manner that was more than multiplicative. In our sample, children with both prenatal tobacco exposure and current lead concentrations in the highest tertile constituted 7.7% (95% CI: 5.7%–9.8%) of the population but accounted for 24.4% (95% CI: 19.3%–30.5%) of ADHD cases.
FIGURE 2.
Joint effects of prenatal tobacco and current lead exposures on ADHD. Subjects with ADHD met DSM-IV criteria for any ADHD subtype. ref indicates reference group.
Population Attributable Fractions
We calculated PAFs to estimate the proportion of ADHD cases that might be attributable to prenatal tobacco exposure and current lead exposure if the toxicant exposures were indeed causally linked to ADHD (Table 3). The PAF for prenatal tobacco exposure was 21.7% (95% CI: 12.1%–27.6%), corresponding to 510 000 cases of DSM-IV–defined ADHD in US children 8 to 15 years of age. We also estimated that 25.4% (95% CI: 13.9%–32.5%) of ADHD cases among 8-to 15-year-old children (corresponding to 598 000 cases) might be attributable to lead exposure in the highest tertile. In addition, we calculated the PAF for having ≥1 toxicant exposure. Our estimates suggested that 38.2% (95% CI: 22.5%–47.0%) of ADHD cases among 8-to 15-year-old children might be attributable to prenatal tobacco exposure, lead concentrations of >1.3 µg/dL, or both, corresponding to 900 000 excess cases.
TABLE 3.
PAFs for Prenatal Tobacco Exposure and Childhood Lead Exposure for ADHD in US Children
| Characteristic | Proportion of Patients With ADHD Exposed, Estimate (95% CI), % |
AORa | PAF, Estimate (95% CI), %a | Excess Casesa |
|---|---|---|---|---|
| Prenatal smoke exposure | 37.7 (31.8–44.2) | 2.4 | 21.7 (12.1–27.6) | 510 000 |
| Blood lead level in third tertile | 44.3 (37.9–50.7) | 2.3 | 25.4 (13.9–32.5) | 598 000 |
| Prenatal smoke exposure and/or blood lead level in third tertile |
58.4 (51.7–64.5) | 2.9 | 38.2 (22.5–47.0) | 900 000 |
| Prenatal smoke exposure and blood lead level in third tertile |
24.4 (19.3–30.5) | 8.1 | 21.4 (17.4–23.1) | 504 000 |
Subjects with ADHD met DSM-IV criteria for any ADHD subtype.
Models were adjusted for current household smoke exposure, gender, age, race/ethnicity, income, preschool attendance, mother’s age at child’s birth, and birth weight.
DISCUSSION
Our results suggested that prenatal tobacco and childhood lead exposures were risk factors for ADHD in a nationally representative sample of US children and that the effects of the 2 exposures were even greater than would be expected if their independent effects were multiplied. These findings provide additional support for the association of prenatal tobacco exposure6,10,16 and childhood lead exposure19,20 with inattention and hyperactivity. In addition, we are the first, to our knowledge, to identify and to quantify the joint effects on ADHD of these common toxicant exposures.
This study had several strengths, including our use of current ADHD diagnostic criteria and a national, population-based sample of children with low-level lead exposures that are relevant for contemporary children.
Our findings agree with previous smaller studies that documented relationships between DSM-IV–defined ADHD and prenatal tobacco exposure10,16 and childhood lead exposure,19,20 and our use of a national, population-based sample yields increased generalizability. Evidence of the link between ADHD and the toxicants remained significant even after adjustment for a range of covariates and exclusion of children with current blood lead levels of >5 µg/dL. It is important to note that, although only a small subset of children (n = 51) in our sample had current lead levels of >5 µg/dL, it is likely that many more had peak lead concentrations above this level, because longitudinal studies documented peak levels that were 1.9 to 2.8 times greater than levels in later childhood (eg, 5 to 6 years of age).47,48
We documented a significant interaction between prenatal tobacco exposure and childhood lead exposure, such that children with both exposures had a more than eightfold increased likelihood of ADHD, compared with children with neither exposure. This finding adds to the small but growing literature documenting the interactive effects of toxicant exposures on neurodevelopmental outcomes,49,50 including previous evidence that hyperactivity was potentiated in animals exposed to both nicotine neonatally and paraoxon during adulthood.51 The published literature includes extensive documentation of the impact of both tobacco27,52 and lead25,26 on brain dopamine systems, which provides a plausible locus for their joint effects. Indeed, previous studies showed both lead53–56 and tobacco16,57–59 effects on dopamine receptors. Additional sites of action, such as the dopamine transporter,60,61 also may play a key role in the toxicants’ ADHD-related interaction. The multihit model of neurotoxicity postulates that insults to different targets within a specific brain system compromise homeostatic and repair capacities, thereby increasing the system’s vulnerability.21
Important limitations to our study must be noted. First, our study cannot verify causality because of its cross-sectional design. It has been postulated that the relationships between the toxicant exposures and ADHD might be explained by unmeasured genetic factors (a propensity to smoke might be associated with maternal ADHD43 that is transmitted genetically to the offspring) or the presence of confounding environmental factors, such as prenatal alcohol exposure.8 However, previous investigations that addressed those factors still found significant associations between the toxicants and ADHD, including studies that accounted for genetic influences62 and studies that adjusted for both parental psychopathologic conditions and prenatal alcohol use.8–10,12,20,63 Moreover, animal studies in which the case and control subjects had identical genetic lineages and differed only in their toxicant exposures documented links between ADHD-related phenotypes and both early tobacco64–67 and lead68–70 exposures.
In addition, our study is limited in that assessment of prenatal tobacco exposure was based on caregiver reports, rather than a biological marker.71 However, some previous studies suggested high reliability of maternal reports of smoking during pregnancy,72 with limited evidence for underreporting.73–76 Furthermore, because social desirability response bias favors misclassification of subjects who smoked during pregnancy as nonsmokers, which likely would attenuate smoking effects, it is notable that we still documented significant effects of prenatal tobacco exposure on ADHD. Additional limitations include our use of a dichotomous variable (yes/no) to measure prenatal tobacco exposure, which rendered us unable to assess dose-response and timing effects.40,77 These limitations underscore the need for future studies incorporating biomarkers of prenatal tobacco exposure and longitudinal assessment from the prenatal period to later childhood, such as in the National Children’s Study.78
Furthermore, we studied the relationship between ADHD and current, rather than peak, lead levels. However, this may prove to be a strength rather than a weakness, given the accumulating evidence that current blood lead levels are stronger predictors of cognitive outcomes than are peak levels.48,79,80 We were unable to determine the association between the toxicant exposures and specific ADHD subtypes because of limited sample size, and we did not have genetic information available for assessment of gene-environment interactions. Future studies with considerable sample sizes are needed to examine whether the toxicant exposures are associated with specific ADHD subtypes and/or endophenotypes12,24,81 and whether certain genetic subgroups are particularly susceptible to ADHD in the setting of lead53 and prenatal tobacco16,82,83 exposures. The study of joint toxicant-gene effects seems particularly promising, because Neuman et al16 recently documented that prenatally smoke-exposed children carrying both the high-risk dopamine transporter (DAT) and dopamine D4 receptor (DRD4) alleles had an elevated odds ratio of 9.0 for population-defined ADHD, combined subtype. Finally, it was beyond the scope of this cross-sectional study to determine what effect decreases in prenatal tobacco and childhood lead exposures over time might have had on ADHD rates. Numerous factors complicate investigation of US changes in ADHD prevalence over time, including changing ADHD diagnostic criteria and heightened awareness of the disorder.
CONCLUSIONS
We found that prenatal tobacco and childhood lead exposures may be significant risk factors for ADHD, especially when individuals are exposed to both toxicants. Although the United States has made considerable strides in reducing these toxicant exposures, 15% of women reported smoking during pregnancy in a large, US population-based study in 2004,84 and an estimated 1.6% of US children had blood lead levels above the Centers for Disease Control and Prevention level of concern (≥10 µg/dL) in 1999–2002, with almost 14% having levels of 5 to 9 µg/dL.85 Our findings suggest that reduction of toxicant exposures may be an important avenue for ADHD prevention, and they underscore the enormous burden that may be associated with continued exposure to tobacco and lead.
WHAT’S KNOWN ON THIS SUBJECT
Prenatal tobacco and childhood lead exposures have been associated with hyperactivity and inattentive symptoms, but little is known about their independent and potential combined effects on attention-deficit/hyperactivity disorder, as defined with current diagnostic criteria.
WHAT THIS STUDY ADDS
This study is the first to determine the independent effects of tobacco and lead exposures on ADHD in a nationally representative sample of US children using DSM-IV criteria for outcome assessment, and provides the first estimate of the joint effects of these common toxicants on ADHD.
ACKNOWLEDGMENTS
This study was supported by a Academic Pediatrics Association Young Investigator Grant (Dr Froehlich), grant K12HD028827 from the National Institutes of Health (Dr Froehlich), grant 1T32PE10027 from the Department of Health and Human Services (Dr Froehlich), grant T32-HD052468-01 from the National Institute of Child Health and Human Development (Mr Braun), and a Robert Wood Johnson Generalist Physician Faculty Scholars Award (Dr Kahn).
We thank Debra J. Brody, MPH (Division of Health Examination Statistics, National Center for Health Statistics, Centers for Disease Control and Prevention, Atlanta, GA), for her assistance with accessing the NHANES data set.
ABBREVIATIONS
- ADHD
attention-deficit/hyperactivity disorder
- CI
confidence interval
- aOR
adjusted odds ratio
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- NHANES
National Health and Nutrition Examination Survey
- DISC
Diagnostic Interview Schedule for Children
- PAF
population attributable fraction
Footnotes
Reprints Information about ordering reprints can be found online: Downloaded from http://www.pediatrics.org/misc/reprints.shtml
Data analyzed for this investigation were collected by the National Center for Health Statistics. All analyses, interpretations, and conclusions expressed in this article are those of the authors and not the National Center for Health Statistics, which is responsible only for the initial data.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Leibson CL, Katusic SK, Barbaresi WJ, Ransom J, O’Brien PC. Use and costs of medical care for children and adolescents with and without attention-deficit/hyperactivity disorder. JAMA. 2001;285(1):60–66. doi: 10.1001/jama.285.1.60. [DOI] [PubMed] [Google Scholar]
- 2.Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. J Pediatr Psychol. 2007;32(6):643–654. doi: 10.1093/jpepsy/jsl054. [DOI] [PubMed] [Google Scholar]
- 3.Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult outcome of hyperactive boys: educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993;50(7):565–576. doi: 10.1001/archpsyc.1993.01820190067007. [DOI] [PubMed] [Google Scholar]
- 4.Sherman DK, McGue MK, Iacono WG. Twin concordance for attention deficit hyperactivity disorder: a comparison of teachers’ and mothers’ reports. Am J Psychiatry. 1997;154(4):532–535. doi: 10.1176/ajp.154.4.532. [DOI] [PubMed] [Google Scholar]
- 5.Braun JM, Kahn RS, Froehlich TE, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in US children. Environ Health Perspect. 2006;114(12):1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linnet KM, Dalsgaard S, Obel C, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160(6):1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- 7.Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry. 1999;38(7):892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolesc Psychiatry. 2002;41(4):378–385. doi: 10.1097/00004583-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Milberger S, Biederman J, Faraone SV, Jones J. Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: findings from a high-risk sample of siblings. J Clin Child Psychol. 1998;27(3):352–358. doi: 10.1207/s15374424jccp2703_11. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz M, Denardin D, Laufer Silva T, et al. Smoking during pregnancy and attention-deficit/hyperactivity disorder, predominantly inattentive type: a case-control study. J Am Acad Child Adolesc Psychiatry. 2006;45(11):1338–1345. doi: 10.1097/S0890-8567(09)61916-X. [DOI] [PubMed] [Google Scholar]
- 11.Needleman HL, Gunnoe C, Leviton A, et al. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N Engl J Med. 1979;300(13):689–695. doi: 10.1056/NEJM197903293001301. [DOI] [PubMed] [Google Scholar]
- 12.Chiodo LM, Jacobson SW, Jacobson JL. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol. 2004;26(3):359–371. doi: 10.1016/j.ntt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Bellinger D, Hu H, Titlebaum L, Needleman HL. Attentional correlates of dentin and bone lead levels in adolescents. Arch Environ Health. 1994;49(2):98–105. doi: 10.1080/00039896.1994.9937461. [DOI] [PubMed] [Google Scholar]
- 14.Fergusson DM, Horwood LJ, Lynskey MT. Early dentine lead levels and subsequent cognitive and behavioural development. J Child Psychol Psychiatry. 1993;34(2):215–227. doi: 10.1111/j.1469-7610.1993.tb00980.x. [DOI] [PubMed] [Google Scholar]
- 15.Wasserman GA, Liu X, Pine DS, Graziano JH. Contribution of maternal smoking during pregnancy and lead exposure to early child behavior problems. Neurotoxicol Teratol. 2001;23(1):13–21. doi: 10.1016/s0892-0362(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 16.Neuman RJ, Lobos E, Reich W, Henderson CA, Sun LW, Todd RD. Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biol Psychiatry. 2007;61(12):1320–1328. doi: 10.1016/j.biopsych.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 17.Knopik VS, Sparrow EP, Madden PA, et al. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychol Med. 2005;35(5):625–635. doi: 10.1017/s0033291704004155. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez A, Bohlin G. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J Child Psychol Psychiatry. 2005;46(3):246–254. doi: 10.1111/j.1469-7610.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- 19.Nigg JT, Knottnerus GM, Martel MM, et al. Low blood lead levels associated with clinically diagnosed attention-deficit/hyperactivity disorder and mediated by weak cognitive control. Biol Psychiatry. 2008;63(3):325–331. doi: 10.1016/j.biopsych.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HL, Chen XT, Yang B, et al. Case-control study of blood lead levels and attention deficit hyperactivity disorder in Chinese children. Environ Health Perspect. 2008;116(10):1401–1406. doi: 10.1289/ehp.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cory-Slechta DA. Studying toxicants as single chemicals: does this strategy adequately identify neurotoxic risk? Neurotoxicology. 2005;26(4):491–510. doi: 10.1016/j.neuro.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Wigle DT, Arbuckle TE, Walker M, Wade MG, Liu S, Krewski D. Environmental hazards: evidence for effects on child health. J Toxicol Environ Health B Crit Rev. 2007;10(1–2):3–39. doi: 10.1080/10937400601034563. [DOI] [PubMed] [Google Scholar]
- 23.Wigle DT, Arbuckle TE, Turner MC, et al. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B Crit Rev. 2008;11(5–6):373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- 24.Nigg JT. What Causes ADHD? Understanding What Goes Wrong and Why. New York, NY: Guilford Press; 2006. [Google Scholar]
- 25.Schneider JS, Huang FN, Vemuri MC. Effects of low-level lead exposure on cell survival and neurite length in primary mesencephalic cultures. Neurotoxicol Teratol. 2003;25(5):555–559. doi: 10.1016/s0892-0362(03)00018-7. [DOI] [PubMed] [Google Scholar]
- 26.Cory-Slechta DA. Relationships between lead-induced learning impairments and changes in dopaminergic, cholinergic, and glutamatergic neurotransmitter system functions. Annu Rev Pharmacol Toxicol. 1995;35:391–415. doi: 10.1146/annurev.pa.35.040195.002135. [DOI] [PubMed] [Google Scholar]
- 27.Muneoka K, Nakatsu T, Fuji J, Ogawa T, Takigawa M. Prenatal administration of nicotine results in dopaminergic alterations in the neocortex. Neurotoxicol Teratol. 1999;21(5):603–609. doi: 10.1016/s0892-0362(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 28.Oliff HS, Gallardo KA. The effect of nicotine on developing brain catecholamine systems. Front Biosci. 1999;4:D883–D897. doi: 10.2741/oliff. [DOI] [PubMed] [Google Scholar]
- 29.Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- 30.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Bravo M, Ribera J, Rubio-Stipec M, et al. Test-retest reliability of the Spanish version of the Diagnostic Interview Schedule for Children (DISC-IV) J Abnorm Child Psychol. 2001;29(5):433–444. doi: 10.1023/a:1010499520090. [DOI] [PubMed] [Google Scholar]
- 32.Canino G, Shrout PE, Rubio-Stipec M, et al. The DSM-IV rates of child and adolescent disorders in Puerto Rico: prevalence, correlates, service use, and the effects of impairment. Arch Gen Psychiatry. 2004;61(1):85–93. doi: 10.1001/archpsyc.61.1.85. [DOI] [PubMed] [Google Scholar]
- 33.Miller DT, Paschal DC, Gunter EW, Stroud PE, D’Angelo J. Determination of lead in blood using electrothermal atomisation atomic absorption spectrometry with a L’vov platform and matrix modifier. Analyst. 1987;112(12):1701–1704. doi: 10.1039/an9871201701. [DOI] [PubMed] [Google Scholar]
- 34.Parsons P, Slavin W. A rapid Zeeman graphite furnace atomic absorption spectrometric method for the determination of lead in blood. Spectrochim Acta. 1993;48B(6/7):925–939. [Google Scholar]
- 35.Pastor PN, Reuben CA. Diagnosed attention deficit hyperactivity disorder and learning disability: United States, 2004 –2006. Vital Health Stat 10. 2008;(237):1–14. [PubMed] [Google Scholar]
- 36.Pastor PN, Reuben CA. Racial and ethnic differences in ADHD and LD in young school-age children: parental reports in the National Health Interview Survey. Public Health Rep. 2005;120(4):383–392. doi: 10.1177/003335490512000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claycomb CD, Ryan JJ, Miller LJ, Schnakenberg-Ott SD. Relationships among attention deficit hyperactivity disorder, induced labor, and selected physiological and demographic variables. J Clin Psychol. 2004;60(6):689–693. doi: 10.1002/jclp.10238. [DOI] [PubMed] [Google Scholar]
- 38.Nigg JT, Breslau N. Prenatal smoking exposure, low birth weight, and disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(3):362–369. doi: 10.1097/01.chi.0000246054.76167.44. [DOI] [PubMed] [Google Scholar]
- 39.Ben Amor L, Grizenko N, Schwartz G, et al. Perinatal complications in children with attention-deficit hyperactivity disorder and their unaffected siblings. J Psychiatry Neurosci. 2005;30(2):120–126. [PMC free article] [PubMed] [Google Scholar]
- 40.Williams GM, O’Callaghan M, Najman JM, et al. Maternal cigarette smoking and child psychiatric morbidity: a longitudinal study. Pediatrics. 1998;102(1) doi: 10.1542/peds.102.1.e11. Available at: www.pediatrics.org/cgi/content/full/102/1/e11. [DOI] [PubMed] [Google Scholar]
- 41.Weitzman M, Gortmaker S, Sobol A. Maternal smoking and behavior problems of children. Pediatrics. 1992;90(3):342–349. [PubMed] [Google Scholar]
- 42.Bernert JT, Jr, McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J Anal Toxicol. 2000;24(5):333–339. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- 43.Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62(10):1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- 44.Benowitz NL, Kuyt F, Jacob PIII, Jones RT, Osman AL. Cotinine disposition and effects. Clin Pharmacol Ther. 1983;34(5):604–611. doi: 10.1038/clpt.1983.222. [DOI] [PubMed] [Google Scholar]
- 45.Rothman KJ. Epidemiology: An Introduction. New York, NY: Oxford University Press; 2002. [Google Scholar]
- 46.Hanley JA. A heuristic approach to the formulas for population attributable fraction. J Epidemiol Community Health. 2001;55(7):508–514. doi: 10.1136/jech.55.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jusko TA, Henderson CR, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL. Blood lead concentrations <10 µg/dL and child intelligence at 6 years of age. Environ Health Perspect. 2008;116(2):243–248. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 µg per deciliter. N Engl J Med. 2003;348(16):1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barlow BK, Richfield EK, Cory-Slechta DA, Thiruchelvam M. A fetal risk factor for Parkinson’s disease. Dev Neurosci. 2004;26(1):11–23. doi: 10.1159/000080707. [DOI] [PubMed] [Google Scholar]
- 50.Thiruchelvam M, Richfield EK, Goodman BM, Baggs RB, Cory-Slechta DA. Developmental exposure to the pesticides paraquat and maneb and the Parkinson’s disease phenotype. Neurotoxicology. 2002;23(4–5):621–633. doi: 10.1016/s0161-813x(02)00092-x. [DOI] [PubMed] [Google Scholar]
- 51.Ankarberg E, Fredriksson A, Eriksson P. Increased susceptibility to adult paraoxon exposure in mice neonatally exposed to nicotine. Toxicol Sci. 2004;82(2):555–561. doi: 10.1093/toxsci/kfh274. [DOI] [PubMed] [Google Scholar]
- 52.Dwoskin LP, Teng L, Buxton ST, Crooks PA. (S)-(—)-Cotinine, the major brain metabolite of nicotine, stimulates nicotinic receptors to evoke [3H]dopamine release from rat striatal slices in a calcium-dependent manner. J Pharmacol Exp Ther. 1999;288(3):905–911. [PubMed] [Google Scholar]
- 53.Froehlich TE, Lanphear BP, Dietrich KN, Cory-Slechta DA, Wang N, Kahn RS. Interactive effects of a DRD4 polymorphism, lead, and sex on executive functions in children. Biol Psychiatry. 2007;62(3):243–249. doi: 10.1016/j.biopsych.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 54.Brockel BJ, Cory-Slechta DA. Lead-induced decrements in waiting behavior: involvement of D2-like dopamine receptors. Pharmacol Biochem Behav. 1999;63(3):423–434. doi: 10.1016/s0091-3057(99)00033-7. [DOI] [PubMed] [Google Scholar]
- 55.Widzowski DV, Finkelstein JN, Pokora MJ, Cory-Slechta DA. Time course of postnatal lead-induced changes in dopamine receptors and their relationship to changes in dopamine sensitivity. Neurotoxicology. 1994;15(4):853–865. [PubMed] [Google Scholar]
- 56.Cory-Slechta DA, Widzowski DV. Low level lead exposure increases sensitivity to the stimulus properties of dopamine D1 and D2 agonists. Brain Res. 1991;553(1):65–74. doi: 10.1016/0006-8993(91)90231-j. [DOI] [PubMed] [Google Scholar]
- 57.Bahk JY, Li S, Park MS, Kim MO. Dopamine D1 and D2 receptor mRNA up-regulation in the caudate-putamen and nucleus accumbens of rat brains by smoking. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(6):1095–1104. doi: 10.1016/s0278-5846(02)00243-9. [DOI] [PubMed] [Google Scholar]
- 58.Dagher A, Bleicher C, Aston JA, Gunn RN, Clarke PB, Cumming P. Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse. 2001;42(1):48–53. doi: 10.1002/syn.1098. [DOI] [PubMed] [Google Scholar]
- 59.Court JA, Lloyd S, Thomas N, et al. Dopamine and nicotinic receptor binding and the levels of dopamine and homovanillic acid in human brain related to tobacco use. Neuroscience. 1998;87(1):63–78. doi: 10.1016/s0306-4522(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 60.Middleton LS, Cass WA, Dwoskin LP. Nicotinic receptor modulation of dopamine transporter function in rat striatum and medial prefrontal cortex. J Pharmacol Exp Ther. 2004;308(1):367–377. doi: 10.1124/jpet.103.055335. [DOI] [PubMed] [Google Scholar]
- 61.Weiss S, Tzavara ET, Davis RJ, et al. Functional alterations of nicotinic neurotransmission in dopamine transporter knock-out mice. Neuropharmacology. 2007;52(7):1496–1508. doi: 10.1016/j.neuropharm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Thapar A, Fowler T, Rice F, et al. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. Am J Psychiatry. 2003;160(11):1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- 63.Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Arch Gen Psychiatry. 1998;55(8):721–727. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- 64.Peters DA, Taub H, Tang S. Postnatal effects of maternal nicotine exposure. Neurobehav Toxicol. 1979;1(3):221–225. [PubMed] [Google Scholar]
- 65.Ajarem JS, Ahmad M. Prenatal nicotine exposure modifies behavior of mice through early development. Pharmacol Biochem Behav. 1998;59(2):313–318. doi: 10.1016/s0091-3057(97)00408-5. [DOI] [PubMed] [Google Scholar]
- 66.Sorenson CA, Raskin LA, Suh Y. The effects of prenatal nicotine on radial-arm maze performance in rats. Pharmacol Biochem Behav. 1991;40(4):991–993. doi: 10.1016/0091-3057(91)90117-k. [DOI] [PubMed] [Google Scholar]
- 67.Yanai J, Pick CG, Rogel-Fuchs Y, Zahalka EA. Alterations in hippocampal cholinergic receptors and hippocampal behaviors after early exposure to nicotine. Brain Res Bull. 1992;29(3–4):363–368. doi: 10.1016/0361-9230(92)90069-a. [DOI] [PubMed] [Google Scholar]
- 68.Rice DC, Karpinski KF. Lifetime low-level lead exposure produces deficits in delayed alternation in adult monkeys. Neurotoxicol Teratol. 1988;10(3):207–214. doi: 10.1016/0892-0362(88)90019-0. [DOI] [PubMed] [Google Scholar]
- 69.Rice DC. Chronic low-lead exposure from birth produces deficits in discrimination reversal in monkeys. Toxicol Appl Pharmacol. 1985;77(2):201–210. doi: 10.1016/0041-008x(85)90319-9. [DOI] [PubMed] [Google Scholar]
- 70.Levin ED, Bowman RE. The effect of pre- or postnatal lead exposure on Hamilton search task in monkeys. Neurobehav Toxi-col Teratol. 1983;5(3):391–394. [PubMed] [Google Scholar]
- 71.Russell T, Crawford M, Woodby L. Measurements for active cigarette smoke exposure in prevalence and cessation studies: why simply asking pregnant women isn’t enough. Nicotine Tob Res. 2004;6 suppl 2:S141–S151. doi: 10.1080/14622200410001669141. [DOI] [PubMed] [Google Scholar]
- 72.Reich W, Todd RD, Joyner CA, Neuman RJ, Heath AC. Reliability and stability of mothers’ reports about their pregnancies with twins. Twin Res. 2003;6(2):85–88. doi: 10.1375/136905203321536209. [DOI] [PubMed] [Google Scholar]
- 73.Heath AC, Knopik VS, Madden PA, et al. Accuracy of mothers’ retrospective reports of smoking during pregnancy: comparison with twin sister informant ratings. Twin Res. 2003;6(4):297–301. doi: 10.1375/136905203322296656. [DOI] [PubMed] [Google Scholar]
- 74.Ershoff DH, Mullen PD, Quinn VP. A randomized trial of a serialized self-help smoking cessation program for pregnant women in an HMO. Am J Public Health. 1989;79(2):182–187. doi: 10.2105/ajph.79.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Price JH, Krol RA, Desmond SM, Losh DP, Roberts SM, Snyder FF. Comparison of three antismoking interventions among pregnant women in an urban setting: a randomized trial. Psychol Rep. 1991;68(2):595–604. doi: 10.2466/pr0.1991.68.2.595. [DOI] [PubMed] [Google Scholar]
- 76.Windsor RA, Cutter G, Morris J, et al. The effectiveness of smoking cessation methods for smokers in public health maternity clinics: a randomized trial. Am J Public Health. 1985;75(12):1389–1392. doi: 10.2105/ajph.75.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Callaghan MJ, Williams GM, Andersen MJ, Bor W, Najman JM. Obstetric and perinatal factors as predictors of child behaviour at 5 years. J Paediatr Child Health. 1997;33(6):497–503. doi: 10.1111/j.1440-1754.1997.tb01658.x. [DOI] [PubMed] [Google Scholar]
- 78.Landrigan PJ, Trasande L, Thorpe LE, et al. The National Children’s Study: a 21-year prospective study of 100 000 American children. Pediatrics. 2006;118(5):2173–2186. doi: 10.1542/peds.2006-0360. [DOI] [PubMed] [Google Scholar]
- 79.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113(7):894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen A, Dietrich KN, Ware JH, Radcliffe J, Rogan WJ. IQ and blood lead from 2 to 7 years of age: are the effects in older children the residual of high blood lead concentrations in 2-year-olds? Environ Health Perspect. 2005;113(5):597–601. doi: 10.1289/ehp.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Langley K, Holmans PA, van den Bree MB, Thapar A. Effects of low birth weight, maternal smoking in pregnancy and social class on the phenotypic manifestation of attention deficit hyperactivity disorder and asso ciated antisocial behaviour: investigation in a clinical sample. BMC Psychiatry. 2007;7:26. doi: 10.1186/1471-244X-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Todd RD, Neuman RJ. Gene-environment interactions in the development of combined type ADHD: evidence for a synapse-based model. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(8):971–975. doi: 10.1002/ajmg.b.30640. [DOI] [PubMed] [Google Scholar]
- 83.Kahn RS, Khoury J, Nichols WC, Lanphear BP. Role of dopamine transporter genotype and maternal prenatal smoking in childhood hyperactive-impulsive, inattentive, and oppositional behaviors. J Pediatr. 2003;143(1):104–110. doi: 10.1016/S0022-3476(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 84.Allen AM, Dietz PM, Tong VT, England L, Prince CB. Prenatal smoking prevalence ascertained from two population-based data sources: birth certificates and PRAMS questionnaires, 2004. Public Health Rep. 2008;123(5):586–592. doi: 10.1177/003335490812300508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Centers for Disease Control and Prevention. Blood lead levels: United States, 1999–2002. MMWR Morb Mortal Wkly Rep. 2005;54(20):513–516. [PubMed] [Google Scholar]