Abstract
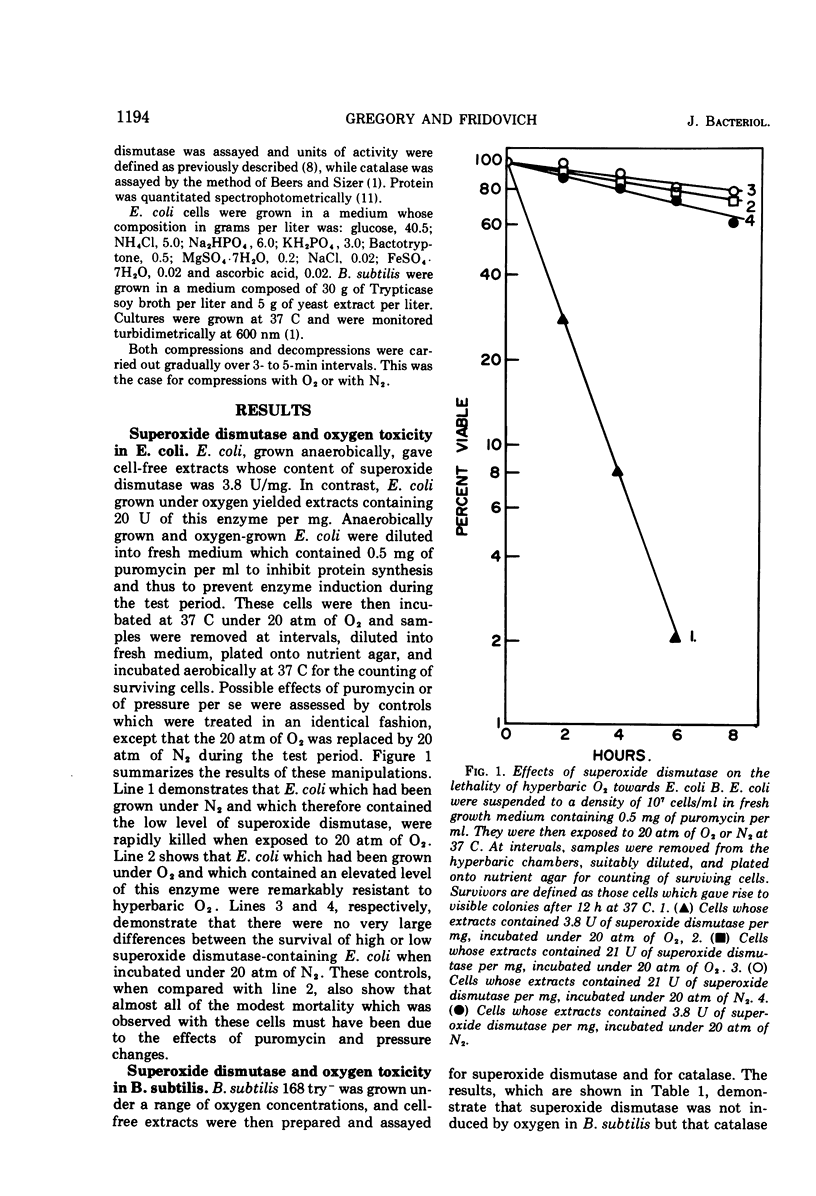
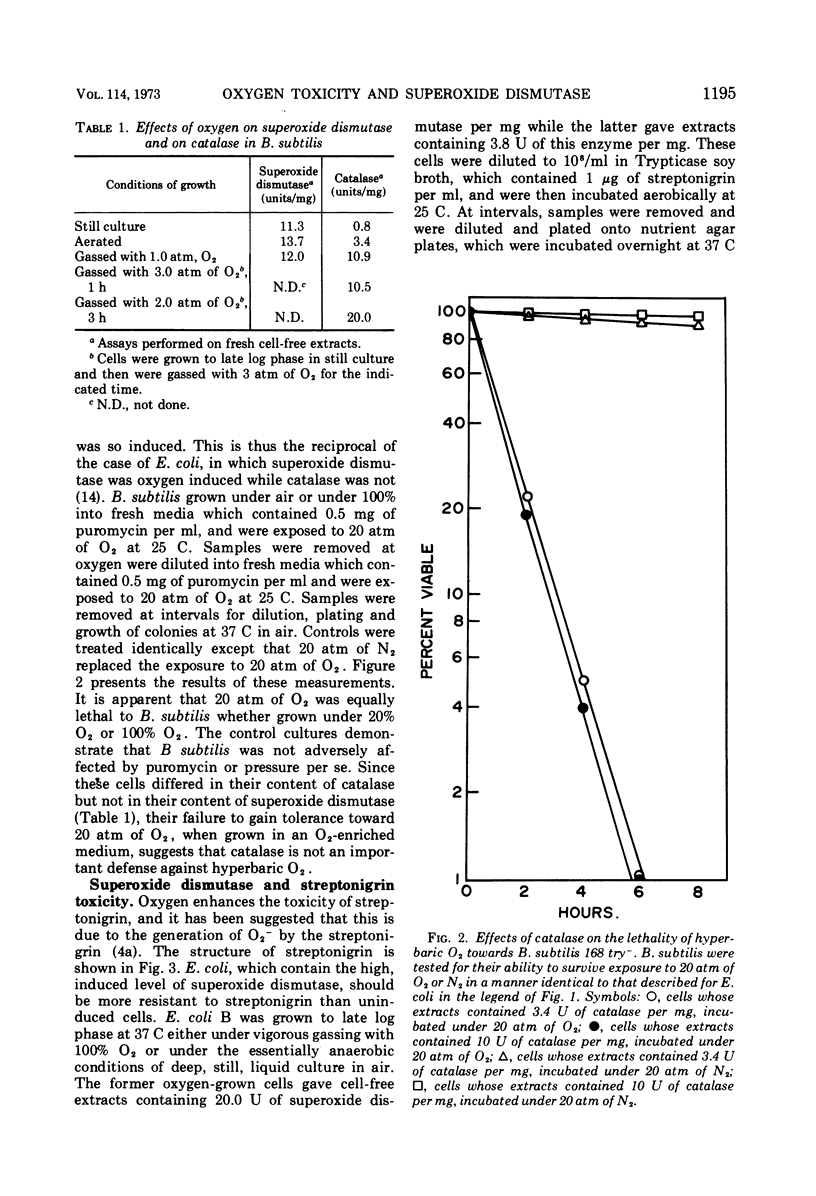
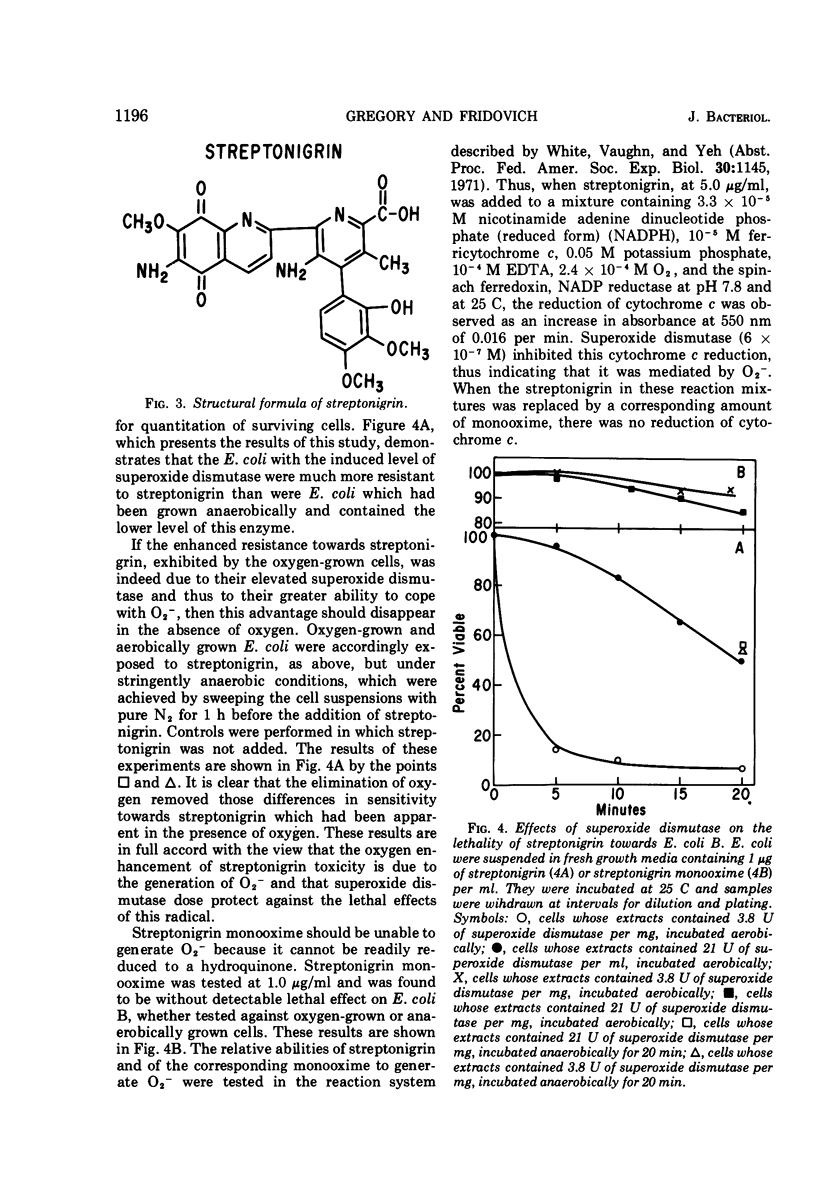
Oxygen caused an increase in the amount of superoxide dismutase in Escherichia coli B but not in Bacillus subtilis. E. coli B cells, induced by growth under 100% O2, were much more resistant to the lethal effects of 20 atm of O2 than were cells which contained the low uninduced level of this enzyme. In contrast, B. subtilis, which could not respond to O2 by increasing its content of superoxide dismutase, remained equally sensitive to hyperbaric O2 whether grown under 100% O2 or areobically. The catalase in these organisms exhibited a reciprocal response to oxygen. Thus, the catalase of E. coli B was not induced by O2, whereas that of B. subtilis was so induced. These results are consistent with the view that superoxide dismutase is an important component of the defenses of these organisms against the toxicity of oxygen, whereas their catalases are of secondary importance in this respect. The ability of streptonigrin to generate O2−, by a cycle of reduction followed by spontaneous reoxidation, has been verified in vitro. It is further observed that E. coli B which contain the high induced level of superoxide dismutase were more resistant to the lethality of this antibiotic, in the presence of oxygen, than were E. coli B which contained the low uninduced level of this enzyme. This difference between induced and uninduced cells was eliminated by the removal of O2. These results are consistent with the proposal that the enhanced lethality of streptonigrin under aerobic conditions may relate to its in vivo generation of O2− by a cycle of reduction and spontaneous reoxidation. In toto, these observations lend support to the hypothesis that O2− is an important agent of oxygen toxicity and that superoxide dismutase functions to blunt the threat posed by this reactive radical.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Gottlieb S. F. Effect of hyperbaric oxygen on microorganisms. Annu Rev Microbiol. 1971;25:111–152. doi: 10.1146/annurev.mi.25.100171.000551. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973 May;114(2):543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugaard N. Cellular mechanisms of oxygen toxicity. Physiol Rev. 1968 Apr;48(2):311–373. doi: 10.1152/physrev.1968.48.2.311. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Turbidity measurements of bacterial cultures in some available commercial instruments. Anal Biochem. 1970 Nov;38(1):252–259. doi: 10.1016/0003-2697(70)90174-0. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem. 1972 Jan 10;247(1):188–192. [PubMed] [Google Scholar]
- White H. L., White J. R. Interaction of streptonigrin with DNA in vitro. Biochim Biophys Acta. 1966 Sep;123(3):648–651. doi: 10.1016/0005-2787(66)90241-3. [DOI] [PubMed] [Google Scholar]
- White J. R., Dearman H. H. Generation of free radicals from phenazine methosulfate, streptonigrin, and riboflavin in bacterial suspensions. Proc Natl Acad Sci U S A. 1965 Sep;54(3):887–891. doi: 10.1073/pnas.54.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]



