Abstract
CD36 participates in macrophage internalization of a variety of particles, and has been implicated in inflammatory responses to many of these ligands. To what extent CD36 co-operates with other receptors in mediating these processes remains unclear. Because CD36 has been shown to co-operate with TLR2, we investigated the roles and interactions of CD36 and TLRs in inflammation and phagocytosis. Using antibody-induced endocytosis of CD36 and phagocytosis of erythrocytes displaying antibodies to CD36, we show that selective engagement and internalization of this receptor did not lead to pro-inflammatory cytokine production by primary human and murine macrophages. In addition, CD36-mediated phagocytosis of Plasmodium falciparum malaria-parasitized erythrocytes (PEs), which contain parasite components that activate TLRs, also failed to induce cytokine secretion from primary macrophages. Furthermore, we demonstrate that CD36-mediated internalization did not require TLR2 or the TLR-signaling molecule IRAK4. However, macrophage pre-treatment with TLR agonists markedly stimulated particle uptake via CD36. Similarly, PE uptake was unaffected by TLR deficiency, but in wild-type cells was increased by pre-treatment with purified P. falciparum glycosylphosphatidylinositols, which activate TLR2. Our findings indicate that CD36 must co-operate with other receptors such as TLRs to participate in cytokine responses. Although purified P. falciparum components activate TLRs, CD36-mediated internalization of intact PEs is not inflammatory. Further, CD36 mediates internalization of particles, including PEs, independently of TLR signaling, but can functionally co-operate with TLRs to enhance internalization.
INTRODUCTION
CD36, a member of the class B scavenger receptor family, is expressed in a variety of cell types and binds a diverse array of ligands (1). CD36 has been shown to participate in the internalization of a number of particles, including β-amyloid (2), oxidized low-density lipoproteins (oxLDL)4 (3), non-opsonized bacteria (4), apoptotic cells (5), and Plasmodium falciparum malaria-infected erythrocytes (6). Binding and uptake of β-amyloid, oxLDL, and bacteria is accompanied by release of pro-inflammatory mediators, and CD36 has also been implicated in these responses (4, 7–10). In some cases, CD36 is thought to directly mediate the observed inflammation: β-amyloid stimulation of microglia was reported to induce a CD36-dependent pro-inflammatory signaling cascade (7).
Despite the implication of CD36 in inflammatory responses to many of its ligands, internalization of apoptotic cells is non-inflammatory (11, 12). It may be that the pro-inflammatory effects of CD36 engagement during apoptotic cell clearance are suppressed by immunoregulatory cytokines such as IL-10 and TGFβ that are secreted during this process (11, 12). An alternative possibility is that CD36 does not directly mediate pro-inflammatory signaling, but rather presents ligands for recognition by other signaling receptors. In support of this hypothesis, CD36 has been shown to augment cytokine responses to Toll-like receptor 2 (TLR2) agonists such as Staphylococcus aureus lipoteichoic acid (LTA) (13, 14). By analogy to the role of CD14 in presenting LPS to TLR4, it has been proposed that CD36 concentrates ligands to facilitate TLR2 recognition (13). Thus, it remains unclear whether CD36 can independently mediate inflammation, or whether it must partner with other receptors such as TLRs to contribute to inflammatory responses.
While recent studies have focused on CD36-TLR2 interactions in the context of inflammation, these receptors could also conceivably co-operate in mediating phagocytosis. Although TLRs do not mediate phagocytosis directly (15), they have been shown to enhance macrophage uptake of bacterial pathogens (16–18), and in one report this was dependent on upregulation of scavenger receptors (17). However, it is not known if TLRs can modulate CD36-mediated internalization.
In this study, we dissected the roles and interactions of CD36 and TLRs in inflammation and phagocytosis. The existing uncertainty regarding the interaction between CD36 and TLRs in mediating inflammatory responses results, at least in part, from the complexity of the experimental models used. To simplify the analysis, we devised model systems that selectively activate CD36 to investigate whether this receptor can mediate inflammation independently of TLRs. We also employed selective, receptor-targeted strategies to assess whether CD36 and TLRs can co-operatively mediate phagocytosis. Furthermore, we explored potential CD36-TLR collaborations in an in vitro malaria-macrophage model, as CD36 mediates internalization of P. falciparum parasitized erythrocytes (PEs) and purified P. falciparum components have been shown to stimulate inflammation via TLRs (19–22). Here, we demonstrate that targeted activation and internalization of CD36 do not by themselves stimulate pro-inflammatory cytokine production. Despite the presence of malaria TLR agonists in PEs, CD36-mediated PE internalization was also found to be non-inflammatory. In addition, we show that CD36 and TLRs can co-operate to promote internalization of particles, including PEs.
MATERIALS AND METHODS
Parasites
P. falciparum (ITG strain) was cultured as previously described (23). Cultures were treated with Mycoplasma-Removal Agent (MP Biochemicals), confirmed to be mycoplasma-free (MycoAlert Mycoplasma Detection Kit, Lonza), and synchronized by alanine treatment (24). Mature-stage cultures were washed and used at a 20:1 PE:macrophage ratio.
Mice
C57BL/6 mice were purchased from Charles River. Cd36−/−, Tlr2−/−, and Irak4−/− mice on the C57BL/6 background were bred in the University of Toronto animal facility. Animal protocols were approved by the Animal Care Committee of the University of Toronto and all animal work was performed in compliance with university institutional guidelines.
Macrophage isolation
Human peripheral blood mononuclear cells were isolated from blood of healthy volunteers by gradient centrifugation using Ficoll-Paque (GE Healthcare). Monocytes were purified by adherence to glass coverslips in 24-well plates (1.5×106 PBMCs/well), and cultured for 3–5 days in RPMI 1640 with 10% fetal bovine serum and gentamicin (Gibco-Invitrogen; medium R10G) to allow for differentiation into macrophages. Peritoneal murine macrophages were isolated (23), adhered to coverslips in 24-well plates (0.125×106/well) in R10G, and used within 5 days.
CD36 cross-linking and endocytosis assay
Human macrophages were incubated on ice for 15 min with mouse-α-human CD36 IgG clone 131.2 (a kind gift from Dr. Narendra N. Tandon, Thrombosis Research Laboratory, Otsuka Maryland Medicinal Laboratories; 3.3 µg/ml) or mouse non-immune matched IgG1 isotype (Ebioscience). For murine macrophages, mouse-α-mouse CD36 IgA clone 63 (Cascade Biosciences; 2 µg/mL) or isotype control antibody (Ebioscience) were used. Cells were washed twice with cold PBS and incubated on ice for 10 min with a secondary antibody to cross-link CD36: goat-α-mouse IgG-Cy2 antibody (Jackson ImmunoResearch Laboratories) and goat-α-mouse IgA-FITC antibody (Sigma) for human and mouse macrophages, respectively. For imaging of cell surface CD36, cells were fixed immediately with 4% PFA. For imaging of endocytosed CD36-antibody complexes, cells were incubated at 37°C for 30 min before fixation, and fluorescent staining of remaining surface CD36 was performed with donkey-α-goat IgG-Cy3 tertiary antibody (Jackson ImmunoResearch Laboratories). Images were acquired with a spinning disk confocal microscope and Volocity acquisition software. To assess cytokine production, macrophages were incubated at 37°C for 24 hrs following cross-linking; supernatants were collected and assayed by ELISA for TNF and IL-6 (Ebioscience, BD Bioscience). LPS (Sigma; 100 ng/mL) was used as a positive control.
Preparation of erythrocytes coated with anti-CD36 antibody
Erythrocytes coated with Biotin, Avidin, and Biotinylated antibody (EBABs) were generated as previously reported (25, 26). Briefly, human erythrocytes were sequentially treated with biotin and avidin; then incubated with a biotinylated mouse-α-human CD36, clone SMO (ID Labs) to target EBABs to human CD36, or with a biotinylated goat-α-mouse Ig F(ab’)2 (Abcam) followed by a mouse-α-mouse CD36 (clone 63, Cascade Bioscience) to target EBABs to murine CD36. Control EBABs for human and murine systems were prepared using a biotinylated mouse IgM isotype-matched antibody (ID Labs) or a mouse IgA isotype-matched antibody (Ebioscience), respectively. Antibody conjugation was confirmed using Cy3-labeled secondary antibodies (Jackson Immunoresearch Laboratories). EBABs were added to macrophages at a 20:1 ratio.
Internalization assays
PE internalization assays were performed as described (23) for 2 hours. Images were acquired with an Olympus BX41 microscope and an Infinity2 camera. EBAB internalization assays were performed at 37°C; macrophages were then washed with PBS, subjected to a hypotonic lysis to remove bound but not internalized EBABs, and fixed with 0.75% glutaraldehyde (Sigma). EBAB binding assays were performed at 4°C and fixed without hypotonic lysis. EBABs were stained with o-dianisidine as described (27), and macrophages were stained with 1% eosin. Differential interference contrast (DIC) microscopy was used for imaging of EBABs. To demonstrate EBAB uptake, murine macrophages were incubated with EBABs for 30 min at 37°C, then incubated for 10 min on ice with donkey-α-goat IgG-Cy3 antibody to stain bound EBABs. A hypotonic lysis was performed, and DIC and fluorescence microscopy were used to identify unstained (i.e. internalized) EBABs. Phagocytic indices were determined as reported (23).
Cytokine release upon CD36-mediated phagocytosis
α-CD36 EBABs or P. falciparum cultures were added to macrophages in R10G. Control EBABs or uninfected red blood cells (uRBC) were used as negative controls, respectively, and LPS (100 ng/mL) was used as a positive control. In some experiments, macrophages were pre-incubated for 12 hrs with recombinant IFN-γ from the corresponding species (Ebioscience; 100 U/mL). After 4 and 8 hrs at 37°C, supernatants were collected and assayed by ELISA.
Treatment with TLR2 agonists
HPLC-purified PfGPI was isolated and coated onto gold beads as previously described (21, 28) to enhance stability and facilitate quantification. For cytokine assays, macrophages were incubated with PfGPI, or gold beads alone as a control, in R10G (200 ng/mL) for 24 hrs at 37°C; supernatants were collected and assayed by ELISA. For internalization assays, macrophages were pre-incubated at 37°C with PfGPI or gold beads (400 ng/mL), FSL-1 (Invivogen; 20 ng/mL), or Pam3CSK4 (Invivogen; 100 ng/mL) in RPMI, followed by addition of EBABs or PEs in a 50 µL volume.
Flow cytometry
Following treatment with media or TLR2 agonists, human or murine macrophages were washed with cold PBS with 2% fetal bovine serum and gently scraped. Human macrophages were blocked with 10% mouse serum for 10 min, then stained with mouse-α-human CD36 conjugated to FITC (clone FA6.152, IOTest; 20 µL/test) for 20 min on ice. A rat IgG isotype-matched antibody conjugated to FITC (Ebioscience; 20 µL/test) was used as a control. Murine macrophages were treated with anti-mouse CD16/32 (Ebioscience) for 10 min on ice to block Fc receptors, then incubated with mouse-α-mouse CD36 IgA (2 µg/mL) for 20 min, followed by rat-α-mouse IgA-PE (Ebioscience; 1.25 µg/mL) for 20 min on ice. A mouse IgA isotype-matched antibody plus secondary antibody was used as a control. Cells were analyzed using the FACSCalibur flow cytometer (Becton Dickinson) and FlowJo software.
Statistical analysis
Using GraphPad Prism software, differences between groups were analyzed using the Student’s t-test or one- or two-way ANOVA with Bonferroni post-tests. Each type of experiment was performed in duplicate or triplicate. Data are presented as means ± SD.
RESULTS
Targeted activation of CD36 and CD36-mediated internalization do not induce pro-inflammatory cytokine secretion
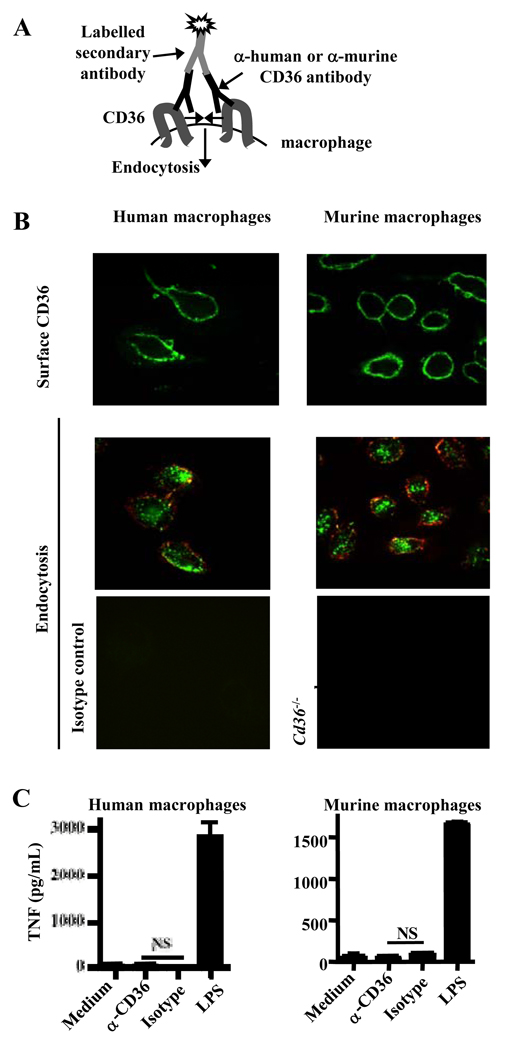
We initially examined whether CD36 could induce cytokine production, independently of TLRs or other pattern recognition receptors, by selectively activating CD36 by antibody-mediated cross-linking, leading to receptor endocytosis (Fig. 1A). Surface CD36 on primary human and murine macrophages was cross-linked on ice with an α-CD36 antibody and a fluorophore-conjugated secondary antibody (Fig. 1B, upper panels), and these antibody-induced complexes were internalized upon incubation at 37°C (Fig. 1B, middle panels). The system was CD36-specific, as no complexes were observed when using an isotype-matched antibody or Cd36−/− murine macrophages (Fig. 1B, lower panels).
Fig. 1. Antibody-induced CD36 cross-linking and endocytosis do not stimulate secretion of pro-inflammatory cytokines.
(A) Diagrammatic representation of antibody-induced CD36 cross-linking and endocytosis model. (B) Upper panels: Surface CD36 was cross-linked on primary human and murine macrophages by incubation at 4°C with α-CD36 antibody, followed by a FITC- or Cy2-conjugated secondary antibody. Middle panels: Following transfer of macrophages to 37°C, CD36-antibody complexes were internalized. Internalization of the complexes was confirmed by labeling the remaining surface CD36 with a tertiary Cy3-coupled antibody and examining vertical sections of cells constructed from Z-stacks (above and to left of individual images). Green signal within the macrophage represents internalized CD36-antibody complexes. Lower panels: The CD36-specific nature of the system was demonstrated by treating human macrophages with an isotype-matched antibody (left) and using Cd36−/− murine macrophages (right). (C) Macrophages were incubated at 37°C for 24 hr following CD36 crosslinking, and TNF levels in supernatants were assessed by ELISA. ND, not detectable. NS, not significant by Student’s t-test. Results are representative of three independent experiments.
Cytokine production was assessed 24 hrs following CD36 cross-linking and endocytosis. While LPS stimulated strong cytokine production, there was no secretion of TNF (Fig. 1C) or IL-6 (Fig. S1A) upon cross-linking with α-CD36 antibody. These data suggest that CD36 activation and endocytosis do not induce pro-inflammatory cytokine release.
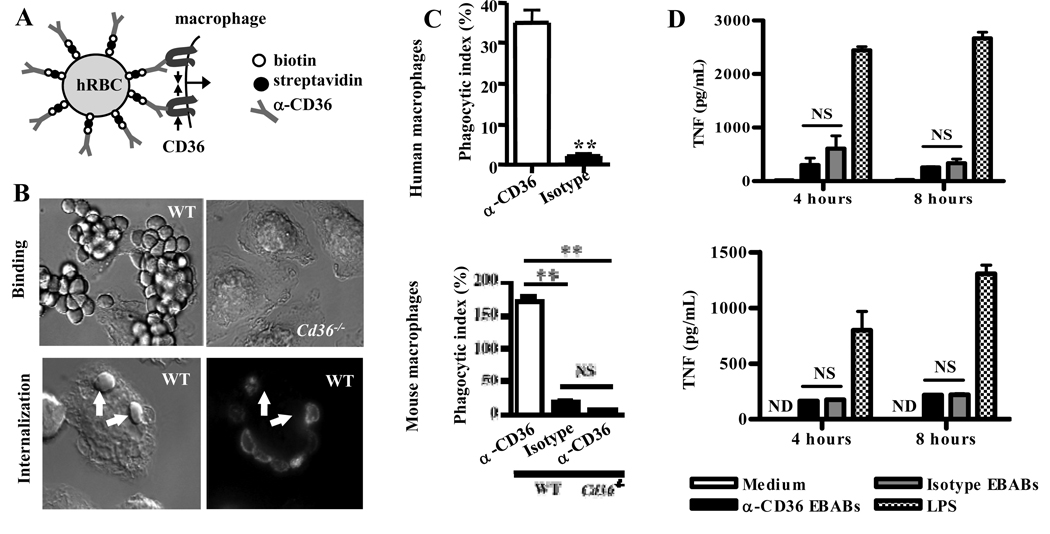
Signaling can differ when receptors are clustered into small vs. large aggregates (29, 30). We therefore investigated whether CD36-mediated phagocytosis of large particles stimulates inflammation. We employed erythrocytes conjugated to α-CD36 antibodies by an Erythrocyte-Biotin-Avidin-Biotinylated antibody (EBAB) coupling procedure ((25), Fig. 2A). α-CD36 EBABs bound to wild-type, but not Cd36−/−, macrophages (Fig. 2B, upper panels). α-CD36 EBAB internalization, confirmed by selective fluorescent labeling (Fig. 2B, lower panels), was CD36-specific. α-CD36 EBABs were poorly internalized by Cd36−/− macrophages, and EBABs prepared with an isotype-matched control antibody were poorly internalized by wild-type macrophages (Fig. 2C, bottom; p<0.01). Similarly, human macrophages bound (data not shown) and internalized α-CD36 EBABs but not control EBABs (Fig. 2C, top; p=0.004).
Fig. 2. CD36-mediated phagocytosis of large particles does not result in secretion of proinflammatory cytokines.
(A) Schematic diagram of human and mouse α-CD36 EBABs. α-CD36 antibodies conjugated to human red blood cells (RBC) via biotin and streptavidin crosslink CD36 on the macrophage surface, resulting in internalization. (B) Differential interference contrast images of α-CD36 EBABs binding to wild-type and Cd36−/− murine macrophages (upper panels) and internalization by wild-type macrophages (lower panels). After an internalization assay, EBAB uptake was confirmed by staining bound EBABs with a fluorophore-conjugated antibody; following a hypotonic lysis, the ghosts of lysed bound EBABs appeared stained, whereas internalized EBABs (indicated by arrows) did not. (C) Human macrophages (top) and wild-type and Cd36−/− murine macrophages (bottom) were incubated with α-CD36 EBABs and EBABs prepared with an isotype-matched control antibody, and phagocytosis was assessed. ** indicates p<0.01 by Student’s t-test (human data) or one-way ANOVA with Bonferroni post-tests (murine data). (D) α-CD36 EBABs or isotype control EBABs were incubated with human (top) or murine (bottom) macrophages at 37°C for 4 and 8 hr, and TNF in supernatants was measured by ELISA. NS, not significant by Student’s t-test. Results are representative of three independent experiments.
To assess the inflammatory outcomes of CD36-mediated phagocytosis, α-CD36 and control EBABs were incubated with macrophages for 4 and 8 hrs. CD36-mediated EBAB uptake did not induce TNF (Fig. 2D) or IL-6 (Fig. S1B) secretion. Thus, like CD36-mediated endocytosis, CD36-mediated phagocytosis of large particles did not stimulate pro-inflammatory cytokine release.
CD36-mediated PE internalization does not induce pro-inflammatory cytokine secretion
We next investigated the inflammatory outcomes of non-opsonic internalization of P. falciparum PEs, which is largely mediated by CD36 ((6, 23), Fig. S2A,B). While selective CD36 activation and internalization did not stimulate cytokine production (Fig. 1, 2), CD36 can participate in inflammatory responses to bacterial and fungal pathogens via collaboration with TLR2 (13, 14, 31). A number of purified Plasmodium components have been characterized as TLR agonists. Of note, TLR2 mediates macrophage TNF and IL-6 responses to purified P. falciparum glycosylphosphatidylinositols (PfGPI; Fig. S2C), which function as membrane anchors for parasite proteins (21). IRAK4, a signaling molecule downstream of most TLRs, is also required for these cytokine responses (Fig. S2C). Interestingly, PfGPI-induced TNF production was facilitated by CD36 ((28), Fig. S2C), indicating some degree of co-operation between CD36 and TLR2 in the macrophage response to malaria. However, it is unclear whether CD36-mediated internalization of whole PEs induces inflammation in co-operation with TLRs.
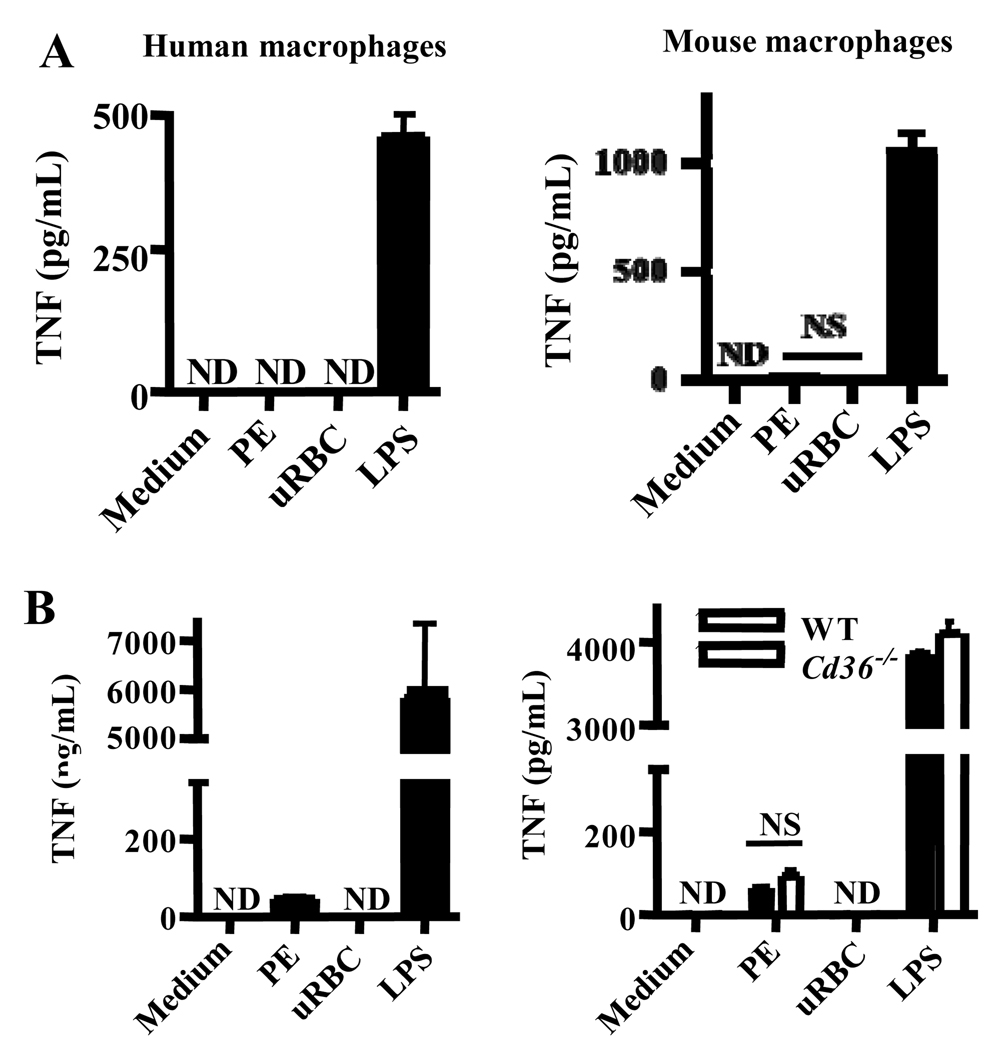
To assess the inflammatory outcomes of PE phagocytosis, we incubated human and murine macrophages with PEs or uninfected red blood cells (uRBC) as a control, then collected supernatants after 4 hrs. Since mature PEs rupture to release parasite progeny as well as inflammatory parasite products, we used washed, synchronized mid-mature-stage PEs to focus on the effects of intact PE internalization. While LPS stimulated TNF and IL-6 production, neither cytokine was produced by macrophages internalizing PEs or macrophages incubated with uRBCs (Fig. 3A; Fig. S1C), consistent with our previous observations (6, 35). Because macrophage priming by other immune cells is necessary for robust cytokine responses to P. falciparum (32), we repeated these experiments using macrophages primed with IFN-γ. This cytokine is produced early in malaria infection and enhances cytokine responses to PfGPI and other TLR agonists (21, 33, 34). Priming did not alter the CD36-dependence of PE uptake (data not shown). Although IFN-γ-primed macrophages internalizing PEs did not produce IL-6 (Fig S1C), they produced more TNF compared to macrophages incubated with uRBC (Fig. 3B). However, this increased TNF production was not a result of CD36-mediated PE internalization, as the same response was observed upon PE incubation with Cd36−/− macrophages (Fig. 3B). Thus, CD36-mediated PE uptake did not induce pro-inflammatory cytokine secretion despite the presence of TLR agonists in PEs, indicating a lack of co-operation between CD36 and TLRs in this context.
Fig. 3. CD36-mediated internalization of P. falciparum PEs by macrophages does not stimulate pro-inflammatory cytokine secretion.
(A) Human macrophages (left) and wild-type murine macrophages (right) were incubated for 4 hr at 37°C with carefully synchronized and washed mature-stage PEs, uninfected red blood cells (uRBC), or LPS. Supernatants were collected and analyzed by ELISA for TNF. NS, not significant by Student’s t-test. (B) To ensure that lack of priming was not obscuring inflammatory consequences of CD36-mediated PE internalization, macrophages were pre-incubated for 12 hr with IFN-γ (100 U/mL) prior to the internalization assay. Cd36−/− murine macrophages were included as a control to assess the contribution of CD36-mediated PE internalization to the low levels of TNF production observed. NS, not significant by Student’s t-test (human data); *** indicates p<0.001 and NS indicates not significant by one-way ANOVA with Bonferroni post-tests (murine data). ND, not detectable. Results are representative of three independent experiments.
CD36-mediated internalization is not impaired by the absence of TLRs, but is enhanced by macrophage pre-treatment with TLR agonists
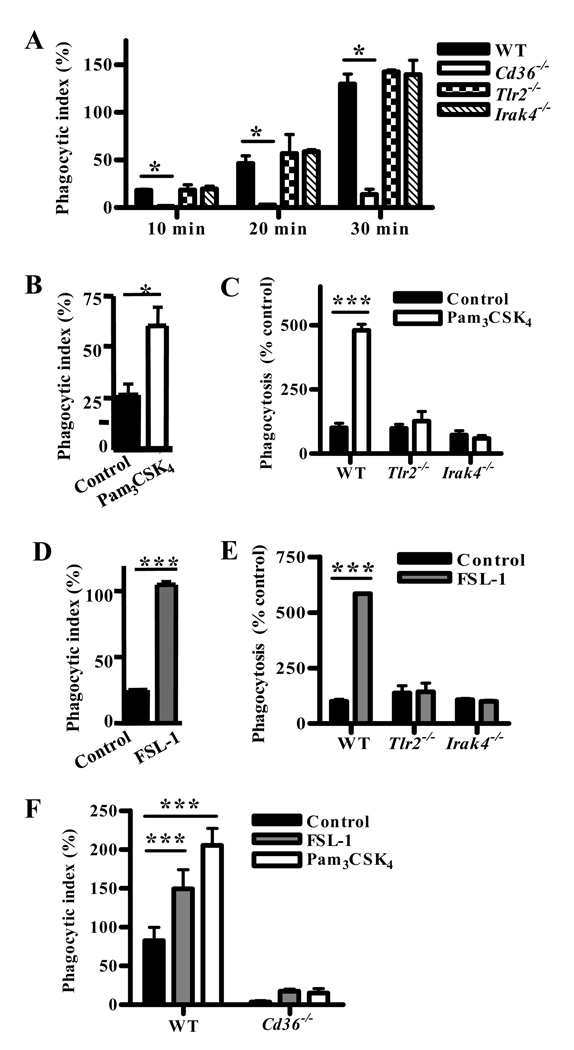
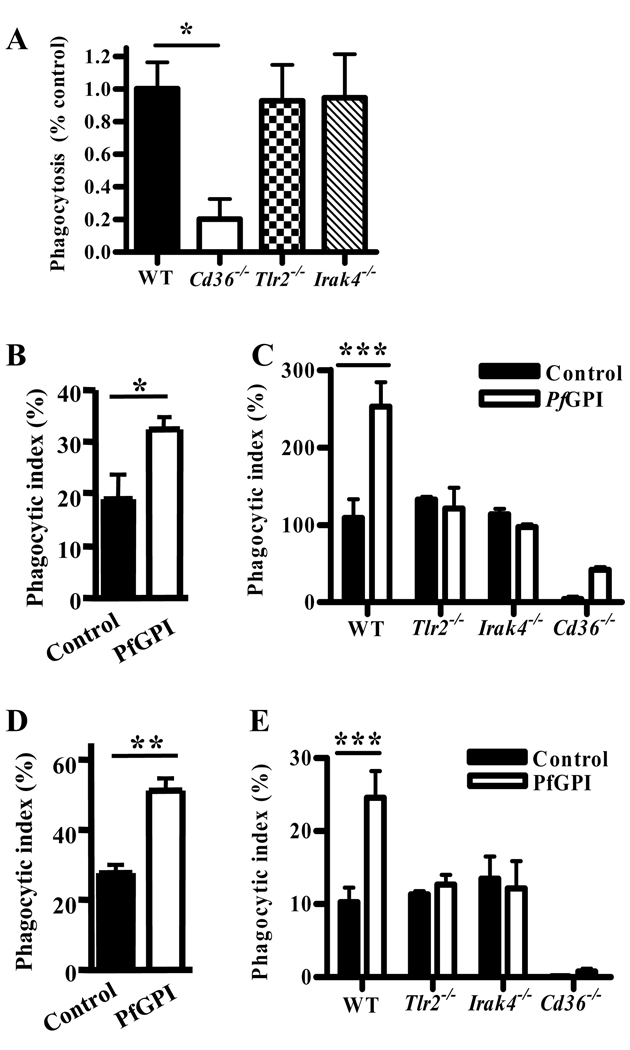
We next examined whether TLR2 or other TLRs co-operate with CD36 in mediating phagocytosis. Macrophage deficiency in TLR2 or IRAK4 did not impair internalization of α-CD36 EBABs by murine macrophages (Fig. 4A), indicating that TLRs are not essential for CD36-mediated internalization.
Fig. 4. Macrophage pre-stimulation by TLR2 agonists enhances CD36-mediated internalization in a TLR2-, IRAK4-, and CD36-dependent manner.
(A) Wild-type, Tlr2−/−, Irak4−/−, and Cd36−/− murine macrophages were incubated with α-CD36 EBABs over a 30 min time course, and phagocytic indices were determined. * indicates p<0.05 by one-way ANOVA with Bonferroni post-tests. (B) Human and (C) murine macrophages were treated with medium alone or TLR2 agonist Pam3CSK4 (100 ng/mL) for 1 hr, followed by a 30 min α-CD36 EBAB internalization assay. (D) Human and (E) murine macrophages were treated with medium alone or CD36-dependent TLR2 agonist FSL-1 (20 ng/mL) for 1 hour prior to α-CD36 EBAB internalization. (F) Wild-type and Cd36−/− murine macrophages were pre-stimulated with medium alone, Pam3CSK4, or FSL-1 for 1 hr prior to α-CD36 EBAB internalization. * indicates p<0.05, ** p<0.01, and *** p<0.001 by Student’s t-test (human data) or two-way ANOVA with Bonferroni post-tests (murine data). Data shown are representative of three independent experiments.
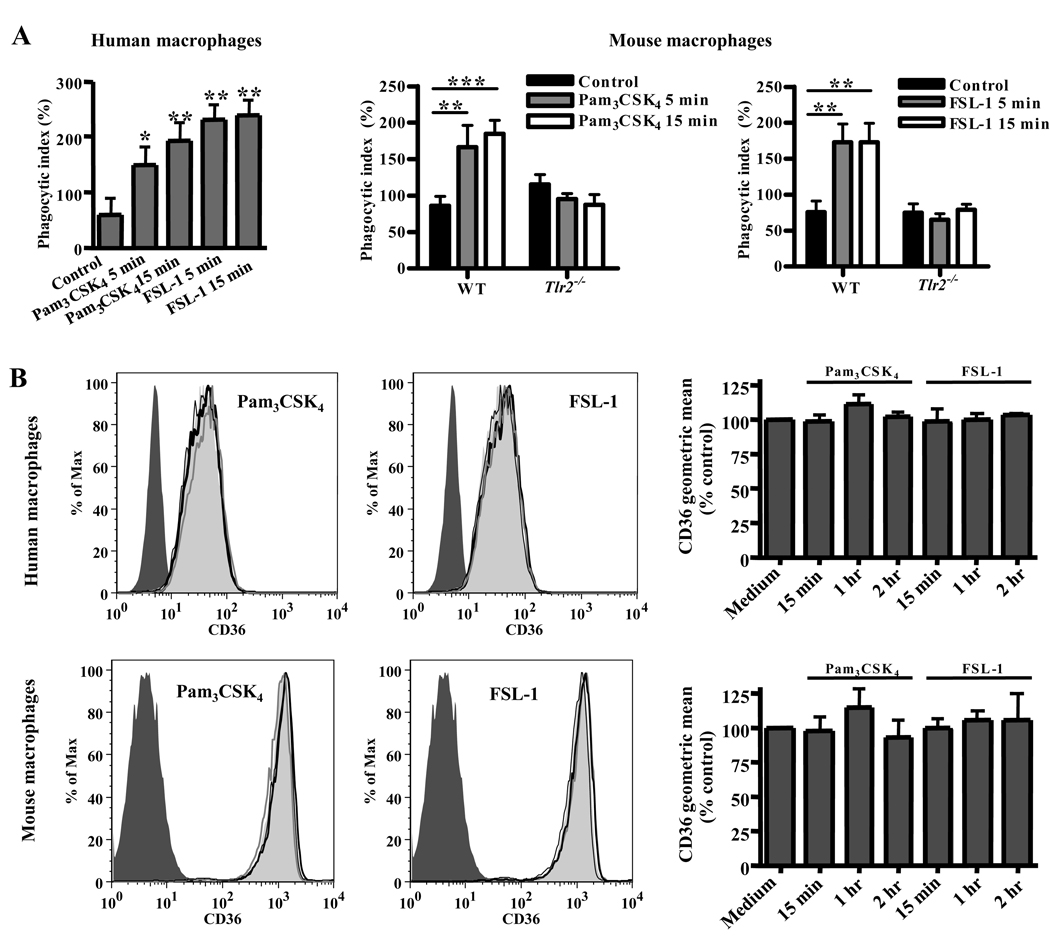
As macrophage pre-stimulation with exogenous TLR agonists has been shown to enhance phagocytosis (17), we next treated macrophages with synthetic TLR2 agonists for 1 hr prior to α-CD36 EBAB internalization. Pre-treatment with Pam3CSK4 increased EBAB internalization by human (Fig. 4B; p<0.05) and wild-type murine macrophages (Fig. 4C; p<0.001) in a TLR2- and IRAK4-dependent manner (Fig. 4C). Similar increases were observed upon pre-treatment of human (Fig. 4D; p<0.001) and murine macrophages (Fig. 4E; p<0.001) with FSL-1, which stimulates TLR2-mediated inflammation in a CD36-dependent manner (14). The enhanced uptake was largely CD36-dependent, as TLR2 pre-stimulation of Cd36−/− macrophages induced only a small increase in EBAB internalization over baseline compared to wild-type macrophages (Fig. 4F). This increase was not statistically significant, although we cannot exclude the minor contribution of other receptors to the observed enhancement of phagocytosis.
Other reports have demonstrated that TLR stimulation can upregulate expression of scavenger receptors, including CD36 (17, 35). We investigated the possibility that TLR2 activation enhanced CD36-mediated internalization in our system by augmenting surface CD36 levels. However, increased gene expression of CD36 seemed unlikely to play a role, as EBAB uptake was enhanced by FSL-1 and Pam3CSK4 pre-treatments as brief as 5 min (Fig. 5A). Further, we did not detect any change in surface CD36 levels after incubation with these ligands for 15 min, 1 hr, and 2hr (Fig. 5B). Thus, TLR2 stimulation does not enhance CD36-mediated internalization by increasing the number of CD36 receptors on the macrophage surface.
Fig. 5. Enhancement of CD36-mediated internalization by TLR2 stimulation occurs with brief pre-treatment periods and is not related to increased surface levels of CD36.
(A) Pre-stimulation with FSL-1 and Pam3CSK4 for 5 min and 15 min significantly increased uptake of α-CD36 EBABs by human macrophages (left) and wild-type murine macrophages, but not Tlr2−/− macrophages (right). * indicates p<0.05, ** p<0.01, and *** p<0.001 by one-way ANOVA (human data) or two-way ANOVA with Bonferroni post-tests (murine data). (B) Human and wild-type murine macrophages were incubated with medium alone (light gray shaded histogram) or with FSL-1 or Pam3CSK4 for 15 min (thick black line), 1 hr (gray line), or 2hr (thin black line). Cells were stained for surface CD36 and analyzed by flow cytometry. The dark gray shaded histogram indicates macrophages incubated with the appropriate isotype-matched antibody control. Histogram plots (left) show data from a representative experiment. For at least three independent experiments, the geometric means of CD36 levels were normalized to medium alone and pooled (right). None of the treatments induced a significant change in CD36 levels as analyzed by one-way ANOVA.
We next examined whether the enhancement of CD36-mediated internalization is specific to TLR2 or if other TLRs can exert a similar effect. Pre-stimulation of human and murine macrophages for 1 hr with LPS, CpG oligonucleotides, and polyI:C (which activate TLR4, TLR9, and TLR3, respectively) significantly enhanced phagocytosis of α-CD36 EBABs (Fig. S3). Collectively, these data demonstrate that multiple TLRs can co-operate with CD36 to promote phagocytosis.
CD36-mediated PE internalization is not dependent on TLRs, but is enhanced by pretreatment with TLR2 agonist PfGPI
We next investigated whether TLRs regulate CD36-mediated uptake of P. falciparum PEs. Unlike EBABs, PEs contain malaria TLR agonists that may stimulate TLRs and thus modulate uptake. However, uptake of PEs was similar in wild-type, Tlr2−/− and Irak4−/− macrophages (Fig. 6A), even upon IFN-γ pre-treatment for 12 hrs to enhance macrophage TLR responses (data not shown). Thus, TLR2 and other IRAK4-dependent TLRs were not essential for CD36-mediated internalization of PEs.
Fig. 6. TLRs are not required for CD36-mediated PE internalization, but macrophage pre-stimulation with a malaria TLR2 agonist enhances internalization of α-CD36 EBABs and PEs.
(A) Wild-type, Tlr2−/−, Irak4−/−, and Cd36−/− murine macrophages were incubated with P. falciparum PEs for 2 hr. Data represent the results of at least 5 independent experiments expressed as a percentage of the phagocytic index of wild-type macrophages and pooled together. * indicates p<0.05 by one-way ANOVA with Bonferroni post-tests. (B) Human and (C) murine macrophages were pre-treated with gold beads alone (control) or HPLC-purified PfGPI (400 ng/mL) for 1 hr, followed by a 30 min α-CD36 EBAB internalization assay. (D) Human and (E) murine macrophages were pre-treated with PfGPI or control beads for 2 hr, followed by 2 hr PE internalization assay. * indicates p<0.05, ** p<0.01, and *** p<0.001 by Student’s t-test (human data) or two-way ANOVA with Bonferroni post-tests (murine data). Results are representative of at least two independent experiments.
Although TLRs were not required for PE uptake, we hypothesized that macrophage pretreatment with the malaria TLR2 agonist PfGPI may modulate PE internalization, similar to TLR2-enhanced α-CD36 EBAB uptake. In support of this premise, human and wild-type murine macrophages pre-treated with purified PfGPI for 1 hr exhibited increased α-CD36 EBAB internalization compared to controls (Fig. 6B,C; p=0.011 and p<0.001, respectively), while uptake by Tlr2−/− and Irak4−/− macrophages was unaffected (Fig. 6C). Similarly, PfGPI pretreatment for 2 hrs enhanced non-opsonic PE internalization by human (Fig. 6D, p=0.005) and murine (Fig. 6E, p<0.001) macrophages in a TLR2- and IRAK4-dependent manner (Fig. 6E). Using Cd36−/− macrophages, we confirmed that the increased uptake was largely dependent on CD36 (Fig. 6C, E), but was not due to a PfGPI-induced increase in surface levels of CD36 (Fig. S4). These data indicate a functional co-operation between CD36 and TLR2 in promoting innate PE internalization by macrophages.
DISCUSSION
CD36 and TLRs: Co-operative roles in inflammation and phagocytosis
The role of CD36 in the regulation of inflammatory responses has been controversial, with some reports implicating direct CD36-mediated pro-inflammatory signaling (4, 7) and other reports suggesting a more passive role for CD36 in presenting ligands to TLRs (13). To address this issue, we used robust model systems to examine selective activation of CD36-induced endocytosis and phagocytosis. We did not observe pro-inflammatory cytokine production in either model (Fig. 1, 2), indicating that CD36 activation can generate signals to induce internalization without inducing cytokine secretion. Our data support the hypothesis that CD36 participates in inflammation by presenting ligands to other receptors such as TLRs, rather than independently initiating pro-inflammatory signals. This model is consistent with the diverse binding capacities of CD36 (1) and the induction of a physical association between CD36 and TLR2 upon FSL-1 treatment (14). Notably, oxLDL and β-amyloid have been found to stimulate TLRs (36, 37), raising the possibility that CD36 contributes to inflammatory responses to these ligands by facilitating their recognition by TLRs.
It was recently reported that transfection of CD36 into TLR2/4-deficient cell lines enhanced IL-8 responses to LPS and Gram negative bacteria, suggesting a role for CD36-mediated TLR-independent pro-inflammatory signaling (4). However, it is possible that in this system CD36 delivers ligands to other innate sensing receptors present in the cell. We cannot formally exclude a direct contribution of CD36-mediated signaling to cytokine responses initiated by other receptors, and CD36 may be able to independently induce production of specific inflammatory mediators, such as reactive oxygen species (38). Nevertheless, in the models we examined, CD36 activation alone was incapable of producing pro-inflammatory cytokines, suggesting that these responses depend on the ability of CD36 ligands to also activate pro-inflammatory signaling receptors such as TLRs.
While CD36 and TLR2 have been shown to co-operatively induce inflammation (8, 13, 14, 39), we demonstrate here a novel functional co-operation between these receptors in mediating phagocytosis. Although CD36-mediated internalization did not require TLRs, exogenous TLR2 activation enhanced CD36-mediated particle uptake (Fig. 4). In our experiments it is unlikely that TLR-mediated transcription of scavenger receptors (17, 35) is the basis for the increased phagocytosis, as surface CD36 levels did not change upon TLR2 stimulation (Fig. 5). Early signaling events via the MyD88-IRAK4 complex appear to be critical for TLR2-mediated enhancement of internalization, as Irak4−/− macrophages did not exhibit increased phagocytosis after stimulation with TLR2 agonists. The observation that TLR3 stimulation also promoted uptake indicates that MyD88-IRAK4-independent pathways can mediate this effect, and suggests the involvement of downstream events common to all TLRs. A number of signaling molecules activated by TLRs participate in phagocytosis, raising the possibility that synergy between TLR-and CD36-mediated signals underlies TLR enhancement of CD36-mediated internalization. For example, MAP kinases p38 and ERK have been implicated in TLR-enhanced internalization (16, 40), are also activated by CD36 cross-linking and play a role in CD36-mediated PE internalization (6). In addition, TLR stimulation activates Rho GTPases (41), which are critical for actin remodeling during phagocytosis. Although some groups have not detected increases in activated Rho GTPases during TLR-enhanced internalization (16, 40), another report using RNAi implicated Rho GTPases in TLR-induced phagocytosis (42).
While future studies are required to address the precise underlying mechanism, our findings may have relevance for various biological systems. TLR2 regulation of S. aureus phagocytosis (16) may occur via co-operation with CD36, which functions as a phagocytic receptor for this pathogen (8). Moreover, TLR2 recognizes β-amyloid (36) and has been implicated in β-amyloid clearance in a murine model of Alzheimer’s disease (43), suggesting that TLR2 and CD36 may collaboratively enhance β-amyloid phagocytosis.
Co-operation between CD36 and TLRs: Implications for malaria
We extended our studies of CD36-TLR interactions to an in vitro malaria-macrophage model in which both CD36 and TLRs are engaged. We found that CD36-mediated PE internalization per se did not induce pro-inflammatory cytokine production (Fig. 3). On the other hand, our group previously implicated CD36 in TNF production induced by PfGPI (28). Given the non-inflammatory nature of selective CD36 activation, we propose that these distinct inflammatory outcomes of CD36 engagement in our malaria-macrophage model are attributable to differential activation of TLRs by distinct CD36 ligands. TLR2 is an essential sensor for PfGPI, and the affinity of CD36 for lipid moieties (1) suggests that it may present this ligand to TLR2. In contrast, CD36-mediated PE binding is non-inflammatory, suggesting that components exposed on the PE surface do not activate TLRs or other pro-inflammatory receptors. Notably, all known malaria TLR agonists are thought to be confined inside the intact PE, and only released upon rupture of mature PEs at the end of the parasite’s intraerythrocytic life cycle (22). Thus, these products are likely inaccessible to cell surface TLRs upon PE binding, and hence would not stimulate inflammation. Additionally, our data suggest that the PE surface components that directly bind macrophage CD36 – likely parasite-encoded variable surface antigens such as P. falciparum erythrocyte membrane protein 1 (6, 44) – do not activate TLRs or other proinflammatory receptors.
TLR2 has been reported to be recruited to phagosomes, where it samples the contents for cognate agonists (45, 46). Thus, one might expect that PE degradation within the phagosome would release malaria TLR agonists and trigger TLR-mediated inflammation, similarly to how CD36-mediated internalization of S. aureus LTA has been postulated to promote cytokine responses by delivering this ligand to phagosomal TLR2 (8). If TLR2 is present in PE-containing phagosomes, it may not be effectively activated in our model due to rapid degradation of PfGPI, inhospitable conditions (e.g. low pH), or PE activation of other pathways that dampen TLR signaling (47). Alternatively, TLR2 may not be recruited to PE-containing phagosomes. We were unable to explore this question due to the low fidelity of available reagents to detect TLR2.
The non-inflammatory nature of PE internalization contrasts with phagocytosis of other pathogens, which is coupled to release of pro-inflammatory mediators that activate other host cells and thus strengthen immune defenses (48). It is tempting to speculate that P. falciparum evolved mechanisms to avoid TLR activation upon innate PE clearance in order to more closely resemble uptake of apoptotic cells rather than bacteria. This strategy would reduce host inflammatory responses and prevent accelerated PE clearance. As excessive production of proinflammatory cytokines has been implicated in the pathophysiology of severe malaria (49), non-inflammatory PE uptake may in fact represent a host-pathogen co-evolution to limit immunopathology.
Despite this potential co-evolution to restrict inflammation, the obligate rupture of PEs to release parasite progeny concomitantly releases parasite products such as PfGPI that stimulate TLRs and induce inflammatory responses. TLR-mediated inflammation has been linked to severe malaria pathogenesis, leading to proposals for inhibition of TLR pathways as an anti-inflammatory adjunctive therapy (50, 51). Here we demonstrate that macrophage TLR2 stimulation with PfGPI enhances PE uptake (Fig. 6), indicating that TLR2 can co-operate with CD36 to integrate inflammatory and phagocytic responses to malaria. Moreover, our finding suggests a novel and potentially beneficial role for TLRs in the innate immune response to malaria: promotion of PE clearance by macrophages, which has been implicated in the control of acute blood stage parasite replication (52–56).
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to staff and patients in the apheresis unit at Princess Margaret Hospital (Toronto, Canada) for providing human serum for parasite culture media; to Dr. Kodjo Ayi (University of Toronto) for his expertise and assistance with parasite culture; to Dr. Thomas R. Hawn (University of Washington) for provision of Tlr2−/− mice; to Dr. Wen-Chen Yeh (Princess Margaret Hospital, Toronto) for provision of Irak4−/− mice; and to Dr. W. Conrad Liles for critical reading of the manuscript.
Footnotes
This work was supported by a Canadian Institutes of Health Research (CIHR) Team Grant in Malaria (K.C.K. and S.G.), a CIHR Operating Grant (MT-13721; K.C.K.), a CIHR MD/PhD Studentship (L.K.E.), and a CRC Chair in Molecular Parasitology (K.C.K.), and by grant from NIAID, NIH (AI 41139; DCG).
Abbreviations used in this paper: EBAB, Erythrocyte-Biotin-Avidin-Biotinylated antibody; LTA, lipoteichoic acid; oxLDL, oxidized low-density lipoproteins; PE, parasitized erythrocyte; PfGPI, Plasmodium falciparum glycosylphosphatidylinositols; uRBC, uninfected red blood cell.
Publisher's Disclaimer: Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org."
REFERENCES
- 1.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci. 2004;24:9838–9846. doi: 10.1523/JNEUROSCI.2557-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 4.Baranova IN, Kurlander R, Bocharov AV, Vishnyakova TG, Chen Z, Remaley AT, Csako G, Patterson AP, Eggerman TL. Role of human CD36 in bacterial recognition, phagocytosis, and pathogen-induced JNK-mediated signaling. J Immunol. 2008;181:7147–7156. doi: 10.4049/jimmunol.181.10.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fadok VA, Warner ML, Bratton DL, Henson PM. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3) J Immunol. 1998;161:6250–6257. [PubMed] [Google Scholar]
- 6.McGilvray ID, Serghides L, Kapus A, Rotstein OD, Kain KC. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood. 2000;96:3231–3240. [PubMed] [Google Scholar]
- 7.Moore KJ, El Khoury J, Medeiros LA, Terada K, Geula C, Luster AD, Freeman MW. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J Biol Chem. 2002;277:47373–47379. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- 8.Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maxeiner H, Husemann J, Thomas CA, Loike JD, El Khoury J, Silverstein SC. Complementary roles for scavenger receptor A and CD36 of human monocyte-derived macrophages in adhesion to surfaces coated with oxidized low-density lipoproteins and in secretion of H2O2. J Exp Med. 1998;188:2257–2265. doi: 10.1084/jem.188.12.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janabi M, Yamashita S, Hirano K, Sakai N, Hiraoka H, Matsumoto K, Zhang Z, Nozaki S, Matsuzawa Y. Oxidized LDL-induced NF-kappa B activation and subsequent expression of proinflammatory genes are defective in monocyte-derived macrophages from CD36-deficient patients. Arterioscler Thromb Vasc Biol. 2000;20:1953–1960. doi: 10.1161/01.atv.20.8.1953. [DOI] [PubMed] [Google Scholar]
- 11.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 13.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 14.Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem. 2006;281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 15.Underhill DM, Gantner B. Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect. 2004;6:1368–1373. doi: 10.1016/j.micinf.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 17.Doyle SE, O'Connell RM, Miranda GA, Vaidya SA, Chow EK, Liu PT, Suzuki S, Suzuki N, Modlin RL, Yeh WC, Lane TF, Cheng G. Toll-like receptors induce a phagocytic gene program through p38. J Exp Med. 2004;199:81–90. doi: 10.1084/jem.20031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajishengallis G, Wang M, Harokopakis E, Triantafilou M, Triantafilou K. Porphyromonas gingivalis fimbriae proactively modulate beta2 integrin adhesive activity and promote binding to and internalization by macrophages. Infect Immun. 2006;74:5658–5666. doi: 10.1128/IAI.00784-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, Halmen KA, Lamphier M, Olivier M, Bartholomeu DC, Gazzinelli RT, Golenbock DT. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A. 2007;104:1919–1924. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furuta T, Imajo-Ohmi S, Fukuda H, Kano S, Miyake K, Watanabe N. Mast cell-mediated immune responses through IgE antibody and Toll-like receptor 4 by malarial peroxiredoxin. Eur J Immunol. 2008;38:1341–1350. doi: 10.1002/eji.200738059. [DOI] [PubMed] [Google Scholar]
- 21.Krishnegowda G, Hajjar AM, Zhu J, Douglass EJ, Uematsu S, Akira S, Woods AS, Gowda DC. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280:8606–8616. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gowda DC. TLR-mediated cell signaling by malaria GPIs. Trends Parasitol. 2007;23:596–604. doi: 10.1016/j.pt.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Patel SN, Serghides L, Smith TG, Febbraio M, Silverstein RL, Kurtz TW, Pravenec M, Kain KC. CD36 mediates the phagocytosis of Plasmodium falciparum-infected erythrocytes by rodent macrophages. J Infect Dis. 2004;189:204–213. doi: 10.1086/380764. [DOI] [PubMed] [Google Scholar]
- 24.Braun-Breton C, Rosenberry TL, da Silva LP. Induction of the proteolytic activity of a membrane protein in Plasmodium falciparum by phosphatidyl inositol-specific phospholipase C. Nature. 1988;332:457–459. doi: 10.1038/332457a0. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vachon E, Martin R, Plumb J, Kwok V, Vandivier RW, Glogauer M, Kapus A, Wang X, Chow CW, Grinstein S, Downey GP. CD44 is a phagocytic receptor. Blood. 2006;107:4149–4158. doi: 10.1182/blood-2005-09-3808. [DOI] [PubMed] [Google Scholar]
- 27.Komatsu N, Kirito K, Shimizu R, Kunitama M, Yamada M, Uchida M, Takatoku M, Eguchi M, Miura Y. In vitro development of erythroid and megakaryocytic cells from a UT-7 subline, UT-7/GM. Blood. 1997;89:4021–4033. [PubMed] [Google Scholar]
- 28.Patel SN, Lu Z, Ayi K, Serghides L, Gowda DC, Kain KC. Disruption of CD36 impairs cytokine response to Plasmodium falciparum glycosylphosphatidylinositol and confers susceptibility to severe and fatal malaria in vivo. J Immunol. 2007;178:3954–3961. doi: 10.4049/jimmunol.178.6.3954. [DOI] [PubMed] [Google Scholar]
- 29.Booth JW, Kim MK, Jankowski A, Schreiber AD, Grinstein S. Contrasting requirements for ubiquitylation during Fc receptor-mediated endocytosis and phagocytosis. Embo J. 2002;21:251–258. doi: 10.1093/emboj/21.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang ZY, Barreda DR, Worth RG, Indik ZK, Kim MK, Chien P, Schreiber AD. Differential kinase requirements in human and mouse Fc-gamma receptor phagocytosis and endocytosis. J Leukoc Biol. 2006;80:1553–1562. doi: 10.1189/jlb.0106019. [DOI] [PubMed] [Google Scholar]
- 31.Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, Tai MF, Stewart CR, Pukkila-Worley R, Hickman SE, Moore KJ, Calderwood SB, Hacohen N, Luster AD, El Khoury J. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med. 2009;206:637–653. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scragg IG, Hensmann M, Bate CA, Kwiatkowski D. Early cytokine induction by Plasmodium falciparum is not a classical endotoxin-like process. Eur J Immunol. 1999;29:2636–2644. doi: 10.1002/(SICI)1521-4141(199908)29:08<2636::AID-IMMU2636>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 33.Artavanis-Tsakonas K, Tongren JE, Riley EM. The war between the malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clin Exp Immunol. 2003;133:145–152. doi: 10.1046/j.1365-2249.2003.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu X, Chakravarty SD, Ivashkiv LB. Regulation of interferon and Tolllike receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol Rev. 2008;226:41–56. doi: 10.1111/j.1600-065X.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mae M, Iyori M, Yasuda M, Shamsul HM, Kataoka H, Kiura K, Hasebe A, Totsuka Y, Shibata K. The diacylated lipopeptide FSL-1 enhances phagocytosis of bacteria by macrophages through a Toll-like receptor 2-mediated signalling pathway. FEMS Immunol Med Microbiol. 2007;49:398–409. doi: 10.1111/j.1574-695X.2007.00218.x. [DOI] [PubMed] [Google Scholar]
- 36.Jana M, Palencia CA, Pahan K. Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer's disease. J Immunol. 2008;181:7254–7262. doi: 10.4049/jimmunol.181.10.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller YI, Chang MK, Binder CJ, Shaw PX, Witztum JL. Oxidized low density lipoprotein and innate immune receptors. Curr Opin Lipidol. 2003;14:437–445. doi: 10.1097/00041433-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Schuepp BJ, Pfister H, Clemetson KJ, Silverstein RL, Jungi TW. CD36-mediated signal transduction in human monocytes by anti-CD36 antibodies but not by anti-thrombospondin antibodies recognizing cell membrane-bound thrombospondin. Biochem Biophys Res Commun. 1991;175:263–270. doi: 10.1016/s0006-291x(05)81229-x. [DOI] [PubMed] [Google Scholar]
- 39.Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, Tai MF, Stewart CR, Pukkila-Worley R, Hickman SE, Moore KJ, Calderwood SB, Hacohen N, Luster AD, El Khoury J. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med. 2009 doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 41.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 42.Kong L, Ge BX. MyD88-independent activation of a novel actin- Cdc42/Rac pathway is required for Toll-like receptor-stimulated phagocytosis. Cell Res. 2008;18:745–755. doi: 10.1038/cr.2008.65. [DOI] [PubMed] [Google Scholar]
- 43.Richard KL, Filali M, Prefontaine P, Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer's disease. J Neurosci. 2008;28:5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baruch DI, Ma XC, Singh HB, Bi X, Pasloske BL, Howard RJ. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood. 1997;90:3766–3775. [PubMed] [Google Scholar]
- 45.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 46.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, Monti P, Piemonti L, Biondi A, Mantovani A, Introna M, Allavena P. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 48.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 49.Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–499. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 50.Coban C, Ishii KJ, Horii T, Akira S. Manipulation of host innate immune responses by the malaria parasite. Trends Microbiol. 2007;15:271–278. doi: 10.1016/j.tim.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Ropert C, Franklin BS, Gazzinelli RT. Role of TLRs/MyD88 in host resistance and pathogenesis during protozoan infection: lessons from malaria. Semin Immunopathol. 2008;30:41–51. doi: 10.1007/s00281-007-0103-2. [DOI] [PubMed] [Google Scholar]
- 52.Quinn TC, Wyler DJ. Intravascular clearance of parasitized erythrocytes in rodent malaria. J Clin Invest. 1979;63:1187–1194. doi: 10.1172/JCI109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith LP, Hunter KW, Oldfield EC, Strickland GT. Murine malaria: blood clearance and organ sequestration of Plasmodium yoelii-infected erythrocytes. Infect Immun. 1982;38:162–167. doi: 10.1128/iai.38.1.162-167.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bach O, Baier M, Pullwitt A, Fosiko N, Chagaluka G, Kalima M, Pfister W, Straube E, Molyneux M. Falciparum malaria after splenectomy: a prospective controlled study of 33 previously splenectomized Malawian adults. Trans R Soc Trop Med Hyg. 2005;99:861–867. doi: 10.1016/j.trstmh.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Demar M, Legrand E, Hommel D, Esterre P, Carme B. Plasmodium falciparum malaria in splenectomized patients: two case reports in French Guiana and a literature review. Am J Trop Med Hyg. 2004;71:290–293. [PubMed] [Google Scholar]
- 56.Su Z, Fortin A, Gros P, Stevenson MM. Opsonin-independent phagocytosis: an effector mechanism against acute blood-stage Plasmodium chabaudi AS infection. J Infect Dis. 2002;186:1321–1329. doi: 10.1086/344576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.