Abstract
Previous studies have demonstrated that infection with HIV-1 clades might differentially contribute to the neuropathogenesis of HIV-1-associated dementia (HAD). HIV-1 transactivator regulatory protein (Tat) plays a major role in the process of disruption of neuronal function. It is not well understood how these HIV-1 subtypes exert different neuropathogenic effects. Activation of indoleamine-2,3-dioxygenase (IDO), the rate-limiting enzyme of the kynurenine pathway, leads to increased tryptophan catabolism and the generation of neurotoxins such as kynurenine (KYN). It is known that KYN plays a crucial role in the neuropathogenesis of HAD. We hypothesize that HIV-1 clade B and C Tat proteins might exert differential effects on human primary astrocytes by the upregulation of the IDO gene and protein expression as well as its activity and production of the neurotoxin KYN. RNA extracted from human primary astrocytes treated with either HIV-1 clade B and C Tat proteins was reverse transcribed and analyzed by quantitative real-time PCR to determine IDO gene expression. In addition, the enzymatic activity of IDO and the concentration of KYN were measured in cell lysates and culture supernatants. Our results indicate that HIV-1 clade B Tat protein significantly upregulated the IDO gene and protein expression, IDO enzyme activity, as well as KYN concentration compared to HIV-1 clade C Tat protein. Thus, our studies for the first time demonstrate that HIV-1 clade B Tat protein in human primary astrocytes appears to increase the level of neuropathogenic agents, such as IDO and KYN, as compared to HIV-1 clade C Tat protein. These results provide further evidence that the prevalence of HAD may be correlated with the difference in clades of HIV-1.
Introduction
HIV remains a global health problem of unprecedented dimensions. Unknown 27 years ago, HIV has already caused an estimated 25 million deaths worldwide and has generated profound demographic changes in the heaviest affected countries. Globally an estimated 36 million people are living with HIV. Overall, 2 million people died due to AIDS in 2007. 1 HIV-1 displays extraordinary genetic variation in global distribution. It is classified into three groups and genetically into nine different subtypes (A–K). Clade B is predominant in North America, Western Europe, and Australia, whereas clade C is common in Africa, Latin America, and Asia.
HIV-1 directly and indirectly affects the central nervous system (CNS) causing neurological impairments, such as AIDS dementia complex (ADC), 2 which are manifested by a massive death of neurons in all regions of the brain. This can be initiated following activation of brain cells such as microglia and astrocytes. Activated HIV-infected brain cells have the ability to secrete neurotoxins such as quinolinic acid and arachidonic acid to enhance cell death. 3 HIV-1 Tat protein is known to cause cellular dysfunction in the immune system and progressively affect the CNS. Tat gene product transactivates viral gene expression and is essential for HIV-1 replication. However, the precise mechanism by which Tat exerts its effect is not yet known. The Tat protein exists in two forms, which in the HXB2 viral isolate consists of 72 and 86 amino acids. The 86-amino acid protein is encoded by two exons, whereas the 72-residue protein, which is identical except for lacking 14 residues from the C terminus, is the product of the first Tat exon. In general, the size of Tat varies up to 101 residues. The extracellular roles of Tat are suspected to be the major reason for the maintenance of HIV-infected cells and could explain the failure of current antiviral therapies to eradicate HIV. 4
HIV-1 Tat induces apoptosis in different cell lines, such as macrophages, cytotoxic T lymphocytes, which are essential for the cellular response of the immune system to eliminate virus-infected cells, and neurons. Tat is known to cause oxidative stress and is associated with disruption of the blood–brain barrier via immortalization of endothelial cells. 5 It has shown that Tat is required for viral replication and it leads to increased production of proinflammatory cytokines, also known to be increased in HIV-1 brain dementia.3–6
Previous studies suggest that HIV-1 infection and host genetic variations play critical roles in influencing differential degrees of neurological problems. 7 Recently, Mishra et al. reported that the HIV-1 clade C Tat gene sequence dicysteine C30C31 changes the motif and alters the functional property. 8 HIV-1 infection activates kynurenine on the intermediate end product of neurotoxins, quinolinic acid; elevated levels of these neurotoxins have been consistently found in the CSF of AIDS patients with ADC. 3 The overstimulation of the enzyme indoleamine-2,3-dioxygenase (IDO) leads to increased production of these neurotoxins. 9 It has been shown that different sequence and genetic polymorphisms in the viral protein and variations in the viral gene enzymes will lead to differential expressions of ADC. However, the underlying mechanisms causing neuronal cell loss or ultimately ADC are not clearly understood. In our study we determined the HIV-1 clade B and C Tat proteins differential modulation of IDO, through a similar or distinct mechanism(s) in human primary astrocytes.
In the present study, we have shown that the HIV-1 clade B Tat protein is involved in increased kynurenine levels, IDO enzyme function, and gene and protein expression of IDO in primary human astrocytes, whereas the HIV-1 clade C Tat protein's contribution to this process was significantly insufficient.
Materials and Methods
HIV-1 clade Tat recombinant proteins
HIV-1 clade B Tat protein was obtained from NIH AIDS Research and Reference Reagent and HIV-1 clade C Tat was obtained from DIATHEVA (Fano, Italy).
Primary human astrocytes cells
Primary astrocyte cells were from the human cerebral cortex (Sciencell, CA). They were cryopreserved at passage one and delivered frozen, guaranteed to further expand for 15 population doublings in this condition. Astrocytes were maintained in basal medium containing 10% fetal bovine serum, 50 units/ml penicillin, astrocytes growth supplement, and 100 μg/ml streptomycin (Sciencell, CA) and the cells were grown to 80–90% confluence.
RNA extraction and quantitative real-time PCR (QRT-PCR)
RNA was extracted using the Qiagen kit (Invitrogen Life Technologies, Carlsbad, CA) and total RNA (5 μg) was used for the synthesis of the first strand of cDNA and the amplification of cDNA was performed using forward and reverse primers for IDO, MCP-1, and β-actin as control (Applied Biosystem, Foster City, CA) for quantitative real-time PCR (QRT-PCR) according to our previously published protocol. 10
| IDO | Forward (5′,5′-GATGAAGAAGTGGGCTTTGC-3′) |
| Reverse (3′,5′-TCCAGTTTGCCAAGACACAG-3′) | |
| MCP-1 | Forward (5′,5′-GTC TCT GTC ACG CTT CTG G-3′) |
| Reverse (3′,5′-GAT CTC TCT CTT GAG CTT GG-3′) | |
| β-actin | Forward (5′,5′-TGACGGGGTCACCCACACTGT GCCCATCTA-3′) |
| Reverse (3′,5′-AGTCATAGTCCGCCTAGAAGC ATTTGCGGT-3′) |
IDO enzymatic activity assay
IDO activity was assayed by the colorimetric method with minor modifications. 11 Briefly, 2 × 106 cells were disrupted by freezing and thawing, the lysate (250 μl) was cleared by centrifugation, and an equal amount of 2 × IDO buffer [100 mM phosphate-buffered saline (PBS), pH 6.5, with 40 mM ascorbate, 20 μM methylene blue, 200 μg/ml catalase, and 800 mM l-tryptophan (Sigma–Aldrich, St. Louis, MO)] was added. After a 30 min incubation at 37°C, 100 μl of 30% trichloroacetic acid was added to stop the reaction, incubated for 30 min at 52°C, and centrifuged. The supernatant was mixed with an equal amount of Ehrlich's reagent, the color was allowed to develop for 10 min, and then the absorbance was read at 490 nm in a spectrophotometer.
Determination of kynurenine
The change in kynurenine levels in the supernatant of HIV-1 Tat clade B- and C-treated cultures was measured spectrophotometrically. 12 Briefly, 100 μl of 30% trichloroacetic acid (Sigma–Aldrich, St. Louis, MO) was added to 200 μl of the culture supernatant, vortexed, and then centrifuged at 10,000 rpm for 5 min. A 125-μl volume of the supernatant was added to 125 μl of Ehrlich's reagent (100 mg of p-dimethylbenzaldehyde, 5 ml of glacial acetic acid) in a microtiter plate well (96-well format). Samples were read against a reagent blank with a 490-nm filter in a microplate reader (Multiskan MS, Lab Systems, CA). The change in kynurenine concentration was obtained by subtracting the control values from the sample value.
Western blot analysis
To assess the IDO protein modification in HIV-1 clade B and C Tat, cells were lysed by lysis buffer (Pierce, IL) with a 1 × complete cocktail of protease inhibitors. Total cellular protein in equal amounts was resolved by 4–15% polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and were incubated with primary mouse monoclonal IDO antibody (Chemicon International, CA), mouse monoclonal β-actin antibody (Santa Cruz Biotechnology, CA) followed by secondary goat antimouse IgG antibody (Santa Cruz Biotechnology, CA). Immunoreactive bands were visualized using a chemiluminescence's Western blotting system according to the manufacturer's instructions (Amersham).
Statistical analysis
Data are expressed as mean ± SEM. Student's t test or analysis of variance was performed where three independent experiments were performed for each set of experiments and each experiment was performed in duplicate or triplicate. Data shown are p < 0.05 and were considered significant.
Results
HIV-1B Tat upregulates IDO gene expression compared to HIV-1C Tat
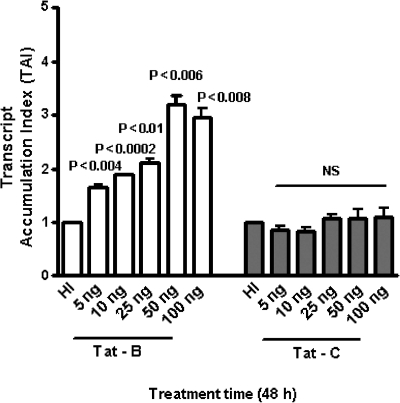
Since HIV-1B infection is reported to induce more neuropathology than HIV-1C infection,8–13 we investigated whether HIV-1B and C clade-derived Tat differentially modulate IDO activation, which may lead to neurotoxic enzyme production by primary human astrocytes. Data presented in Fig. 1 show the kinetics of B and C Tat effects on IDO gene expression by astrocytes at 24, 48, and 72 h as analyzed by quantitative real-time PCR. Astrocytes cultured with 50ng/ml of B Tat demonstrated a significant increase in IDO gene expression at 24 h (p < 0.005), 48 h (p < 0.01), and 72 h (p < 0.02) compared to C Tat or heat-inactivated control, with the maximum upregulation being at 48 h of culture. Data presented in Fig. 2 show the dose–response effects of B and C Tat on IDO gene expression at 48 h. Results showed that B Tat produced a significant increase in IDO gene expression at 5 (p < 0.004), 10 (p < 0.0002), 25 (p < 0.01), 50 (p < 0.006), and 100 ng/ml (p < 0.008) compared to C Tat at similar concentrations, although C Tat showed slight upregulation (statistically nonsignificant) of IDO gene expression at 25–100 ng/ml compared to the heat-inactivated control. We observed that the maximum response was found at 50 ng/ml, and this concentration was used by other investigators using human fetal microglia, U937 monocytic cells, and HUVEC cells.14–16
FIG. 1.
Effect of HIV-1 clade B and C Tat protein on IDO gene expression by human astocytes. Astrocytes (1 × 106 cells/ml) were separately treated with heat-inactivated (HI) control and HIV-1 clade B and C Tat at 50 ng/ml for 24, 48, and 72 h. RNA was extracted and reverse transcribed followed by quantitative real-time PCR for IDO and housekeeping β-actin-specific primers. Data are expressed as mean ± SD of TAI values of three independent experiments.
FIG. 2.
Effect of HIV-1 clade B and C Tat protein on IDO gene expression by human astocytes. Astrocytes (1 × 106cells/ml) were separately treated with heat-inactivated (HI) control and HIV-1 clade B and C Tat at 5–100 ng/ml for 48 h. Data are expressed as mean ± SD of TAI values of three independent experiments.
HIV-1 B Tat induces more IDO enzyme activity in astrocytes
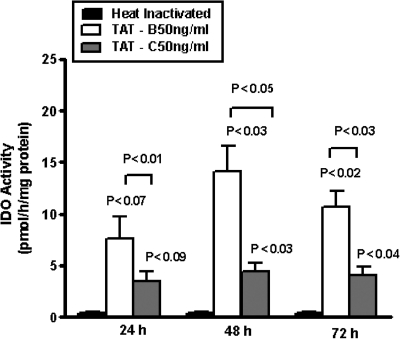
Since IDO is one of the major rate-limiting enzymes in tryptophan catabolism, we investigated the capacity of clade B and C Tat to induce IDO enzyme activity in astrocytes. Data presented in Fig. 3 show that astrocytes treated with Tat B showed significant upregulation of IDO enzyme activity at 24 (p < 0.07), 48 (p < 0.03), and 72 h (p < 0.02) compared to heat-inactivated control. Tat C also showed significant upregulation of IDO enzyme activity at 24 (p < 0.09), 48 (p < 0.03), and 72 h (p < 0.04) compared to heat-inactivated control. However, B Tat significantly induced IDO enzyme activity at 24 (p < 0.01), 48 (p < 0.05), and 72 h (p < 0.03) compared to C Tat-treated cells at similar time periods.
FIG. 3.
Effect of HIV-1 clade B and C Tat protein on IDO enzyme activity. Astrocytes (1 × 106 cells/ml) were separately treated with heat-inactivated (HI) control and HIV-1 clade B and C Tat at 50 ng/ml for 24, 48, and 72 h and the cell lysates were examined for enzyme activity. Data are expressed as mean ± SD of three independent experiments.
Effect of B and C Tat on Kynurenine production
To determine whether IDO gene expression and IDO enzyme induction were mediated through the kynurenine pathway, the kynurenine concentrations were measured by Ehrlich's assay in the supernatant of cultures treated separately with B and C Tat proteins at 24, 48, and 72 h. Data presented in Fig. 4 show that astrocytes cultured separately with 50 ng/ml of B and C Tat produced significantly higher levels of kynurenine compared to the heat-inactivated control at 24, 48, and 72 h. However, the kynurenine levels induced by B Tat were significantly higher compared to C Tat at 24 (p < 0.04), 48 (p < 0.01), and 72 h (p < 0.01). This suggests that the increased levels of kynurenine in the supernatant may be conditional on increased IDO gene expression (Figs. 1 and 2) and enzymatic activity (Fig. 3).
FIG. 4.
Effect of HIV-1 clade B and C Tat proteins on kynurenine concentration. Astrocytes (1 × 106 cells/ml) were separately treated with heat-inactivated (HI) control and HIV-1 clade B and C Tat at 50 ng/ml for 24, 48, and 72 h and the supernatant was examined for kynurenine levels. Data are expressed as mean ± SD of three independent experiments.
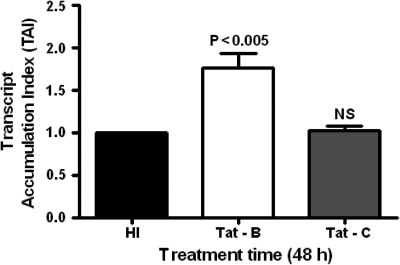
HIV-1B Tat upregulates MCP-1 gene expression compared to HIV-1C Tat
To determine whether the activation in clade B and C Tat protein is due to induction, the functional aspects were analyzed by MCP-1 (CCL-2) gene expression. As shown in Fig. 5 B Tat was able to induce MCP-1 gene expression more than clade C Tat in astrocytes (p < 0.005). This suggests that B Tat was more potent than C Tat in the induction of MCP-1 expression.
FIG. 5.
Effect of HIV-1 clade B and C Tat protein on MCP-1 gene expression. Astrocytes (1 × 106 cells/ml) were separately treated with heat-inactivated (HI) control and HIV-1 clade B and C Tat at 50 ng/ml for 48 h. Data are expressed as mean ± SD of three independent experiments.
Effect of B and C Tat on IDO protein expression
We also studied the induction of the tryptophan-kynurenine pathway in terms of the differential IDO protein expression by astrocytes via B and C Tat of HIV-1. Data presented in Fig. 6 show that B Tat induced protein expression significantly, confirmed by Western blot analysis. Astrocytes treated with B Tat showed increasing IDO protein expression (lane 2, OD = 72.6), which was significantly sufficient compared to C Tat (lane 3, OD = 46.2) or heat-inactivated control (lane 1, OD = 47.6) (Fig. 6A). Data presented in Fig. 6B show the densitometry evaluation of IDO protein levels in B Tat-treated cultures compared to C Tat-treated cultures. B Tat-treated astrocytes showed a significant increase (p < 0.01) in IDO protein levels compared to clade C Tat-treated astrocytes.
FIG. 6.
Effect of HIV-1 clade B and C Tat protein on IDO protein expression. Astrocytes (1 × 106 cells/ml) were separately treated with heat-inactivated (HI) control and HIV-1 clade B and C Tat at 50 ng/ml for 48 h and analyzed by Western blot. (A) A representative experiment: lane 1, control; lane 2, Tat B; lane 3, Tat C. Data presented in (B) are the mean ± SD of three independent experiments.
Discussion
The present study provides new insights into the functional role of IDO in the clade-specific Tat proteins modulation of the kynurenine pathway and production of IDO in primary astrocytes. However, there are no reports on the molecular mechanisms of IDO expression by HIV-1 clade B and C Tat protein. We demonstrated that clade B Tat increased IDO mRNA expression compared with clade C Tat. These findings are of considerable interest, because not only IDO mRNA, but also levels of protein, enzyme, and kynurenine are less compared to clade B Tat.
It has been shown that different sequence and genetic polymorphisms in HIV-1 may lead to differential expressions of HIV-associated dementia (HAD). HIV-1 clade B is predominant in North America, Western Europe, and Australia, whereas HIV-1 clade C is common in Africa, Latin America, and Asia. 17 Current estimates place the prevalence of HAD in the United States and Western Europe at 10–20%. 18 In India, several studies demonstrate that the prevalence of HAD is just over 1%.19,20 HAD appears to be most common in HIV-1 clade B infection prevalent areas but not HIV-1 clade C infection prevalent areas, suggesting that the prevalence of HAD may be correlated with the difference in subtypes of HIV-1.
HIV-1 infection activates glial cells to overstimulate IDO, leading to depletion of tryptophan and increased production of kynurenine, an intermediate of the end product quinolinic acid, to enhance brain cell death. 9 Increased IDO activity in the frontal cortex of HAD patients has already been reported.21,22 Moreover, previous studies have shown that elevated levels of the neurotoxin quinolinic acid have been consistently found in the cerebrospinal fluid (CSF) of HIV-infected patients with HAD, 23 suggesting that quinolinic acid plays a major role in the pathogenesis of HAD.13,24,25
To our knowledge there are no previous reports on the molecular mechanisms of IDO expression following exposure to HIV-1 clade B and C Tat proteins. The present study provides new insights on HIV-1 clade differences by studying the modulation of the kynurenine pathway and IDO production in primary human astrocytes. We demonstrated that HIV-1 clade B Tat increased IDO mRNA expression as compared to HIV-1 clade C Tat (Figs. 1 and 2). Furthermore, our results also demonstrated that the induction of IDO activity and the levels of kynurenine were significantly higher with HIV-1 clade B Tat than with HIV-1 clade C Tat (Figs. 3 and 4). Although clade C Tat showed a low level of kynurenine, there was a lack of significant induction of IDO gene expression.
Our results suggest that HIV-1 genetic variations play critical roles in influencing HIV-1 infection and differentially modify disease progression. 7 The sequential differences in structural and functional aspects of HIV-1 clade C Tat compared to HIV-1 clade B Tat occur especially in the dicysteine motif, 8 binding to CCR2 receptors, 26 and the capacity to induce proinflammatory cytokine TNF-α and MCP-1. 24 Recent studies have shown that HIV-1 clade B Tat protein induces more secretion of TNF-α, IL-6, and the chemokine coreceptor CCR5 compared to HIV-1 clade C Tat 25 . We consistently found that HIV-1 clade B Tat significantly increased MCP-1 compared to HIV-1 clade C Tat (Fig. 5), therefore raising the possibility that these factors may be involved in the observed effects. It is possible that these factors contribute to the differential modulation of kynurenine and IDO activity. There are several stages at which IDO expression can be regulated at transcriptional, translational, and posttranslational levels,27,28 which may have an impact on IDO enzyme activation. It has been shown that various proinflammatory cytokines such as IFN-γ, TNF-α, and IL-1β increase IDO activity. 29 These cytokines are also known to rapidly activate signaling pathways that promote neuropathogenesis. 30 This suggests a possible role for neuropathogenic molecules regulated by IDO activation in HAD. Further studies are needed to fully define the mechanistic effect of HIV-1 clade B- and C Tat-induced IDO enzyme activation.
Our results show that HIV-1 clade B Tat-induced activation of IDO proteins (Fig. 6A and B) was associated with a concomitant elevation of kynurenine (Fig. 4). In contrast, differences in protein modification in intracellular signal transductions results in differential IDO mRNA expression and enzyme activations in Tat clade B- and Tat clade C-exposed cells. However, the mechanisms underlying these alterations and their protein modifications are yet to be elucidated. Because the main observation in this report is that Tat clade B and C both increase kynurenine concentrations but yet result in different patterns of IDO gene expression, these two different clades may have a distinct in vitro mechanism. Our studies show that the Tat protein expression level did not correlate with either the mRNA expression or enzyme levels in Tat clade C (Figs. 1, 2, and 3). Previous studies indicate that without IDO enzyme activation, the IDO protein was still expressed in mouse splenic cells and human DC.31,32 However, Thomas et al. indicated that IDO activity and protein expression are not dependent on IDO mRNA expressions but are limited by the cell heme protein availability for IDO activity. 28 In our study of CD115 astrocytes treated by Tat B, we observed a high degree of expression of the IDO gene and protein and a significant increase in IDO enzyme activity and kynurenine concentration simultaneously, whereas astrocytes treated by Tat C demonstrated that increasing only IDO enzyme activity and kynurenine concentration resulted in no significant changes in terms of gene or protein expression. Overall, the data provide evidence of a connection between IDO gene expression and enzyme activation, enhancing the formation of kynurenine concentrations in Tat clade B-exposed cells.
In conclusion, HIV-1 Tat protein treatment increased IDO expression and activation in primary human astrocytes. Furthermore, HIV-1 clade B Tat could induce more kynurenine as compared to HIV-1 clade C Tat in human primary astrocytes. Based on these results, this might be the reason why HIV-1 clade B has been suggested to be involved in neuropathogenesis in terms of the development of HAD. So far it has been demonstrated that an increase in IDO enzymatic activity in the brain could lead to the enhanced production of neurotoxins, 33 resulting in neurocognitive dysfunction and HAD.
Acknowledgments
The present study was supported by grants from the National Institutes of Health (NIH): DA012366, DA 021537, DA 015628, and DA 014218.
Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS/WHO: AIDS epidemic update: December 2007. UNAIDS. 2008. UNAIDS/07.27E. JC1322E. ISBN 9789291736218.
- 2. Price RW. Brew B. Sidtis J, et al. The brain in AIDS: Central nervous system HIV-1 infection and AIDS dementia complex. Science. 1998;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- 3. Nath A. Haughey NJ. Jones M, et al. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: Protection by memantine. Ann Neuron. 2000;47:186–194. [PubMed] [Google Scholar]
- 4. Liang C. Wainberg MA. The role of Tat in HIV-1 replication: An activator and/or a suppressor? AIDS Rev. 2002;4:41–49. [PubMed] [Google Scholar]
- 5. Price TO. Ercal N. Nakake R, et al. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005;1045:57–63. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 6. Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl. 2):S193–198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 7. Satishchandra P. Nalini A. Gourie-Devi M, et al. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989–1996) Indian J Med Res. 2000;111:14–23. [PubMed] [Google Scholar]
- 8. Mishra M. Vetrivel S. Siddappa NB, et al. Clade-specific differences in neurotoxicity of human immunodeficiency virus-1 B and C Tat of human neurons: Significance of dicysteine C30C31 motif. Ann Neurol. 2008;63:366–376. doi: 10.1002/ana.21292. [DOI] [PubMed] [Google Scholar]
- 9. Guillemin GJ. Smythe G. Takikawa O, et al. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2004;49:15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- 10. Shively CA. Mirkes SJ. Lu NZ, et al. Soy and social stress affect serotonin neurotransmission in primates. Pharmacogenomics J. 2003;3:114–121. doi: 10.1038/sj.tpj.6500166. [DOI] [PubMed] [Google Scholar]
- 11. Lee HJ. Jeong Y. Lee TH, et al. Rosmarinic acid inhibits indoleamine 2,3-dioxygenase expression in murine dendritic cells. Biochem Pharmacol. 2007;73:1412–1421. doi: 10.1016/j.bcp.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 12. Grant RS. Naif H. Thuruthyil SJ, et al. Induction of indolamine [sic] 2,3-dioxygenase in primary human macrophages by human immunodeficiency virus type 1 is strain dependent. J Virol. 2000;74:4110–4115. doi: 10.1128/jvi.74.9.4110-4115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rao VR. Sas AR. Eugenin EA, et al. HIV-1 clade-specific differences in the induction of neuropathogenesis. J Neurosci. 2008;28:10010–10016. doi: 10.1523/JNEUROSCI.2955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hegg CC. Hu S. Peterson PK, et al. Beta-chemokines and human immunodeficiency virus type-1 proteins evoke intracellular calcium increases in human microglia. Neuroscience. 2000;98:191–199. doi: 10.1016/s0306-4522(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 15. Caldwell RL. Gadipatti R. Lane KB, et al. HIV-1 TAT represses transcription of the bone morphogenic protein receptor-2 in U937 monocytic cells. J Leukoc Biol. 2006;79:192–201. doi: 10.1189/jlb.0405194. [DOI] [PubMed] [Google Scholar]
- 16. Tadayuki O. Sonia FC. Gisela V. HIV-1 Tat increases endothelial solute permeability through tyrosine kinase and mitogen-activated protein kinase-dependent pathways. AIDS. 2000;14:475–482. doi: 10.1097/00002030-200003310-00002. [DOI] [PubMed] [Google Scholar]
- 17.Robertson D.Anderson J.Bradac J.Carr J.Foley B.Funkhouser R.Gao F.Hahn B.Kalish M.Kuiken C.Learn G.Leitner T.McCutchan F.Osmanov S.Peeters M.Pieniazek D.Salminen OM.Sharp P.Wolinsky SM.Korber B. A Reference Guide to HIV-1 Classification. Los Alamos National Laboratory; Los Alamos, NM: 2001. [Google Scholar]
- 18.Grant I.Sacktor H.McArthur J. HIV neurocognitive disorders. In: Gendelman HE, editor; Grant I, editor; Everall I, editor; Lipton SA, editor; Swindells S, editor. The Neurology of AIDS. 2nd. Oxford University Press; London, UK: 2005. pp. 357–373. [Google Scholar]
- 19. Shankar AV. Sastry J. Erande A, et al. Making the choice: The translation of global HIV and infant feeding policy to local practice among mothers in Pune, India. J Nutr. 2005;135:960–965. doi: 10.1093/jn/135.4.960. [DOI] [PubMed] [Google Scholar]
- 20. Wadia RS. Pujari SN. Kothari S, et al. Neurological manifestations of HIV disease. J Assoc Physicians India. 2001;49:343–348. [PubMed] [Google Scholar]
- 21. Sardar AM. Reynolds GP. Frontal cortex indoleamine-2,3-dioxygenase activity is increased in HIV-1-associated dementia. Neurosci Lett. 1995;187:9–12. doi: 10.1016/0304-3940(95)11324-p. [DOI] [PubMed] [Google Scholar]
- 22. Smith DG. Guillemin GJ. Pemberton L, et al. Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. J Neurovirol. 2001;7:56–60. doi: 10.1080/135502801300069692. [DOI] [PubMed] [Google Scholar]
- 23. Valle M. Price RW. Nilsson A, et al. CSF quinolinic acid levels are determined by local HIV infection: Cross-sectional analysis and modelling of dynamics following antiretroviral therapy. Brain. 2004;127:1047–1060. doi: 10.1093/brain/awh130. [DOI] [PubMed] [Google Scholar]
- 24. Campbell GR. Watkins JD. Singh KK, et al. Human immunodeficiency virus type 1 subtype C Tat fails to induce intracellular calcium flux and induces reduced tumor necrosis factor production from monocytes. J Virol. 2007;81:5919–5928. doi: 10.1128/JVI.01938-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siddappa NB. Venkatramanan M. Venkatesh P, et al. Transactivation and signaling functions of Tat are not correlated: Biological and immunological characterization of HIV-1 subtype-C Tat protein. Retrovirology. 2006;18:3:53–73. doi: 10.1186/1742-4690-3-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albini A. Ferrini S. Benelli R, et al. HIV-1 Tat protein mimicry of chemokines. Proc Natl Acad Sci USA. 1998;95:13153–13158. doi: 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miwa N. Hayakawa S. Miyazaki S, et al. IDO expression on decidual and peripheral blood dendritic cells and monocytes/macrophages after treatment with CTLA-4 or interferon-γ increase in normal pregnancy but decrease in spontaneous abortion. Mol Hum Reprod. 2005;11:865–870. doi: 10.1093/molehr/gah246. [DOI] [PubMed] [Google Scholar]
- 28. Thomas SR. Salahifar H. Mashima R, et al. Antioxidants inhibit indoleamine-2,3-dioxygenase in IFN-gamma-activated human macrophages: Posttranslational regulation by pyrrolidine dithiocarbamate. J Immunol. 2001;166:6332–6340. doi: 10.4049/jimmunol.166.10.6332. [DOI] [PubMed] [Google Scholar]
- 29. Shirey KA. Jung JY. Maeder GS, et al. Upregulation of IFN-gamma receptor expression by proinflammatory cytokines influences IDO activation in epithelial cells. J Interferon Cytokine Res. 2006;26:53–62. doi: 10.1089/jir.2006.26.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saha RN. Pahan K. Tumor necrosis factor-alpha at the crossroads of neuronal life and death during HIV-associated dementia. J Neurochem. 2003;86:1057–1071. doi: 10.1046/j.1471-4159.2003.01942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mellor AL. Munn DH. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 32. Fallarino F. Vacca C. Orabona C, et al. Functional expression of indoleamine 2,3-dioxygenase by murine CD8α + dendritic cells. Int Immunol. 2002;14:65–68. doi: 10.1093/intimm/14.1.65. [DOI] [PubMed] [Google Scholar]
- 33. Potula R. Poluektova L. Knipe B, et al. Inhibition of indoleamine 2,3-dioxgygenase (IDO) enhances elimination of virus-infected macrophages in animal model of HIV-1 encephalitis. Blood. 2005;106:2382–2390. doi: 10.1182/blood-2005-04-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]