Abstract
Many genes and molecules that drive tissue patterning during organogenesis and tissue regeneration have been discovered. Yet, we still lack a full understanding of how these chemical cues induce the formation of living tissues with their unique shapes and material properties. Here, we review work based on the convergence of physics, engineering and biology that suggests that mechanical forces generated by living cells are as crucial as genes and chemical signals for the control of embryological development, morphogenesis and tissue patterning.
Keywords: Cytoskeleton, Mechanical signaling, Morphogenesis, Pattern formation, Tension
Introduction
We now know many genes, morphogens and signaling molecules that govern tissue genesis. However, we do not fully understand how these chemical cues drive the formation of living tissues and organs with specialized forms and unique physical properties (e.g. rigidity, elastic recoil or viscoelasticity) required to pump blood, withstand repetitive movements or lift our bodies up against the force of gravity. A century ago, much of developmental control was explained in mechanical terms. In his classic treatise On Growth and Form, D'Arcy Thompson described how patterns are “diagrams of underlying forces” (Thompson, 1917). This is because a change in the three-dimensional (3D) shape of any structure, including living cells and tissues, must, at some level, result from the action of a force acting on a mass. Although this view was pushed aside by the advance of molecular biology, the relationship between physicality and developmental control is now coming into focus once again as a result of powerful new alliances between biologists, geneticists, engineers and physicists. This interdisciplinary approach has led to the discovery of fundamental links between mechanical forces, molecular biochemistry, gene expression and tissue patterning that drive embryogenesis and play a central role in morphogenesis and tissue maintenance throughout the life of an organism.
In this article, we highlight some of the recent advances in this emerging field of mechanical biology. In particular, we focus on the role of mechanical forces that are generated in the contractile actin cytoskeleton of living cells and that act on the adhesions of these cells to neighboring cells and to the extracellular matrix (ECM). We describe how both traction forces exerted locally by single cells and more generalized forces (e.g. fluid shear, hydrostatic pressure) resulting from tension generated within the cytoskeletons of large groups of cells in tissues and organs are central to the control of tissue pattern formation during virtually all stages of embryogenesis. We also explore how mechanical signals are converted into changes in intracellular biochemistry and gene expression so that they influence fundamental mechanisms of cell fate determination and morphogenetic control that are also controlled by genes, soluble morphogens and chemical factors.
Mechanical forces in early development
The shaping of the living embryo results from cell division and progressive structural remodeling, beginning with the fertilization of the egg. Mechanical forces mold this process from the start, as they can influence egg activation, early asymmetric cell divisions and the establishment of initial embryonic polarity. For example, the first intracellular signaling event can be activated mechanically: the sperm of the horseshoe crab (Limulus polyphemus) penetrates the physical barrier of the egg using spring forces (see Box 1), which are stored in a specialized cytoskeletal structure known as the acrosome bundle and released by conformational changes of the actin cross-linking scruin-calmodulin protein complex (Shin et al., 2007; Sanders et al., 1996). The physical penetration of the barrier by the sperm triggers a large transient increase in cytoplasmic calcium, which acts as a second messenger to drive downstream egg activation. Interestingly, the application of various types of physical forces (Box 1), including mechanical tension, osmotic pressure or hydrostatic pressure (Horner and Wolfner, 2008a) (reviewed by Horner and Wolfner, 2008b), can also activate Drosophila and sea urchin eggs. In Drosophila, this response is similarly triggered by inducing calcium influx, in this case through the physical activation of mechanosensitive ion channels on the cell surface, which induces downstream chemical signaling events (Horner and Wolfner, 2008a). Although a similar role for physical forces has not been demonstrated directly in mammals, both enzymatic and mechanical mechanisms have been postulated to mediate sperm penetration through the egg barrier (Kim et al., 2008). In any case, these experimental findings in lower organisms suggest that mechanical forces can play a significant signaling role from the earliest stage of embryological development.
Box 1. Mechanical forces involved in development
Spring forces
Spring forces are generated when a spring is compressed or stretched, and act to return the spring to its natural length. The sperm cells of horseshoe crabs penetrate and activate egg cells as a result of spring forces generated by the uncoiling of an actin bundle (Shin et al., 2007; Sanders et al., 1996).
Osmotic pressure
Egg cells become more hydrated as they move from the ovary to the uterus, and associated changes in osmotic pressure activate egg cells mechanically by altering cell shape, membrane tension and mechanosensitive ion channel activity (Horner and Wolfner, 2008a).
Surface tension
Prior to extracellular matrix (ECM) deposition and tissue stabilization, embryonic tissues behave as liquids governed by an effective surface tension, which is determined by differences in intercellular adhesion and cytoskeletal prestress governed by cadherins and actomyosin-based contractility (Foty and Steinberg, 2005; Krieg et al., 2008).
Tensional forces, traction and prestress
Cell shape stability requires the establishment of a mechanical force balance within the cytoskeleton. Tensional pulling forces that are generated within contractile microfilaments are balanced partly by traction forces exerted on the cell's external tethers to the ECM substrate, and partly by internal microtubules that resist compression inside the cell. By balancing these forces among microfilaments, microtubules and the ECM, the cell generates a ‘prestress’, or state of isometric tension, in the cytoskeleton that mechanically stabilizes cell shape and regulates cell fate determination (reviewed by Ingber, 2006).
Shear stress
Shear stress is the frictional force of fluid flow on the surface of cells. The shear stress generated by the heart pumping blood through the systemic circulation plays a key role in endothelial cell and hematopoietic cell fate determination (le Noble et al., 2004; North et al., 2009; Adamo et al., 2009).
Following activation, the fertilized egg divides repeatedly, and its progeny undergoes asymmetric cell divisions that contribute to lineage specification and to the development of axial asymmetries (Siller and Doe, 2009). Although maternal signals are thought to generate asymmetry in early Drosophila embryos (St Johnston and Nusslein-Volhard, 1992), in the single-cell-stage Caenorhabditis elegans embryo, axial asymmetry is established through the mechanochemical control exerted by PAR polarity proteins (Kozlowski et al., 2007) (Fig. 1). Upon fertilization, the sperm donates CYK-4 RhoGAP, an inhibitor of the small Rho GTPase that controls cytoskeletal tension generation, which mechanically loosens the actin cortex near its entry point by inhibiting myosin. This results in the flow of cortical actin towards the opposite side of the cell (the potential anterior pole), owing to the contraction of the remaining intact cytoskeleton (Jenkins et al., 2006). This cortical actin flow results in the movement of cortical PAR protein complexes (PAR-3/6) towards the anterior pole, as well as in the recruitment of cytoplasmic PAR-2 to the posterior pole, thereby generating PAR polarity (Munro et al., 2004). After cell division, another RhoGAP, PAC-1, is recruited to the contact site between the daughter cells, whereas PAR-6 protein accumulates at the contact-free surface by inactivating another Rho GTPase, CDC-42 (Anderson et al., 2008). Subsequent spindle orientation results from the generation of a net pulling force that pulls the spindle towards the cortex at the posterior pole. This tensional force (Box 1) is generated by the depolymerization of the astral microtubules and by a dynein-dynactin motor complex that is anchored to the cell cortex through the LIN-5–GRP-1/2–Gα complex (Gonczy, 2008; Nguyen-Ngoc et al., 2007). The position of this complex is governed by PAR polarity (Colombo et al., 2003; Panbianco et al., 2008), which indicates that physical interactions between microtubules, the contractile actin cortex and external physical cues determine spindle orientation in these cells (Kozlowski et al., 2007).
Fig. 1.
Regulation of asymmetric cell division by external physical cues in C. elegans eggs. In the C. elegans egg, contact with other cells causes the local accumulation of PAC-1 RhoGAP, which inhibits CDC-42 GTPase activity, leading to PAR-6 accumulation with activated CDC-42 at sites free of cell-cell contact. In addition, sperm donates the CYK-4 RhoGAP to the egg, which induces the formation of a loose actin network domain around the entry site (potential posterior pole) that generates a contractile cortical actin flow towards the opposite site (anterior pole). This cortical actin flow moves the PAR-3/6 complex to the anterior pole, and the cytoplasmic PAR-2 to the posterior pole. This PAR polarity modulates the pulling force generated by dynein-dynactin motor complexes that is exerted on depolymerizing microtubules through the LIN-5–GRP-1/2–Gα complex to determine spindle orientation (see inset).
Interestingly, in vitro studies show that mitotic spindle orientation can be controlled mechanically by applying tension to transmembrane integrin receptors and their cytoskeletal linkages in cultured adult mammalian cells (Maniotis et al., 1997), or by changing cell geometry and altering cytoplasmic microtubule alignment in yeast (Daga and Nurse, 2008). By culturing cells on microengineered adhesive substrates (see Box 2), researchers have confirmed that the orientation of the spindle axis and of cell divisions is governed by the spatial distribution of ECM adhesions that resist cell traction forces, and not by chemical signals generated in response to ECM binding (Thery et al., 2005).
Box 2. Experimental approaches in mechanical biology
Micromanipulation
Fine glass microneedles oriented with a micromanipulator can be used to deform individual cells by applying suction to their membranes (Shao and Hochmuth, 1996), or, when coated with integrin-binding ECM proteins, to apply tensional forces to integrin cell surface receptors, the cytoskeleton and nuclear scaffolds (Maniotis et al., 1997).
Surface tensiometry
Tissues are placed between parallel plates, and compressional forces are applied to the tissues and then released; to calculate effective surface tension, cell shape changes are measured as the tissues restore their shape (Foty and Steinberg, 2005).
Magnetic forces
Magnetic nanoparticles are injected into the embryo; subsequently, external magnetic field gradients are applied to cause the deformation of developing tissues (Desprat et al., 2008). Magnetic microparticles coated with specific receptor ligands can also be used to apply controlled tensional or shear stresses to cells via ligated surface receptors by using magnetic tweezers (Matthews et al., 2006) or magnetic twisting cytometry (Wang et al., 1993).
Atomic force microscopy (AFM)
AFM is used to measure tension in the cell cortex by deforming the surface of single cells and recording the force-indentation curves (Krieg et al., 2008). It can also be used to determine the force required to deform ECM-integrin adhesions (Choquet et al., 1997), to physically separate adherent single cells (Krieg et al., 2008), or to stretch single molecules (Puchner et al., 2008).
Traction force microscopy
The traction forces of single cells can be visualized and quantitated by culturing cells on a thin flexible substrate containing fiduciary markers (e.g. fluorescent nanobeads) if the elasticity (Young's modulus) of the substrate is known and marker displacements can be measured (Dembo and Wang, 1999; Wang and Li, 2009).
Microengineered adhesive substrates
Cell shape distortion can be precisely controlled by culturing cells on ECM-coated adhesive islands (the shape, size and position of which can be determined on the micrometer scale) that are engineered using microcontact printing techniques and surrounded by non-adhesive regions (Chen et al., 1997). Microfabricated arrays of ECM protein-coated pillars can be used to measure piconewton-scale forces exerted by single cells at individual adhesion sites by measuring the deformation of the pillars (Tan et al., 2003). Such methods permit the investigation of how cell shape, traction force and prestress contribute to cell fate determination.
Microfluidic systems
Microfluidic systems can be used to explore the role of fluid shear stress and suction forces on cell and tissue behavior (Bao et al., 2008; Yi et al., 2006).
The role of physical forces in controlling asymmetry in early-stage mammalian blastocysts remains controversial (Piotrowska-Nitsche et al., 2005; Torres-Padilla et al., 2007). However, mechanical pressure due to spatial constraints imposed by the zona pellucida (the specialized ECM that surrounds the forming egg) has been suggested to influence cell alignment and divisions that specify early embryonic axis formation (Kurotaki et al., 2007). This is supported by the finding that artificially distorting the egg to mimic the flattened form induced by cytoskeletal changes following fertilization is sufficient to orient the first cleavage plane in mice (Gray et al., 2004).
These findings suggest that mechanical forces that physically deform cells can control their asymmetric division, and thereby dictate axis orientation at the tissue level. Cell distortion induced either by cell migration or fluid flow has been suggested to trigger the initial left-right (L/R) body plan symmetry-breaking event that takes place in the node of the primitive streak in chicks (Gros et al., 2009) and mice (Nonaka et al., 2002; Tanaka et al., 2005). In chicks, the myosin II-driven leftward migration of the cells, which generates an asymmetric distribution of Sonic Hedgehog (Shh)- and Fgf8-producing cells around the node, appears to be induced by another type of physical signal: changes in bioelectrical activity regulated by a membrane H+/K+ ATPase (Gros et al., 2009). In mouse embryos, the left-right dynein (Lrd)-dependent rotation of motile cilia on the cells in the node in a consistent clockwise direction generates leftwardly directed, extra-embryonic fluid flow (Nonaka et al., 2002). This flow generates a L/R morphogen gradient and asymmetric calcium signaling by transporting ‘nodal vesicular parcels’ that encapsulate Shh and retinoic acid, which induce changes in tissue and organ morphology responsible for L/R organ patterning (Tanaka et al., 2005). The flow also causes the asymmetric deformation of the non-motile cilia at the periphery of the node, which activates asymmetric calcium influx through mechanosensitive polycystin-2 ion channels (McGrath et al., 2003). Thus, initial symmetry breaking during the establishment of the body plan appears to result from highly orchestrated interplay between both chemical and mechanical cues.
Mechanical forces in mid-embryogenesis
How a clump of cells is organized into 3D tissues with specialized form and function has been a long-standing question in the field of developmental biology. Although early embryologists had a keen interest in the role of mechanical forces in developmental control (Lenoir, 1982), interest waned with the advent of biochemistry and molecular biology, and as a result most current work on tissue patterning focuses on genetic programming and chemical signaling. But mechanical models of morphogenesis and embryological development continued to be explored (Steinberg, 1963; Beloussov et al., 1975; Keller, 1980; Oster et al., 1983; Ingber and Jamieson, 1985), and some early experimental studies suggested that cell-generated contractile forces play a central role in the control of tissue morphogenesis and 3D organ formation (Ash et al., 1973). In recent years, increasing numbers of investigators have begun to test these ideas through rigorous experimentation. As a result, it now evident that the formation of the first organized tissue structures during processes such as gastrulation and dorsal closure involve multiple biomechanical mechanisms that are crucial to developmental control. These processes that form the primitive metazoan body plan and that are regulated through reciprocal interplay between mechanical and chemical signals include: the sorting of progenitor cells into three distinct germ layers; patterning along the anteroposterior axis using a convergence-extension (CE) mechanism; invagination; folding; and the closure of cell layers into a hollow tube (Fig. 2). For example, integrated mechanochemical control can be seen in the mechanism by which conserved chemical morphogens (e.g. Wnts) modulate the generation of cell tension by altering actomyosin activity in the cytoskeleton, while resulting tension-dependent changes in cell shape feed back to alter gene expression and the production of crucial signaling molecules. The diffusion of soluble factors and the transmission of mechanical forces over longer distances also work together to propagate shape transformations and to drive polarized cell differentiation [e.g. by using a planar cell polarity (PCP) mechanism], which establishes tissue patterning across larger size scales.
Fig. 2.
Mechanical control of cell sorting and gastrulation. (A) Progenitor cell sorting. Different types of cells (depicted as light blue and green) sort and aggregate depending on the level of traction forces exerted on cell surface cadherins. Tensional forces generated by actomyosin interactions in the cortical cytoskeleton, which are controlled by TGFβ/Nodal signaling and exerted on cadherins, play a crucial role in cell sorting. G protein-coupled receptors and p120 catenin control the assembly of cadherins and cortical actin. (B) Axis formation. As mediolateral intercalation begins, cells exert traction on adjacent cells in the mediolateral direction through actin-based filopodia at cell-cell junctions. These tensional forces, which are regulated by the Wnt/planar cell polarity (PCP) and Rho/Rho-associated kinase (ROCK) signaling pathways, stabilize the junctions and drive cell rearrangements. Pulling by neighboring cells shortens the cell aggregate in the mediolateral direction (convergence) and extends it in the anteroposterior direction (extension). (C) Tissue folding. In Drosophila, the mechanical compression of cells due to germ band extension through cell intercalation within a limiting boundary induces the expression of the gene encoding the basic helix-loop-helix transcription factor Twist through β-catenin nuclear translocation. This causes the rearrangement of cortical actin and cell-cell junctions, which drives apical constriction through the secreted protein Folded gastrulation (Fog), the zinc-finger protein Snail and ROCK. This, in turn, results in ventral furrow formation. Morphogens (e.g. BMPs, Wnt and Shh) modulate this signaling mechanism. (D) Dorsal closure. The migrating cells at the leading edge of the Drosophila dorsal epidermis extend actin-based filopodia that promote the formation of new cell-cell junctions when they contact cells on the opposing leading edge. Cytoskeletal tensional forces exerted on, or transmitted across, adhesions under the control of the Wnt/PCP and Rho/ROCK signaling pathways might help to drive tissue closure; underlying amnioserosa cells also contribute by mechanically pulling on the overlying epidermal cells. Although mechanically driven, the entire process of dorsal closure is controlled by soluble morphogens, such as Dpp.
Progenitor cell sorting
Early work in the field of mechanical biology by M. S. Steinberg suggested that the sorting of early progenitor cells into discrete cell groupings and positions might be driven by tissue surface tension acting at intercellular membrane adhesions, much as surface tension controls the shape and sorting of liquid drops with different surface energies (Steinberg, 2007). Interestingly, this ‘differential adhesion hypothesis’ has been recently confirmed experimentally using quantitative engineering analysis techniques (Box 2) (Ninomiya and Winklbauer, 2008; Foty and Steinberg, 2005). Tissue surface tension increases linearly with the surface expression level of cadherin cell-cell adhesion receptors in cultured cells (Foty and Steinberg, 2005), which are also crucial for progenitor cell sorting into distinct germ layers, both in vitro (Schotz et al., 2008) and during vertebrate gastrulation in vivo (Gumbiner, 2005).
Intercellular surface tension results from a balance between adhesion forces generated by cadherin receptors and intracellular tension generated by the contractile actomyosin cytoskeleton (Lecuit and Lenne, 2007). This is a complex process, as the strength of cell-cell (and cell-ECM) adhesions can also be enhanced by altering cytoskeletal tension, which promotes molecular assembly into junctional complexes (and focal adhesions) (Lele et al., 2006a; Miyake et al., 2006). Chemical morphogens, such as Nodal and transforming growth factor beta (TGFβ), control cell sorting during the initial phases of gastrulation by modulating cell contractility, which alters the level of surface tension on these cells (Krieg et al., 2008). Cadherins colocalize with cortical actin, and the amount of cadherins on the cell surface influences cortical actin assembly, which maintains cell shape, rigidity and tension in the embryo via a mechanism that involves G protein-coupled receptors (Tao et al., 2007) (Fig. 2A). Tension generated in the actomyosin cytoskeleton appears to be as crucial for the control of surface tension during germ layer organization in zebrafish as adhesion forces between cadherin receptors on neighboring cells (Schotz et al., 2008). Moreover, recent work suggests that actomyosin-based contractility is the primary mechanism responsible for boundary maintenance in proliferating tissues during compartmental cell sorting in the course of embryogenesis. For example, in early Drosophila embryos, myosin II-based contractile forces result in a stiffening of cells that physically restricts mitotic cells from intermixing with neighboring compartments (Monier et al., 2010). In the developing Drosophila wing, Rho/ROCK/myosin II-dependent tension exerted on cell-cell adhesions also appears to contribute to compartmental boundary maintenance, but in this case by physically guiding cell rearrangements after cell division (Landsberg et al., 2009).
Living cells control their form through a tensegrity mechanism (a term first used in architecture that is short for ‘tensional integrity’), meaning that shape stability results from a balance between tensile and compressive forces acting on and inside the cell that establish a state of isometric tension or prestress (Box 1) in the cytoskeleton (reviewed by Ingber, 2006). Taken together, these observations suggest that tensional forces generated via mechanochemical mechanisms in the contractile cytoskeleton oppose adhesive forces exerted on cadherins by the tensile cytoskeleton of neighboring cells in cell-cell junctions and thereby establish a mechanical force balance. This cellular mechanical force equilibrium appears to be crucial for both early progenitor cell sorting and tissue remodeling during later development. For example, recombination experiments in Xenopus embryos indicate that epithelial tissues modulate the surface tension that drives the pattern formation of underlying mesenchymal tissues (Ninomiya and Winklbauer, 2008), and the surface tension of the embryonic heart is crucial for cardiac loop formation in chick embryos ex vivo (Voronov and Taber, 2002). The level of cytoskeletal prestress also continues to be important for the control of cell, tissue and organ shape stability throughout adult life (reviewed by Ingber, 2006).
Patterning of the anteroposterior axis
The basic metazoan body plan is established during gastrulation by a set of highly conserved morphogenetic movements, and several lines of evidence suggest that these shape transformations are driven by cell-generated traction forces. For instance, CE movements induce tissue narrowing along the mediolateral axis, a process that concomitantly lengthens the gastrula stage embryo in the anteroposterior direction. During Drosophila germ band extension (GBE), epithelial cells intercalate and remodel their apical junctions to elongate the germ band (Bertet et al., 2004), and CE movements are also crucial for Xenopus gastrulation, in which intercalation occurs in the presumptive mesoderm to form a notochord with less pronounced junctional remodeling (Keller et al., 2003; Skoglund et al., 2008). These shape transformations are driven by cells that exert traction forces (Box 1) on the ECM or on adjacent cells (Keller et al., 2008) (Fig. 2B). To accomplish these movements, cells protrude actin-based filopodial processes medially and laterally that extend between neighboring cells to make new cell-cell contacts via the binding of cadherins at their tips (Hogan et al., 2004; Brevier et al., 2008). The exertion of local tensional forces on these adhesions drives the planar remodeling of cell-cell junctions, which prevents cells from reverting to their original orientation and ensures long-distance force transfer during Drosophila GBE (Bertet et al., 2004; Blankenship et al., 2006). Embryonic axis formation is stabilized by the generation of actomyosin-based tension, which organizes and stiffens the cytoskeleton in the paraxial somatic mesoderm (Zhou et al., 2009). Myosin II is also enriched at sites of tension application at the surface of intercalating cells (Fernandez-Gonzalez et al., 2009). These observations suggest that the anisotropy of the micromechanical environment contributes significantly to controlling tissue pattern formation in Drosophila embryos (Rauzi et al., 2008).
The mechanical forces that drive CE movements in Xenopus and zebrafish are regulated by the Wnt/PCP pathway (Takeuchi et al., 2003). The effects of these signaling molecules are mediated by the small GTPase Rho (Unterseher et al., 2004; Berger et al., 2009), an upstream RhoGAP inhibitor (Denholm et al., 2005) and the downstream effector Rho-associated kinase (ROCK) (Tanegashima et al., 2008), which modulate myosin-dependent tension generation in cells. These studies indicate that conserved morphogens, such as Wnts, induce cells undergoing CE to continually generate cytoskeletal tension and to exert this force on their adhesions. As a result, all cells change their shape, reorient and align their actin cytoskeleton, which further amplifies this contractile response. The transmission of these forces over multiple cells drives cell realignment and induces tissue restructuring, as observed in Drosophila GBE (Butler et al., 2009).
Tissue folding, invagination and dorsal closure
During gastrulation, tissue folding is initiated by a subset of cells that physically constrict their apical membranes and expand their basal domains along the ventral midline of the embryo, resulting in the inward folding (invagination) of the presumptive mesoderm to form the epithelium of the ventral furrow (Fig. 2C). In C. elegans, this apical constriction is regulated by the local accumulation of myosin II at the apical surface of the invaginating cells (Sawyer et al., 2009; Young et al., 1991). This local enrichment of myosin II is established by the recruitment of PAR proteins (PAR-3 and PAR-6) to the free apical membrane (Goldstein and Macara, 2007; Nance et al., 2003), much as asymmetry is established in the egg and in earlier embryonic tissues, as described above. Moreover, soluble morphogens appear to control invagination by activating actomyosin-based contractility (Chisholm, 2006; Corrigall et al., 2007; Lee et al., 2006). For example, in C. elegans, the Wnt signaling pathway controls gastrulation by altering myosin light chain phosphorylation and actin network contractility at the apical side of endodermal precursor cells (Lee et al., 2006), whereas in the morphogenetic furrow of the developing Drosophila eye, Hedgehog (Hh) signaling regulates apical constriction and ingression by activating Rho, ROCK and myosin II (Corrigall et al., 2007).
Importantly, mechanical forces can also activate chemical signaling pathways that are crucial for developmental control. The mechanical deformation of Drosophila embryos with magnetic tweezers, which mimics the compression of anterior pole stomodeal cells due to GBE through cell intercalation, activates the expression of the basic helix-loop-helix transcription factor Twist by inducing β-catenin to translocate from cell junctions to the nucleus, and this mechanical induction process is necessary for the differentiation of the anterior mid-gut organ derived from these cells (Desprat et al., 2008). In the mesoderm, Twist activates downstream signaling molecules, including the zinc-finger transcription factor Snail and the secreted protein Folded gastrulation (Fog), which leads to tissue invagination (Seher et al., 2007; Dawes-Hoang et al., 2005; Kolsch et al., 2007). Snail generates reversible pulsed apical constrictions that are subsequently followed by a Twist-dependent collective cell constriction through the stimulation of actomyosin-based contractility via Rho/ROCK pathway activation, which leads to tissue invagination in Drosophila (Martin et al., 2009). Twist-dependent constriction also appears to be sensitive to the mechanical strain produced in the mesoderm by the Snail-dependent contraction pulses, which in turn result from an amplification of Fog signaling attributable to the mechanical inhibition of Fog endocytosis (Pouille et al., 2009). The endocytosis of another crucial morphogen, Bone morphogenetic protein 2 (Bmp2), is also mechanosensitive, and mechanical forces influence the fate of mouse mesenchymal stem cells via this mechanism (Rauch et al., 2002). These findings provide a good example for how soluble morphogens exert their effects, in part, by inducing changes in cell and tissue mechanics that, in turn, feed back to modulate the chemical signals governing collective morphogenetic cell movements.
At the end of gastrulation, the dorsal surface of the Drosophila embryo is covered by a large flat amnioserosa surrounded by the dorsal epidermis, and dorsal closure is controlled mechanically by forces that drive the sealing of the two symmetrical lateral epithelial sheets (Fig. 2D). This closure process involves an orchestrated interplay between the lateral epidermis and the amnioserosa, which are both extremely sensitive to their micromechanical environment (Kiehart et al., 2000). Dorsal closure begins with the accumulation of actin and myosin at the leading edge of the lateral epidermis, which increases cell traction forces that pull the edges toward the midline (Jacinto et al., 2002) and cause the cell apices to constrict, resulting in a purse-string closure of the epidermis (Kiehart et al., 2000). The apical constriction of the amnioserosa cells and the subsequent reduction of their surface area also contribute to dorsal closure (Gorfinkiel et al., 2009; Kiehart et al., 2000).
Importantly, local decapentaplegic (Dpp), a Drosophila TGFβ/BMP homolog, triggers the local myosin II-dependent contraction of cells in the epidermis and amnioserosa during dorsal closure (Fernandez et al., 2007; Franke et al., 2005). Apical constrictions of the amnioserosa also generate ratchet-like pulling forces on the actin cables of the lateral epidermis that move the epithelial sheets towards each other (Solon et al., 2009). The initially columnar amnioserosa cells flatten and elongate by reorienting their internal microtubules, and these dynamic cytoskeletally driven cell shape changes are crucial for the tissue closure process to proceed efficiently and smoothly (Pope and Harris, 2008). Interestingly, microtubules resist significant compressive forces in adult mammalian cells (Brangwynne et al., 2006), and by resisting actomyosin-based tensile forces via a tensegrity mechanism, they contribute to the control of the cytoskeletal prestress that maintains cell shape stability in embryonic and adult tissues and organs (reviewed by Ingber, 2006).
During the closing stages of dorsal closure, cells at the leading edge of the embryonic Drosophila epidermis come within close proximity of each other and protrude finger-like filopodia over the amnioserosa (Jacinto et al., 2000). Interestingly, past studies with cultured nerve cells using direct mechanical measurement techniques have revealed that filopodial extensions constantly exert strong traction forces on their adhesions (Box 1), even though they actively grow outward from the cell membrane, as a result of actin polymerization within the filopodial core (Heidemann and Buxbaum, 1994). Thus, filopodia that span the gap between opposing leading edges of the approaching epithelial layers might similarly exert traction and help to pull these tissues together (Jacinto et al., 2002). In support of this idea, adhesive interactions between the filopodia of matching cells along this leading edge lead to the formation of tethers that appear to pull epithelial sheets into alignment during dorsal closure (Millard and Martin, 2008; Jacinto et al., 2000; Kiehart et al., 2000). This process of ‘filopodial matching’ results in the formation of mechanical connections between stiffened cytoskeletal elements and cell-cell adhesions, which support tensional force transmission over long distances spanning multiple cells; again, this guides sheet movement and tissue patterning at even larger size scales. Concomitant with these movements, the two flanks of the lateral epidermis zip together through the dynamic remodeling of cell-cell junctions mediated by interactions between β-catenin and cadherins. Again, cell traction forces (Box 1) are central to this process (Gorfinkiel and Arias, 2007). Additional tensional forces are generated by the apoptosis of amnioserosa cells, which results in the distortion of neighboring cells and eventually in a pull on the surrounding group of cells as the apoptotic cells involute. This volume contraction generates about half of the total force required for dorsal closure and influences the speed of closure (Toyama et al., 2008). In this manner, both tension generated in the cytoskeleton of individual cells and more generalized mechanical forces that result from cell loss or multicellular deformation help to drive crucial morphogenetic changes in the developing Drosophila embryo.
Establishment of planar cell polarity
During later development, cell tensional forces also contribute significantly to the coordinated orientation of multiple differentiated cells that underlies PCP establishment, which plays a crucial role in the formation of multiple organ systems in Drosophila, including wings, the auditory epithelium and eyes (Karner et al., 2006). For example, during wing development, epithelial cells polarize using cytoskeletal traction forces (Box 1) to pull against neighboring cells, which results in the alignment of intercellular junctions. This process is mediated by junctional remodeling regulated by PCP signaling molecules, such as Wnt and the small GTPase Rab11, that control cell packing geometry as cells convert from irregular forms into a hexagonal array shortly before hair formation (Classen et al., 2005; Farhadifar et al., 2007).
The asymmetric distribution of cytoskeletal and junctional proteins also contributes to polarized cell behavior during hexagonal packing (Blankenship et al., 2006), and actomyosin-dependent contractility as well as long-distance force transmission across the tissue appear to be crucial for this process. For example, myosin II is enriched in a bipolar manner within the aligned cells of the prospective denticle field and contributes to cell rearrangements during the establishment of PCP in the forming wing by acting in concert with denticle field-specific effectors (Walters et al., 2006). Thus, these epithelial packing patterns, which govern functional wing formation, also appear to be determined by a balance between cytoskeletal pulling forces and resisting adhesive tethers that attach to neighboring cells and the underlying ECM (Farhadifar et al., 2007). Interestingly, studies on adult mammalian cells indicate that endothelial cells similarly polarize their cytoskeleton, nucleus and membrane components when they pull against an adhesive substrate (Ingber et al., 1986), and that they also use mechanical feedback and Rho signaling to sense these cell shape asymmetries (Mammoto et al., 2007). The modulation of cytoskeletal tension through the Rho/ROCK/myosin II pathway regulates Wnt/PCP signaling, which controls junctional resistance sites (Figs 2, 3) crucial to the establishment of cell orientation. This, in turn, is pivotal for the control of cell division (Karner et al., 2009), directional cell migration (Phillips et al., 2005; Bastock and Strutt, 2007) and packing geometry (Chen et al., 2006). In mouse kidney development, a similar mechanochemical PCP signaling mechanism underpins the consistent orientation of mitotic cells along the tubular axis that drives the lengthening of developing renal tubules. A disturbance of mechanosensing by the primary cilia, which is mediated by polycystin-1/2 mechanosensitive ion channels on the cell surface, can affect mitotic alignment, cause renal tubular enlargement and result in cyst formation, as observed in mice with polycystic kidney disease (Fischer et al., 2006; Nauli et al., 2003).
Fig. 3.
Cytoskeletal tension and ECM mechanics in lung development. (A) Graph showing the effects of the Rho activator and tension promoter, cytotoxic necrotizing factor 1 (CNF-1), on epithelial branch formation during mouse lung development. Note that low doses of CNF-1 (2 and 20 ng/ml) increase terminal bud number, whereas a high dose (200 ng/ml), which results in high levels of tension in the growing cells, inhibits this process. (B) Photomicrographs of lung rudiments cultured for two days with or without 2 or 200 ng/ml CNF-1. There is an increase in distal lung buds at the low dose and a large-scale contraction of the entire gland at the high CNF-1 dose that greatly enhances cell contractility. Scale bar: 500 μm. (C) Immunofluorescence micrograph of a section through a normal lung rudiment stained for the basement membrane protein laminin. The arrow indicates a region of the basement membrane at the periphery of one epithelial bud, showing that the thinnest regions of the basement membrane typically appose the part of the epithelium with the most rapid cell growth and tissue expansion rates. Reproduced, with permission, from Moore et al. (Moore et al., 2005).
Organogenesis
Virtually all organs are sensitive to mechanical cues during their formation in the later stages of embryological development. Mechanical forces are crucial for the formation of blood vessels (Lucitti et al., 2007; Mammoto et al., 2009), lungs (Cohen and Larson, 2006; Gutierrez et al., 2003; Inanlou and Kablar, 2003; Moore et al., 2005), kidneys (Serluca et al., 2002; Vasilyev et al., 2009), muscle (Kahn et al., 2009), mammary glands (Alcaraz et al., 2008), brain (Anava et al., 2009; Moore et al., 2009; Wilson et al., 2007), cartilage and bone (Ohashi et al., 2002; Stokes et al., 2002), as well as of the hematopoietic system (Adamo et al., 2009; North et al., 2009) and the heart (Forouhar et al., 2006; Hove et al., 2003; Voronov et al., 2004). For example, in zebrafish embryos, the heart starts pumping blood using a hydroelastic impedance pumping mechanism in which waves of contraction and elastic deformation of the heart chamber, generated by myocytes located near the heart tube entrance, travel forward and reflect back to generate dynamic suction forces that drive blood flow even before valves form (Forouhar et al., 2006). Shear stress (Box 1) generated by blood flow due to heart pumping is also crucial for arterio-venous cell fate determination, as indicated by the activation of arterial markers, such as ephrin B2, in the endothelial cells of the chick yolk sac (le Noble et al., 2004). Blood flow is also required for the normal development of the hematopoietic system in mice and zebrafish; this effect appears to be mediated by the induction of the transcription factor Runx1 through flow-sensitive nitric oxide (NO) production (Adamo et al., 2009; North et al., 2009). Mechanical pressure applied to the vascular wall also influences blood vessel formation in chicken embryos (Lucitti et al., 2006).
Mechanical forces play a similarly central role in the developmental control of other forming organs. Fluid shear forces promote kidney development by orchestrating collective epithelial movements to form functional nephrons in zebrafish (Vasilyev et al., 2009). Increased amniotic fluid pressure and cystic fibrosis transmembrane conductive regulator-dependent muscle contractions similarly accelerate lung maturation in rats (Cohen and Larson, 2006), and pressure caused by the inspiration of air with the first breath after birth appears to be crucial for lung maturation, as rat lung epithelium increases surfactant synthesis and secretion when mechanically distorted (Gutierrez et al., 2003).
In the developing mouse heart, nonmuscle myosin heavy chains IIA and IIB are asymmetrically expressed in the embryonic heart tube, and their position appears to be closely correlated with the direction of heart looping regardless of the distribution of the key transcription factor Pitx2 (Linask et al., 2003; Linask et al., 2002; Lu et al., 2008). Moreover, the disruption of myosin-based tension generation with a ROCK inhibitor during the initial stages of heart looping disturbs morphogenesis in this system (Wei et al., 2002); however, tension inhibitors do not interfere with heart looping in chicks, so the conservation of this mechanism remains uncertain (Remond et al., 2006). In rats, the heart also alters its stiffness according to its developmental stage by changing the isoform ratios of the cytoskeletal protein titin, the elastic properties of which influence the physical compliance of heart tissue (Lahmers et al., 2004). The C-terminal kinase domain of titin unfolds in response to mechanical strain, leading to the exposure of an ATP-binding site for autophosphorylaton; in this manner, titin might work as a strain-sensing molecule for force adaptation in muscle (Puchner et al., 2008). Thus, embryonic organs appear to adapt their material properties in response to the changes in the physical demands on their function that occur during each developmental stage, and cytoskeletal prestress and mechanotransducer molecules play central roles in these responses, much as they do in adult tissues (reviewed by Ingber, 2006).
The application of bioengineering approaches to cell and developmental control have revealed that cytoskeletal tension-dependent changes in cell shape and variations in ECM compliance also alter the direction in which cells move and grow, which are crucial behaviors that drive tissue patterning during organogenesis in vitro and in vivo (Parker et al., 2002; Mammoto et al., 2009; Nelson et al., 2005). The analysis of embryonic mouse lungs has also confirmed that epitheliogenesis and angiogenesis can be altered by modulating Rho signaling and myosin-dependent tension generation in the cytoskeleton in vivo (Moore et al., 2005). Altering cytoskeletal tension changes the forces that cells exert on their basement membrane (BM) adhesions in the tissues of forming lungs, and if cytoskeletal tension is too high, the expansion of the whole lung organ is suppressed (Fig. 3A,B). The BM at the tips of growing epithelial buds in the lung is thinner than the surrounding BM regions (Fig. 3C), and actin stress fiber alignment is more pronounced in mesenchymal cells beneath these same sites, suggesting that the cellular force balance might be altered in these regions as well. Moreover, inhibiting cytoskeletal tension with Rho/ROCK antagonists blocks BM thinning in these regions and prevents both epithelial budding morphogenesis and branching angiogenesis in the developing lungs (Moore et al., 2005). Thus, the ability to generate spatial variations in the mechanical force balance between epithelial cells and their underlying BM, by altering either cytoskeletal tension or ECM mechanics, could be responsible for the establishment of local growth differentials that drive tissue patterning in many developing organs, both in embryos and adults (Huang and Ingber, 1999; Moore et al., 2005).
Tensional forces and ECM mechanics also contribute to epithelial cell differentiation and morphogenesis during mammary gland development (Alcaraz et al., 2008; Nelson et al., 2006). For example, both the correct composition and elasticity of the ECM are required for the maintenance of mammary epithelial cell differentiation, as measured by β-casein expression in cultured breast epithelial cells (Alcaraz et al., 2008). A complete loss of ECM structure (and hence of resistance to cell traction forces; see Box 1) also leads to mammary gland regression (Wicha, 1984; Wicha et al., 1982), as well as to the involution of capillary blood vessels (Ingber et al., 1986). Local variations in myosin II-dependent cortical tension guides branching in forming capillary blood vessels (Fischer et al., 2009), which is crucial for the development of almost all organs in vertebrates, as well as for many pathological processes, including cancer and macular degeneration. Variations in ECM elasticity also control angiogenesis in vitro and in vivo by regulating p190RhoGAP signaling, which in turn alters the balance between two antagonistic transcription factors, TFII-I and Gata2, that control the expression of a receptor for angiogenic factors, vascular endothelial growth factor receptor 2 (Mammoto et al., 2009).
Interestingly, neoplastic transformation might also involve alterations in the physical interactions between cells and the ECM that increase cell tension (Paszek et al., 2005; Butcher et al., 2009; Ingber, 2008; Bissell et al., 2005; Levental et al., 2009). Moreover, in mouse colon tissues deficient in the tumor suppressor gene adenomatosis polyposis coli, the physical deformation of cells and tissues, as might occur in a disturbed micromechanical environment within premalignant lesions, induces the transcriptional upregulation of oncogenes, such as Myc and Twist1, through the nuclear translocation of β-catenin (Whitehead et al., 2008). The abnormal Rho-mediated sensing of mechanical cues in the tumor microenvironment also appears to contribute to the aberrant behavior of tumor capillary endothelial cells, resulting in the development of characteristic structural abnormalities in the cancer microvasculature that interfere with drug delivery and radiation therapy (Ghosh et al., 2008). Taken together, these findings suggest that cancer could be seen as a disease of tissue development that results from the deregulation of both chemical and mechanical cues in the local tissue microenvironment.
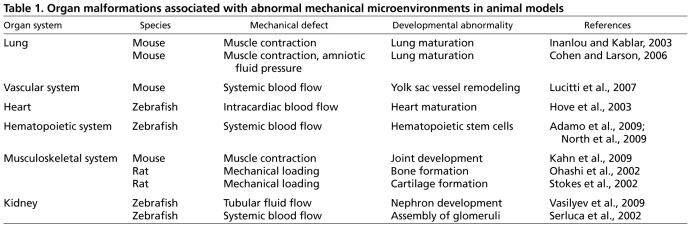
Finally, it is important to note that abnormal mechanical environments can interfere with normal organogenesis and produce organ malformations (Bartman et al., 2004; Cohen and Larson, 2006; Hove et al., 2003; Inanlou and Kablar, 2003; Lucitti et al., 2007; Moore et al., 2005; Vasilyev et al., 2009) (Table 1). In addition, certain developmental abnormalities, such as esophageal atresia, a blind-ended pouch of the esophagus, can be repaired clinically solely by restoring normal tension distributions using tensed surgical sutures that mechanically stretch the remaining tissue structures (Foker et al., 1997). Thus, just as mechanical forces generated by the cells of the developing embryo play a central role in normal organogenesis, the disturbance or loss of normal mechanical cues can lead to severe developmental defects.
Table 1.
Organ malformations associated with abnormal mechanical microenvironments in animal models
Molecular basis of cellular mechanotransduction
During organogenesis, individual cells sense changes in physical forces and transduce them into intracellular signals that drive the alterations in cell shape, polarity, growth, migration and differentiation required for tissue development. The molecular mechanisms by which forces applied to living cells alter the intracellular biochemistry and gene expression crucial for developmental control remain to be fully defined. However, great advances have been made in this field of mechanotransduction over the past two decades (reviewed by Ingber, 2006; Orr et al., 2006). These studies have revealed that cell surface adhesion receptors that link the internal cytoskeleton to the ECM and to neighboring cells (e.g. integrins and cadherins, respectively) provide preferred molecular pathways for mechanical signal transfer across the cell surface (Fig. 4). Cell tensional forces that are generated in the actomyosin cytoskeleton are also resisted by these adhesive tethers to neighboring cells and the ECM, and by internal cytoskeletal structures that resist being compressed, such as microtubules or cross-linked actin filaments within filopodia (Wang et al., 2001; Brangwynne et al., 2006; Ingber, 2006). This results in the establishment of a tensegrity-based mechanical force balance that generates cytoskeletal prestress ensuring cell, tissue and organ shape stability (reviewed by Ingber, 2006).
Fig. 4.
Model of the mechanical control of cell fate switching. Mechanical forces generated by acto-myosin interactions within the cytoskeleton are resisted by integrin adhesions to the ECM, cadherin adhesions to neighboring cells and internal cytoskeletal struts (e.g. microtubules and cross-linked actin bundles as in filopodia), thereby establishing a tensional prestress that stabilizes cell and tissue structure through a tensegrity mechanism (reviewed by Ingber, 2006). Alterations in the mechanical forces that are balanced between the ECM, neighboring cells and opposing cytoskeletal elements modulate intracellular biochemistry and gene expression (Ingber, 2006; Stamenovic and Ingber, 2009). Molecules involved in cytoskeletal tension generation, including actin, myosin II, Rho, ROCK and the Rho modulator p190RhoGAP, play a central role in this form of mechanical signaling. External forces (e.g. fluid shear stress) also can modulate gene transcription, for example through changes in nitric oxide (NO) signaling. The binding of growth factors and ECM ligands to their respective cell surface receptors can alter cellular biochemistry and gene expression independently of changes in cell prestress or external forces; however, mechanical stresses govern the final biochemical response and determine cell fate (e.g. whether stem cells differentiate into bone, muscle, nerve, blood or other cells). Physical forces exerted on surface adhesion receptors are also transmitted directly to the nucleus along cytoskeletal filaments and molecules that connect the cytoskeleton to the nucleus, such as nesprin (Wang et al., 2009). Nuclear envelope molecules, such as lamin, stabilize nuclear architecture under mechanical strain, and defects in nuclear mechanical signaling can lead to developmental abnormalities.
Because cells and tissues are tensionally prestressed, forces transmitted between cells across ECM and cell-cell junctions are transmitted to multiple sites in the cell over cytoskeletal linkages, and the efficiency of this signal transfer is sensitive to the level of tensional prestress in the cells, which ‘tunes’ the cellular response, much as mechanical tension tunes a violin string (Box 1) (Wang et al., 2001; Wang et al., 2009). Mechanical forces exerted on surface adhesion receptors and channeled along cytoskeletal filaments can be converted into changes in molecular biochemistry at the cell surface or in junctional complexes (e.g. focal adhesions, cell-cell adhesion complexes) via tension- or strain-dependent changes of molecular shape that expose new binding sites, as seen, for example, in p130Cas, talin and fibronectin (Tamada et al., 2004; Sawada et al., 2006; Brown and Discher, 2009; Zhong et al., 1998; Gee et al., 2008; del Rio et al., 2009), that alter molecular binding or unbinding kinetics (Lele et al., 2006b) or that modulate ion flux through membrane channels (Thodeti et al., 2009). These forces might also be concentrated at distant sites in the nucleus, where they can alter gene activity or stem cell fate through the modulation of nuclear ion channels (Itano et al., 2003) or through the nuclear transport of key signaling molecules, such as of β-catenin (Farge, 2003; Kahn et al., 2009), or of transcription factors, such as TFII-I or Gata2 (Mammoto et al., 2009), to determine cell fate and drive tissue development. Again, the efficiency of force transmission depends directly on the level of isometric tension in the cytoskeleton (reviewed by Ingber, 2006; Wang et al., 2009). Importantly, such a hard-wired mechanism for nuclear mechanotransduction in response to forces acting on the cell surface is much faster than conventional chemical signal propagation (Na et al., 2008).
Members of the nesprin family of outer nuclear membrane proteins, which have been implicated in nuclear mechano-transduction because they physically link the intermediate filament lamin proteins of the nuclear scaffold to cytoskeletal filaments in the cytoplasm (Wang et al., 2009), are crucial for musculogenesis in Drosophila (Zhang et al., 2002). Other molecules that anchor the nuclear membrane to lamins, such as Kugelkern (Brandt et al., 2006), also regulate nuclear architecture and dictate epithelial morphology during cellularization (Pilot et al., 2006). This could be relevant for developmental control because nuclear envelope lamins stabilize nuclear architecture under mechanical strain through mechanically activated NF-κB transcription (Lammerding et al., 2004). Moreover, mutations in the genes that encode certain lamins and other key nuclear structural molecules cause the altered nuclear mechanics that underlie various human congenital developmental disorders, including Emery-Dreifuss muscular dystrophy, dilated cardiomyopathy and Hutchinson-Gilford progeria syndrome (Lammerding and Lee, 2009).
Cytoskeletal tension as a fundamental bioregulator
As discussed above, it appears that cell shape and cytoskeletal structure might be altered by changing the level of cell contractility or prestress (Box 1), the mechanical compliance of the ECM (e.g. through biochemical remodeling), the strength of cell-cell or cell-ECM adhesions, or the number or size of cells packed within a tissue volume that is bounded by a relatively rigid ECM (Fig. 4). Studies with cultured anchorage-dependent cells that require adhesion to an ECM substrate to survive, such as endothelial or mesenchymal stem cells, have revealed that changes in cell fate between growth, differentiation and apoptosis can be controlled by altering similar parameters, including cell shape (Chen et al., 1997; Dike et al., 1999), the actin or microtubule cytoskeleton (Mooney et al., 1994), ECM elasticity (Engler et al., 2006), tissue patterns (Ruiz and Chen, 2008; Nelson et al., 2005) or Rho/ROCK signaling (McBeath et al., 2004; Mammoto et al., 2004; Numaguchi et al., 2003). For example, single endothelial cells proliferate when spread on large (>1500 μm2) microfabricated ECM adhesive islands, undergo apoptosis when cultured on small islands that completely prevent cell extension (<500 μm2), and become quiescent and differentiate when spread to a moderate degree on a substrate of intermediate size (Dike et al., 1999).
Neuronal cells exert mechanical tension on their substrate adhesions, which is crucial for nerve guidance, and tension distributions and the ECM area available for spreading govern the formation and function of mature neuronal networks (Anava et al., 2009; Moore et al., 2009; Wilson et al., 2007). Mechanical tension within Drosophila axons is also pivotal for the formation and clustering of neurotransmitter vesicles (Siechen et al., 2009), suggesting that the micromechanical environment is central to the maintenance and functionality of neuronal information processing.
The mechanical control of lineage switching appears to play a key role in the differentiation of stem cells. For example, mesenchymal stem cells held in different shapes or cultured on artificial polyacrylamide gels of different stiffnesses that are coated with ECM proteins decide their fate depending on the elasticity of their ECM substrate, even when cultured in the presence of soluble inducing factors, and this mechanical control mechanism depends on the generation of Rho-dependent cytoskeletal tension (McBeath et al., 2004). Interestingly, cells preferentially differentiate into distinct cell types (e.g. neuron versus bone or muscle) when cultured on ECMs with a mechanical stiffness that is most similar to that of the respective in vivo tissue (Engler et al., 2006). Undifferentiated mouse embryonic stem cells also appear to be highly mechanosensitive, and they rapidly change their mechanics and lose pluripotency (as measured by the suppression of Oct3/4 gene expression) when exposed to mechanical stress (Chowdhury et al., 2009). These findings suggest that stem cells are exquisitely sensitive to their physical microenvironment and that mechanical cues are as important for the control of stem cell growth and function as soluble factors.
Mechanical adhesive interactions between stem cells and surrounding supporting stromal cells (or their intervening ECM) might similarly promote asymmetric cell divisions that are crucial for the maintenance of stem cell niches in the embryo, as has been described for the Drosophila testis (Tanentzapf et al., 2007) and for neuroblasts (Siegrist and Doe, 2006). This possibility is supported by the finding that the loss of cadherin function, which disrupts cell-cell junctions within germ line stem cells, causes the subsequent loss of ovarian and testicular stem cells in Drosophila (Song et al., 2002) and mice (Karpowicz et al., 2009). However, these mechanical cues must be integrated with other chemical signals, such as growth factors (e.g. insulin-like growth factor or fibroblast growth factor) (Bendall et al., 2007), to exert effective developmental control.
Conclusions
The myriad findings described above illustrate the point that mechanical forces play a central role in morphogenesis and tissue patterning, and that physical cues are as important as chemical factors for developmental control throughout virtually all stages of embryogenesis. Although the contribution of physical forces to cell and tissue deformation in the embryo has been recognized for over a century, it is only recently that mechanical stresses have been shown to function as informative signals that produce specific changes in molecular biochemistry and gene expression through the process of cellular mechanotransduction. Moreover, although virtually all physical forces in the developing embryo must, at some level, result from the action of its constituent cells, it is only now becoming clear that cytoskeletal tension is the driving force behind many of these key mechanical processes and mechano-chemical transduction events. Cells sense changes in mechanical signals based on their ability to alter biochemistry and to induce remodeling in the cytoskeleton and the ECM at the molecular level, but the extent of this response and the efficiency of mechanical signal transfer throughout the cell and nucleus depend on the level of isometric tension or prestress in the cytoskeleton. At the same time, cell-generated tensile forces alter the chemical signals that are conveyed by cells, in addition to producing the distortion of neighboring cells and ECM molecules that propagate mechanochemical signaling over long distances, thus driving tissue patterning and organ formation at the scale of the whole embryo.
Therefore, although developmental biology has been dominated by a focus on genes and chemical interactions over the past century, it is time to explore further how mechanical forces that act on cell surface receptors and linked cytoskeletal networks can exert their potent effects on tissue development during embryogenesis, as well as throughout adult life. These findings also raise the possibility that a better understanding of these mechanochemical control mechanisms might increase our chances of reversing developmental defects and of treating diseases, such as cancer, by restoring normal mechanical loading conditions or by correcting abnormal mechanochemical signaling mechanisms. Thus, the field of mechanical developmental biology would benefit greatly from carrying out more studies that involve the application of controlled mechanical stresses at specific locations in whole developing organs and embryos, and the measurement of the resulting changes in molecular events and in shape transformations that are key to developmental control. It is also necessary to create new methods to measure endogenous mechanical forces and local variations of the material properties of cells, the ECM and tissues within forming organs, and to analyze how mechanical cues and chemical signals dynamically interact in situ during important morphogenetic and embryological patterning events. These goals will best be advanced by combining approaches from cell and developmental biology with those from physics, engineering and computer science, and by training students to be conversant in all of these diverse disciplines.
Acknowledgements
This work was supported by grants from the NIH and the Department of Defense. Dr Ingber is a recipient of a Department of Defense Breast Cancer Innovator Award. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- Adamo L., Naveiras O., Wenzel P. L., McKinney-Freeman S., Mack P. J., Gracia-Sancho J., Suchy-Dicey A., Yoshimoto M., Lensch M. W., Yoder M. C., et al. (2009). Biomechanical forces promote embryonic haematopoiesis. Nature 459, 1131-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz J., Xu R., Mori H., Nelson C. M., Mroue R., Spencer V. A., Brownfield D., Radisky D. C., Bustamante C., Bissell M. J. (2008). Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 27, 2829-2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anava S., Greenbaum A., Ben Jacob E., Hanein Y., Ayali A. (2009). The regulative role of neurite mechanical tension in network development. Biophys. J. 96, 1661-1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Gill J. S., Cinalli R. M., Nance J. (2008). Polarization of the C. elegans embryo by RhoGAP-mediated exclusion of PAR-6 from cell contacts. Science 320, 1771-1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash J. F., Spooner B. S., Wessells N. K. (1973). Effects of papaverine and calcium-free medium on salivary gland morphogenesis. Dev. Biol. 33, 463-469 [DOI] [PubMed] [Google Scholar]
- Bartman T., Walsh E. C., Wen K. K., McKane M., Ren J., Alexander J., Rubenstein P. A., Stainier D. Y. (2004). Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2, E129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock R., Strutt D. (2007). The planar polarity pathway promotes coordinated cell migration during Drosophila oogenesis. Development 134, 3055-3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloussov L. V., Dorfman J. G., Cherdantzev V. G. (1975). Mechanical stresses and morphological patterns in amphibian embryos. J. Embryol. Exp. Morphol. 34, 559-574 [PubMed] [Google Scholar]
- Bendall S. C., Stewart M. H., Menendez P., George D., Vijayaragavan K., Werbowetski-Ogilvie T., Ramos-Mejia V., Rouleau A., Yang J., Bosse M., et al. (2007). IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature 448, 1015-1021 [DOI] [PubMed] [Google Scholar]
- Berger C. D., Marz M., Kitzing T. M., Grosse R., Steinbeisser H. (2009). Detection of activated Rho in fixed Xenopus tissue. Dev. Dyn. 238, 1407-1411 [DOI] [PubMed] [Google Scholar]
- Bertet C., Sulak L., Lecuit T. (2004). Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667-671 [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Kenny P. A., Radisky D. C. (2005). Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harb. Symp. Quant. Biol. 70, 343-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship J. T., Backovic S. T., Sanny J. S., Weitz O., Zallen J. A. (2006). Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev. Cell 11, 459-470 [DOI] [PubMed] [Google Scholar]
- Brandt A., Papagiannouli F., Wagner N., Wilsch-Brauninger M., Braun M., Furlong E. E., Loserth S., Wenzl C., Pilot F., Vogt N., et al. (2006). Developmental control of nuclear size and shape by Kugelkern and Kurzkern. Curr. Biol. 16, 543-552 [DOI] [PubMed] [Google Scholar]
- Brangwynne C. P., MacKintosh F. C., Kumar S., Geisse N. A., Talbot J., Mahadevan L., Parker K. K., Ingber D. E., Weitz D. A. (2006). Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J. Cell Biol. 173, 733-741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevier J., Montero D., Svitkina T., Riveline D. (2008). The asymmetric self-assembly mechanism of adherens junctions: a cellular push-pull unit. Phys. Biol. 5, 16005 [DOI] [PubMed] [Google Scholar]
- Brown A. E., Discher D. E. (2009). Conformational changes and signaling in cell and matrix physics. Curr. Biol. 19, R781-R789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher D. T., Alliston T., Weaver V. M. (2009). A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler L. C., Blanchard G. B., Kabla A. J., Lawrence N. J., Welchman D. P., Mahadevan L., Adams R. J., Sanson B. (2009). Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat. Cell Biol. 11, 859-864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. S., Mrksich M., Huang S., Whitesides G. M., Ingber D. E. (1997). Geometric control of cell life and death. Science 276, 1425-1428 [DOI] [PubMed] [Google Scholar]
- Chen Y., Stump R. J., Lovicu F. J., McAvoy J. W. (2006). A role for Wnt/planar cell polarity signaling during lens fiber cell differentiation? Semin. Cell Dev. Biol. 17, 712-725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm A. D. (2006). Gastrulation: Wnts signal constriction. Curr. Biol. 16, R874-R876 [DOI] [PubMed] [Google Scholar]
- Chowdhury F., Na S., Li D., Poh Y. C., Tanaka T. S., Wang F., Wang N. (2009). Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 9, 82-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen A. K., Anderson K. I., Marois E., Eaton S. (2005). Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev. Cell 9, 805-817 [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Larson J. E. (2006). Cystic fibrosis transmembrane conductance regulator (CFTR) dependent cytoskeletal tension during lung organogenesis. Dev. Dyn. 235, 2736-2748 [DOI] [PubMed] [Google Scholar]
- Colombo K., Grill S. W., Kimple R. J., Willard F. S., Siderovski D. P., Gonczy P. (2003). Translation of polarity cues into asymmetric spindle positioning in Caenorhabditis elegans embryos. Science 300, 1957-1961 [DOI] [PubMed] [Google Scholar]
- Corrigall D., Walther R. F., Rodriguez L., Fichelson P., Pichaud F. (2007). Hedgehog signaling is a principal inducer of Myosin-II-driven cell ingression in Drosophila epithelia. Dev. Cell 13, 730-742 [DOI] [PubMed] [Google Scholar]
- Daga R. R., Nurse P. (2008). Interphase microtubule bundles use global cell shape to guide spindle alignment in fission yeast. J. Cell Sci. 121, 1973-1980 [DOI] [PubMed] [Google Scholar]
- Dawes-Hoang R. E., Parmar K. M., Christiansen A. E., Phelps C. B., Brand A. H., Wieschaus E. F. (2005). folded gastrulation, cell shape change and the control of myosin localization. Development 132, 4165-4178 [DOI] [PubMed] [Google Scholar]
- del Rio A., Perez-Jimenez R., Liu R., Roca-Cusachs P., Fernandez J. M., Sheetz M. P. (2009). Stretching single talin rod molecules activates vinculin binding. Science 323, 638-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denholm B., Brown S., Ray R. P., Ruiz-Gomez M., Skaer H., Hombria J. C. (2005). crossveinless-c is a RhoGAP required for actin reorganisation during morphogenesis. Development 132, 2389-2400 [DOI] [PubMed] [Google Scholar]
- Desprat N., Supatto W., Pouille P. A., Beaurepaire E., Farge E. (2008). Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev. Cell 15, 470-477 [DOI] [PubMed] [Google Scholar]
- Dike L. E., Chen C. S., Mrksich M., Tien J., Whitesides G. M., Ingber D. E. (1999). Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cell Dev. Biol. Anim. 35, 441-448 [DOI] [PubMed] [Google Scholar]
- Engler A. J., Sen S., Sweeney H. L., Discher D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677-689 [DOI] [PubMed] [Google Scholar]
- Farge E. (2003). Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr. Biol. 13, 1365-1377 [DOI] [PubMed] [Google Scholar]
- Farhadifar R., Roper J. C., Aigouy B., Eaton S., Julicher F. (2007). The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr. Biol. 17, 2095-2104 [DOI] [PubMed] [Google Scholar]
- Fernandez B. G., Arias A. M., Jacinto A. (2007). Dpp signalling orchestrates dorsal closure by regulating cell shape changes both in the amnioserosa and in the epidermis. Mech. Dev. 124, 884-897 [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R., Simoes Sde M., Roper J. C., Eaton S., Zallen J. A. (2009). Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell 17, 736-743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Legue E., Doyen A., Nato F., Nicolas J. F., Torres V., Yaniv M., Pontoglio M. (2006). Defective planar cell polarity in polycystic kidney disease. Nat. Genet. 38, 21-23 [DOI] [PubMed] [Google Scholar]
- Fischer R. S., Gardel M., Ma X., Adelstein R. S., Waterman C. M. (2009). Local cortical tension by myosin II guides 3D endothelial cell branching. Curr. Biol. 19, 260-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foker J. E., Linden B. C., Boyle E. M., Jr, Marquardt C. (1997). Development of a true primary repair for the full spectrum of esophageal atresia. Ann. Surg. 226, 533-541; discussion 541-543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouhar A. S., Liebling M., Hickerson A., Nasiraei-Moghaddam A., Tsai H. J., Hove J. R., Fraser S. E., Dickinson M. E., Gharib M. (2006). The embryonic vertebrate heart tube is a dynamic suction pump. Science 312, 751-753 [DOI] [PubMed] [Google Scholar]
- Foty R. A., Steinberg M. S. (2005). The differential adhesion hypothesis: a direct evaluation. Dev. Biol. 278, 255-263 [DOI] [PubMed] [Google Scholar]
- Franke J. D., Montague R. A., Kiehart D. P. (2005). Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Curr. Biol. 15, 2208-2221 [DOI] [PubMed] [Google Scholar]
- Gee E. P., Ingber D. E., Stultz C. M. (2008). Fibronectin unfolding revisited: modeling cell traction-mediated unfolding of the tenth type-III repeat. PLoS One 3, e2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K., Thodeti C. K., Dudley A. C., Mammoto A., Klagsbrun M., Ingber D. E. (2008). Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc. Natl. Acad. Sci. USA 105, 11305-11310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B., Macara I. G. (2007). The PAR proteins: fundamental players in animal cell polarization. Dev. Cell 13, 609-622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P. (2008). Mechanisms of asymmetric cell division: flies and worms pave the way. Nat. Rev. Mol. Cell Biol. 9, 355-366 [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N., Arias A. M. (2007). Requirements for adherens junction components in the interaction between epithelial tissues during dorsal closure in Drosophila. J. Cell Sci. 120, 3289-3298 [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N., Blanchard G. B., Adams R. J., Martinez Arias A. (2009). Mechanical control of global cell behaviour during dorsal closure in Drosophila. Development 136, 1889-1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D., Plusa B., Piotrowska K., Na J., Tom B., Glover D. M., Zernicka-Goetz M. (2004). First cleavage of the mouse embryo responds to change in egg shape at fertilization. Curr. Biol. 14, 397-405 [DOI] [PubMed] [Google Scholar]
- Gros J., Feistel K., Viebahn C., Blum M., Tabin C. J. (2009). Cell movements at Hensen's node establish left/right asymmetric gene expression in the chick. Science 324, 941-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B. M. (2005). Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6, 622-634 [DOI] [PubMed] [Google Scholar]
- Gutierrez J. A., Suzara V. V., Dobbs L. G. (2003). Continuous mechanical contraction modulates expression of alveolar epithelial cell phenotype. Am. J. Respir. Cell Mol. Biol. 29, 81-87 [DOI] [PubMed] [Google Scholar]
- Heidemann S. R., Buxbaum R. E. (1994). Mechanical tension as a regulator of axonal development. Neurotoxicology 15, 95-107 [PubMed] [Google Scholar]
- Hogan C., Serpente N., Cogram P., Hosking C. R., Bialucha C. U., Feller S. M., Braga V. M., Birchmeier W., Fujita Y. (2004). Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol. Cell. Biol. 24, 6690-6700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner V. L., Wolfner M. F. (2008a). Mechanical stimulation by osmotic and hydrostatic pressure activates Drosophila oocytes in vitro in a calcium-dependent manner. Dev. Biol. 316, 100-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner V. L., Wolfner M. F. (2008b). Transitioning from egg to embryo: triggers and mechanisms of egg activation. Dev. Dyn. 237, 527-544 [DOI] [PubMed] [Google Scholar]
- Hove J. R., Koster R. W., Forouhar A. S., Acevedo-Bolton G., Fraser S. E., Gharib M. (2003). Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421, 172-177 [DOI] [PubMed] [Google Scholar]
- Huang S., Ingber D. E. (1999). The structural and mechanical complexity of cell-growth control. Nat. Cell Biol. 1, E131-E138 [DOI] [PubMed] [Google Scholar]
- Inanlou M. R., Kablar B. (2003). Abnormal development of the diaphragm in mdx:MyoD−/−(9th) embryos leads to pulmonary hypoplasia. Int. J. Dev. Biol. 47, 363-371 [PubMed] [Google Scholar]
- Ingber D. E. (2006). Cellular mechanotransduction: putting all the pieces together again. FASEB J. 20, 811-827 [DOI] [PubMed] [Google Scholar]
- Ingber D. E. (2008). Can cancer be reversed by engineering the tumor microenvironment? Semin. Cancer Biol. 18, 356-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E., Jamieson J. D. (1985). Cells as tensegrity structures: architectural regulation of histodifferentiation by physical forces tranduced over basement membrane. In Gene Expression During Normal and Malignant Differentiation (ed. Andersson L. C., Gahmberg C. G., Ekblom P.), pp. 13-32 Orlando, FL: Academic Press; [Google Scholar]
- Ingber D. E., Madri J. A., Folkman J. (1986). A possible mechanism for inhibition of angiogenesis by angiostatic steroids: induction of capillary basement membrane dissolution. Endocrinology 119, 1768-1775 [DOI] [PubMed] [Google Scholar]
- Itano N., Okamoto S., Zhang D., Lipton S. A., Ruoslahti E. (2003). Cell spreading controls endoplasmic and nuclear calcium: a physical gene regulation pathway from the cell surface to the nucleus. Proc. Natl. Acad. Sci. USA 100, 5181-5186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto A., Wood W., Balayo T., Turmaine M., Martinez-Arias A., Martin P. (2000). Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr. Biol. 10, 1420-1426 [DOI] [PubMed] [Google Scholar]
- Jacinto A., Wood W., Woolner S., Hiley C., Turner L., Wilson C., Martinez-Arias A., Martin P. (2002). Dynamic analysis of actin cable function during Drosophila dorsal closure. Curr. Biol. 12, 1245-1250 [DOI] [PubMed] [Google Scholar]
- Jenkins N., Saam J. R., Mango S. E. (2006). CYK-4/GAP provides a localized cue to initiate anteroposterior polarity upon fertilization. Science 313, 1298-1301 [DOI] [PubMed] [Google Scholar]
- Kahn J., Shwartz Y., Blitz E., Krief S., Sharir A., Breitel D. A., Rattenbach R., Relaix F., Maire P., Rountree R. B., et al. (2009). Muscle contraction is necessary to maintain joint progenitor cell fate. Dev. Cell 16, 734-743 [DOI] [PubMed] [Google Scholar]
- Karner C., Wharton K. A., Jr, Carroll T. J. (2006). Planar cell polarity and vertebrate organogenesis. Semin. Cell Dev. Biol. 17, 194-203 [DOI] [PubMed] [Google Scholar]
- Karner C. M., Chirumamilla R., Aoki S., Igarashi P., Wallingford J. B., Carroll T. J. (2009). Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat. Genet. 41, 793-799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz P., Willaime-Morawek S., Balenci L., DeVeale B., Inoue T., van der Kooy D. (2009). E-Cadherin regulates neural stem cell self-renewal. J. Neurosci. 29, 3885-3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. E. (1980). The cellular basis of epiboly: an SEM study of deep-cell rearrangement during gastrulation in Xenopus laevis. J. Embryol. Exp. Morphol. 60, 201-234 [PubMed] [Google Scholar]
- Keller R., Davidson L. A., Shook D. R. (2003). How we are shaped: the biomechanics of gastrulation. Differentiation 71, 171-205 [DOI] [PubMed] [Google Scholar]
- Keller R., Shook D., Skoglund P. (2008). The forces that shape embryos: physical aspects of convergent extension by cell intercalation. Phys. Biol. 5, 15007 [DOI] [PubMed] [Google Scholar]
- Kiehart D. P., Galbraith C. G., Edwards K. A., Rickoll W. L., Montague R. A. (2000). Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J. Cell Biol. 149, 471-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Yamashita M., Kimura M., Honda A., Kashiwabara S., Baba T. (2008). Sperm penetration through cumulus mass and zona pellucida. Int. J. Dev. Biol. 52, 677-682 [DOI] [PubMed] [Google Scholar]
- Kolsch V., Seher T., Fernandez-Ballester G. J., Serrano L., Leptin M. (2007). Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science 315, 384-386 [DOI] [PubMed] [Google Scholar]
- Kozlowski C., Srayko M., Nedelec F. (2007). Cortical microtubule contacts position the spindle in C. elegans embryos. Cell 129, 499-510 [DOI] [PubMed] [Google Scholar]
- Krieg M., Arboleda-Estudillo Y., Puech P. H., Kafer J., Graner F., Muller D. J., Heisenberg C. P. (2008). Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 10, 429-436 [DOI] [PubMed] [Google Scholar]
- Kurotaki Y., Hatta K., Nakao K., Nabeshima Y., Fujimori T. (2007). Blastocyst axis is specified independently of early cell lineage but aligns with the ZP shape. Science 316, 719-723 [DOI] [PubMed] [Google Scholar]
- Lahmers S., Wu Y., Call D. R., Labeit S., Granzier H. (2004). Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ. Res. 94, 505-513 [DOI] [PubMed] [Google Scholar]
- Lammerding J., Lee R. T. (2009). Mechanical properties of interphase nuclei probed by cellular strain application. Methods Mol. Biol. 464, 13-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J., Schulze P. C., Takahashi T., Kozlov S., Sullivan T., Kamm R. D., Stewart C. L., Lee R. T. (2004). Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Invest. 113, 370-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg K. P., Farhadifar R., Ranft J., Umetsu D., Widmann T. J., Bittig T., Said A., Julicher F., Dahmann C. (2009). Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr. Biol. 19, 1950-1955 [DOI] [PubMed] [Google Scholar]
- le Noble F., Moyon D., Pardanaud L., Yuan L., Djonov V., Matthijsen R., Breant C., Fleury V., Eichmann A. (2004). Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development 131, 361-375 [DOI] [PubMed] [Google Scholar]
- Lecuit T., Lenne P. F. (2007). Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 8, 633-644 [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Marston D. J., Walston T., Hardin J., Halberstadt A., Goldstein B. (2006). Wnt/Frizzled signaling controls C. elegans gastrulation by activating actomyosin contractility. Curr. Biol. 16, 1986-1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele T. P., Pendse J., Kumar S., Salanga M., Karavitis J., Ingber D. E. (2006a). Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J. Cell Physiol. 207, 187-194 [DOI] [PubMed] [Google Scholar]
- Lele T. P., Thodeti C. K., Ingber D. E. (2006b). Force meets chemistry: analysis of mechanochemical conversion in focal adhesions using fluorescence recovery after photobleaching. J. Cell. Biochem. 97, 1175-1183 [DOI] [PubMed] [Google Scholar]
- Lenoir T. (1982). The Strategy of Like: Teleology and Mechanics in Nineteenth-Century German Chicago, IL: University of Chicago Press; [DOI] [PubMed] [Google Scholar]
- Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F., Csiszar K., Giaccia A., Weninger W., et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linask K. K., Yu X., Chen Y., Han M. D. (2002). Directionality of heart looping: effects of Pitx2c misexpression on flectin asymmetry and midline structures. Dev. Biol. 246, 407-417 [DOI] [PubMed] [Google Scholar]
- Linask K. K., Han M. D., Linask K. L., Schlange T., Brand T. (2003). Effects of antisense misexpression of CFC on downstream flectin protein expression during heart looping. Dev. Dyn. 228, 217-230 [DOI] [PubMed] [Google Scholar]
- Lu W., Seeholzer S. H., Han M., Arnold A. S., Serrano M., Garita B., Philp N. J., Farthing C., Steele P., Chen J., et al. (2008). Cellular nonmuscle myosins NMHC-IIA and NMHC-IIB and vertebrate heart looping. Dev. Dyn. 237, 3577-3590 [DOI] [PubMed] [Google Scholar]
- Lucitti J. L., Visconti R., Novak J., Keller B. B. (2006). Increased arterial load alters aortic structural and functional properties during embryogenesis. Am. J. Physiol. Heart Circ. Physiol. 291, H1919-H1926 [DOI] [PubMed] [Google Scholar]
- Lucitti J. L., Jones E. A., Huang C., Chen J., Fraser S. E., Dickinson M. E. (2007). Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134, 3317-3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A., Huang S., Moore K., Oh P., Ingber D. E. (2004). Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J. Biol. Chem. 279, 26323-26330 [DOI] [PubMed] [Google Scholar]
- Mammoto A., Huang S., Ingber D. E. (2007). Filamin links cell shape and cytoskeletal structure to Rho regulation by controlling accumulation of p190RhoGAP in lipid rafts. J. Cell Sci. 120, 456-467 [DOI] [PubMed] [Google Scholar]
- Mammoto A., Connor K. M., Mammoto T., Yung C. W., Huh D., Aderman C. M., Mostoslavsky G., Smith L. E., Ingber D. E. (2009). A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 457, 1103-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis A. J., Bojanowski K., Ingber D. E. (1997). Mechanical continuity and reversible chromosome disassembly within intact genomes removed from living cells. J. Cell. Biochem. 65, 114-130 [PubMed] [Google Scholar]
- Martin A. C., Kaschube M., Wieschaus E. F. (2009). Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495-499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K., Chen C. S. (2004). Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483-495 [DOI] [PubMed] [Google Scholar]
- McGrath J., Somlo S., Makova S., Tian X., Brueckner M. (2003). Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114, 61-73 [DOI] [PubMed] [Google Scholar]
- Millard T. H., Martin P. (2008). Dynamic analysis of filopodial interactions during the zippering phase of Drosophila dorsal closure. Development 135, 621-626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y., Inoue N., Nishimura K., Kinoshita N., Hosoya H., Yonemura S. (2006). Actomyosin tension is required for correct recruitment of adherens junction components and zonula occludens formation. Exp. Cell Res. 312, 1637-1650 [DOI] [PubMed] [Google Scholar]
- Monier B., Pelissier-Monier A., Brand A. H., Sanson B. (2010). An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat. Cell Biol. 12, 1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney D. J., Hansen L. K., Langer R., Vacanti J. P., Ingber D. E. (1994). Extracellular matrix controls tubulin monomer levels in hepatocytes by regulating protein turnover. Mol. Biol. Cell 5, 1281-1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. A., Polte T., Huang S., Shi B., Alsberg E., Sunday M. E., Ingber D. E. (2005). Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev. Dyn. 232, 268-281 [DOI] [PubMed] [Google Scholar]
- Moore S. W., Biais N., Sheetz M. P. (2009). Traction on immobilized netrin-1 is sufficient to reorient axons. Science 325, 166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro E., Nance J., Priess J. R. (2004). Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev. Cell 7, 413-424 [DOI] [PubMed] [Google Scholar]
- Na S., Collin O., Chowdhury F., Tay B., Ouyang M., Wang Y., Wang N. (2008). Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc. Natl. Acad. Sci. USA 105, 6626-6631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J., Munro E. M., Priess J. R. (2003). C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development 130, 5339-5350 [DOI] [PubMed] [Google Scholar]
- Nauli S. M., Alenghat F. J., Luo Y., Williams E., Vassilev P., Li X., Elia A. E., Lu W., Brown E. M., Quinn S. J., et al. (2003). Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129-137 [DOI] [PubMed] [Google Scholar]
- Nelson C. M., Jean R. P., Tan J. L., Liu W. F., Sniadecki N. J., Spector A. A., Chen C. S. (2005). Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl. Acad. Sci. USA 102, 11594-11599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. M., Vanduijn M. M., Inman J. L., Fletcher D. A., Bissell M. J. (2006). Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 314, 298-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Ngoc T., Afshar K., Gonczy P. (2007). Coupling of cortical dynein and G alpha proteins mediates spindle positioning in Caenorhabditis elegans. Nat. Cell Biol. 9, 1294-1302 [DOI] [PubMed] [Google Scholar]
- Ninomiya H., Winklbauer R. (2008). Epithelial coating controls mesenchymal shape change through tissue-positioning effects and reduction of surface-minimizing tension. Nat. Cell Biol. 10, 61-69 [DOI] [PubMed] [Google Scholar]
- Nonaka S., Shiratori H., Saijoh Y., Hamada H. (2002). Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 418, 96-99 [DOI] [PubMed] [Google Scholar]
- North T. E., Goessling W., Peeters M., Li P., Ceol C., Lord A. M., Weber G. J., Harris J., Cutting C. C., Huang P., et al. (2009). Hematopoietic stem cell development is dependent on blood flow. Cell 137, 736-748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numaguchi Y., Huang S., Polte T. R., Eichler G. S., Wang N., Ingber D. E. (2003). Caldesmon-dependent switching between capillary endothelial cell growth and apoptosis through modulation of cell shape and contractility. Angiogenesis 6, 55-64 [DOI] [PubMed] [Google Scholar]
- Ohashi N., Robling A. G., Burr D. B., Turner C. H. (2002). The effects of dynamic axial loading on the rat growth plate. J. Bone Miner. Res. 17, 284-292 [DOI] [PubMed] [Google Scholar]
- Orr A. W., Helmke B. P., Blackman B. R., Schwartz M. A. (2006). Mechanisms of mechanotransduction. Dev. Cell 10, 11-20 [DOI] [PubMed] [Google Scholar]
- Oster G. F., Murray J. D., Harris A. K. (1983). Mechanical aspects of mesenchymal morphogenesis. J. Embryol. Exp. Morphol. 78, 83-125 [PubMed] [Google Scholar]
- Panbianco C., Weinkove D., Zanin E., Jones D., Divecha N., Gotta M., Ahringer J. (2008). A casein kinase 1 and PAR proteins regulate asymmetry of a PIP(2) synthesis enzyme for asymmetric spindle positioning. Dev. Cell 15, 198-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K. K., Brock A. L., Brangwynne C., Mannix R. J., Wang N., Ostuni E., Geisse N. A., Adams J. C., Whitesides G. M., Ingber D. E. (2002). Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 16, 1195-1204 [DOI] [PubMed] [Google Scholar]
- Paszek M. J., Zahir N., Johnson K. R., Lakins J. N., Rozenberg G. I., Gefen A., Reinhart-King C. A., Margulies S. S., Dembo M., Boettiger D., et al. (2005). Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241-254 [DOI] [PubMed] [Google Scholar]
- Phillips H. M., Murdoch J. N., Chaudhry B., Copp A. J., Henderson D. J. (2005). Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ. Res. 96, 292-299 [DOI] [PubMed] [Google Scholar]
- Pilot F., Philippe J. M., Lemmers C., Chauvin J. P., Lecuit T. (2006). Developmental control of nuclear morphogenesis and anchoring by charleston, identified in a functional genomic screen of Drosophila cellularisation. Development 133, 711-723 [DOI] [PubMed] [Google Scholar]
- Piotrowska-Nitsche K., Perea-Gomez A., Haraguchi S., Zernicka-Goetz M. (2005). Four-cell stage mouse blastomeres have different developmental properties. Development 132, 479-490 [DOI] [PubMed] [Google Scholar]
- Pope K. L., Harris T. J. D. (2008). Control of cell flattening and junctional remodeling during squamous epithelial morphogenesis in Drosophila. Development 135, 2227-2238 [DOI] [PubMed] [Google Scholar]
- Pouille P. A., Ahmadi P., Brunet A. C., Farge E. (2009). Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci. Signal 2, ra16 [DOI] [PubMed] [Google Scholar]
- Puchner E. M., Alexandrovich A., Kho A. L., Hensen U., Schafer L. V., Brandmeier B., Grater F., Grubmuller H., Gaub H. E., Gautel M. (2008). Mechanoenzymatics of titin kinase. Proc. Natl. Acad. Sci. USA 105, 13385-13390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch C., Brunet A. C., Deleule J., Farge E. (2002). C2C12 myoblast/osteoblast transdifferentiation steps enhanced by epigenetic inhibition of BMP2 endocytosis. Am. J. Physiol. Cell Physiol. 283, C235-C243 [DOI] [PubMed] [Google Scholar]
- Rauzi M., Verant P., Lecuit T., Lenne P. F. (2008). Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat. Cell Biol. 10, 1401-1410 [DOI] [PubMed] [Google Scholar]
- Remond M. C., Fee J. A., Elson E. L., Taber L. A. (2006). Myosin-based contraction is not necessary for cardiac c-looping in the chick embryo. Anat. Embryol. (Berlin) 211, 443-454 [DOI] [PubMed] [Google Scholar]
- Ruiz S. A., Chen C. S. (2008). Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells 26, 2921-2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. C., Way M., Sakai J., Matsudaira P. (1996). Characterization of the actin cross-linking properties of the scruin-calmodulin complex from the acrosomal process of Limulus sperm. J. Biol. Chem. 271, 2651-2657 [DOI] [PubMed] [Google Scholar]
- Sawada Y., Tamada M., Dubin-Thaler B. J., Cherniavskaya O., Sakai R., Tanaka S., Sheetz M. P. (2006). Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer J. M., Harrell J. R., Shemer G., Sullivan-Brown J., Roh-Johnson M., Goldstein B. (2009). Apical constriction: A cell shape change that can drive morphogenesis. Dev. Biol. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotz E. M., Burdine R. D., Julicher F., Steinberg M. S., Heisenberg C. P., Foty R. A. (2008). Quantitative differences in tissue surface tension influence zebrafish germ layer positioning. HFSP J. 2, 42-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seher T. C., Narasimha M., Vogelsang E., Leptin M. (2007). Analysis and reconstitution of the genetic cascade controlling early mesoderm morphogenesis in the Drosophila embryo. Mech. Dev. 124, 167-179 [DOI] [PubMed] [Google Scholar]
- Serluca F. C., Drummond I. A., Fishman M. C. (2002). Endothelial signaling in kidney morphogenesis: a role for hemodynamic forces. Curr. Biol. 12, 492-497 [DOI] [PubMed] [Google Scholar]
- Shin J. H., Tam B. K., Brau R. R., Lang M. J., Mahadevan L., Matsudaira P. (2007). Force of an actin spring. Biophys. J. 92, 3729-3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siechen S., Yang S., Chiba A., Saif T. (2009). Mechanical tension contributes to clustering of neurotransmitter vesicles at presynaptic terminals. Proc. Natl. Acad. Sci. USA 106, 12611-12616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist S. E., Doe C. Q. (2006). Extrinsic cues orient the cell division axis in Drosophila embryonic neuroblasts. Development 133, 529-536 [DOI] [PubMed] [Google Scholar]
- Siller K. H., Doe C. Q. (2009). Spindle orientation during asymmetric cell division. Nat. Cell Biol. 11, 365-374 [DOI] [PubMed] [Google Scholar]
- Skoglund P., Rolo A., Chen X., Gumbiner B. M., Keller R. (2008). Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development 135, 2435-2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon J., Kaya-Copur A., Colombelli J., Brunner D. (2009). Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell 137, 1331-1342 [DOI] [PubMed] [Google Scholar]
- Song X., Zhu C. H., Doan C., Xie T. (2002). Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 296, 1855-1857 [DOI] [PubMed] [Google Scholar]
- St Johnston D., Nusslein-Volhard C. (1992). The origin of pattern and polarity in the Drosophila embryo. Cell 68, 201-219 [DOI] [PubMed] [Google Scholar]
- Stamenovic D., Ingber D. E. (2009). Tensegrity-guided self assembly: from molecules to living cells. Soft Matter 5, 1137-1145 [Google Scholar]
- Steinberg M. S. (1963). Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science 141, 401-408 [DOI] [PubMed] [Google Scholar]
- Steinberg M. S. (2007). Differential adhesion in morphogenesis: a modern view. Curr. Opin. Genet. Dev. 17, 281-286 [DOI] [PubMed] [Google Scholar]
- Stokes I. A., Mente P. L., Iatridis J. C., Farnum C. E., Aronsson D. D. (2002). Growth plate chondrocyte enlargement modulated by mechanical loading. Stud. Health Technol. Inform. 88, 378-381 [PubMed] [Google Scholar]
- Takeuchi M., Nakabayashi J., Sakaguchi T., Yamamoto T. S., Takahashi H., Takeda H., Ueno N. (2003). The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr. Biol. 13, 674-679 [DOI] [PubMed] [Google Scholar]
- Tamada M., Sheetz M. P., Sawada Y. (2004). Activation of a signaling cascade by cytoskeleton stretch. Dev. Cell 7, 709-718 [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Okada Y., Hirokawa N. (2005). FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature 435, 172-177 [DOI] [PubMed] [Google Scholar]
- Tanegashima K., Zhao H., Dawid I. B. R. (2008). WGEF activates Rho in the Wnt-PCP pathway and controls convergent extension in Xenopus gastrulation. EMBO J. 27, 606-617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G., Devenport D., Godt D., Brown N. H. (2007). Integrin-dependent anchoring of a stem-cell niche. Nat. Cell Biol. 9, 1413-1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q., Nandadasa S., McCrea P. D., Heasman J., Wylie C. (2007). G-protein-coupled signals control cortical actin assembly by controlling cadherin expression in the early Xenopus embryo. Development 134, 2651-2661 [DOI] [PubMed] [Google Scholar]
- Thery M., Racine V., Pepin A., Piel M., Chen Y., Sibarita J. B., Bornens M. (2005). The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 7, 947-953 [DOI] [PubMed] [Google Scholar]
- Thodeti C. K., Matthews B., Ravi A., Mammoto A., Ghosh K., Bracha A. L., Ingber D. E. (2009). TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ. Res. 104, 1123-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. A. (1917). On Growth and Form Cambridge, UK: Cambridge University Press; [Google Scholar]
- Torres-Padilla M. E., Parfitt D. E., Kouzarides T., Zernicka-Goetz M. (2007). Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature 445, 214-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama Y., Peralta X. G., Wells A. R., Kiehart D. P., Edwards G. S. (2008). Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science 321, 1683-1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterseher F., Hefele J. A., Giehl K., De Robertis E. M., Wedlich D., Schambony A. (2004). Paraxial protocadherin coordinates cell polarity during convergent extension via Rho A and JNK. EMBO J. 23, 3259-3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilyev A., Liu Y., Mudumana S., Mangos S., Lam P. Y., Majumdar A., Zhao J., Poon K. L., Kondrychyn I., Korzh V., et al. (2009). Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol. 7, e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronov D. A., Taber L. A. (2002). Cardiac looping in experimental conditions: effects of extraembryonic forces. Dev. Dyn. 224, 413-421 [DOI] [PubMed] [Google Scholar]
- Voronov D. A., Alford P. W., Xu G., Taber L. A. (2004). The role of mechanical forces in dextral rotation during cardiac looping in the chick embryo. Dev. Biol. 272, 339-350 [DOI] [PubMed] [Google Scholar]
- Walters J. W., Dilks S. A., DiNardo S. (2006). Planar polarization of the denticle field in the Drosophila embryo: roles for Myosin II (zipper) and fringe. Dev. Biol. 297, 323-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Naruse K., Stamenovic D., Fredberg J. J., Mijailovich S. M., Tolic-Norrelykke I. M., Polte T., Mannix R., Ingber D. E. (2001). Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl. Acad. Sci. USA 98, 7765-7770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Tytell J. D., Ingber D. E. (2009). Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10, 75-82 [DOI] [PubMed] [Google Scholar]
- Wei L., Imanaka-Yoshida K., Wang L., Zhan S., Schneider M. D., DeMayo F. J., Schwartz R. J. (2002). Inhibition of Rho family GTPases by Rho GDP dissociation inhibitor disrupts cardiac morphogenesis and inhibits cardiomyocyte proliferation. Development 129, 1705-1714 [DOI] [PubMed] [Google Scholar]
- Whitehead J., Vignjevic D., Futterer C., Beaurepaire E., Robine S., Farge E. (2008). Mechanical factors activate beta-catenin-dependent oncogene expression in APC mouse colon. HFSP J. 2, 286-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha M. S. (1984). Interaction of rat mammary epithelium with extracellular matrix components. Prog. Clin. Biol. Res. 145, 129-142 [PubMed] [Google Scholar]
- Wicha M. S., Lowrie G., Kohn E., Bagavandoss P., Mahn T. (1982). Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc. Natl. Acad. Sci. USA 79, 3213-3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N. R., Ty M. T., Ingber D. E., Sur M., Liu G. (2007). Synaptic reorganization in scaled networks of controlled size. J. Neurosci. 27, 13581-13589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P. E., Pesacreta T. C., Kiehart D. P. (1991). Dynamic changes in the distribution of cytoplasmic myosin during Drosophila embryogenesis. Development 111, 1-14 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Ragnauth C., Greener M. J., Shanahan C. M., Roberts R. G. (2002). The nesprins are giant actin-binding proteins, orthologous to Drosophila melanogaster muscle protein MSP-300. Genomics 80, 473-481 [PubMed] [Google Scholar]
- Zhong C., Chrzanowska-Wodnicka M., Brown J., Shaub A., Belkin A. M., Burridge K. (1998). Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell Biol. 141, 539-551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Kim H. Y., Davidson L. A. (2009). Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development 136, 677-688 [DOI] [PMC free article] [PubMed] [Google Scholar]