Abstract
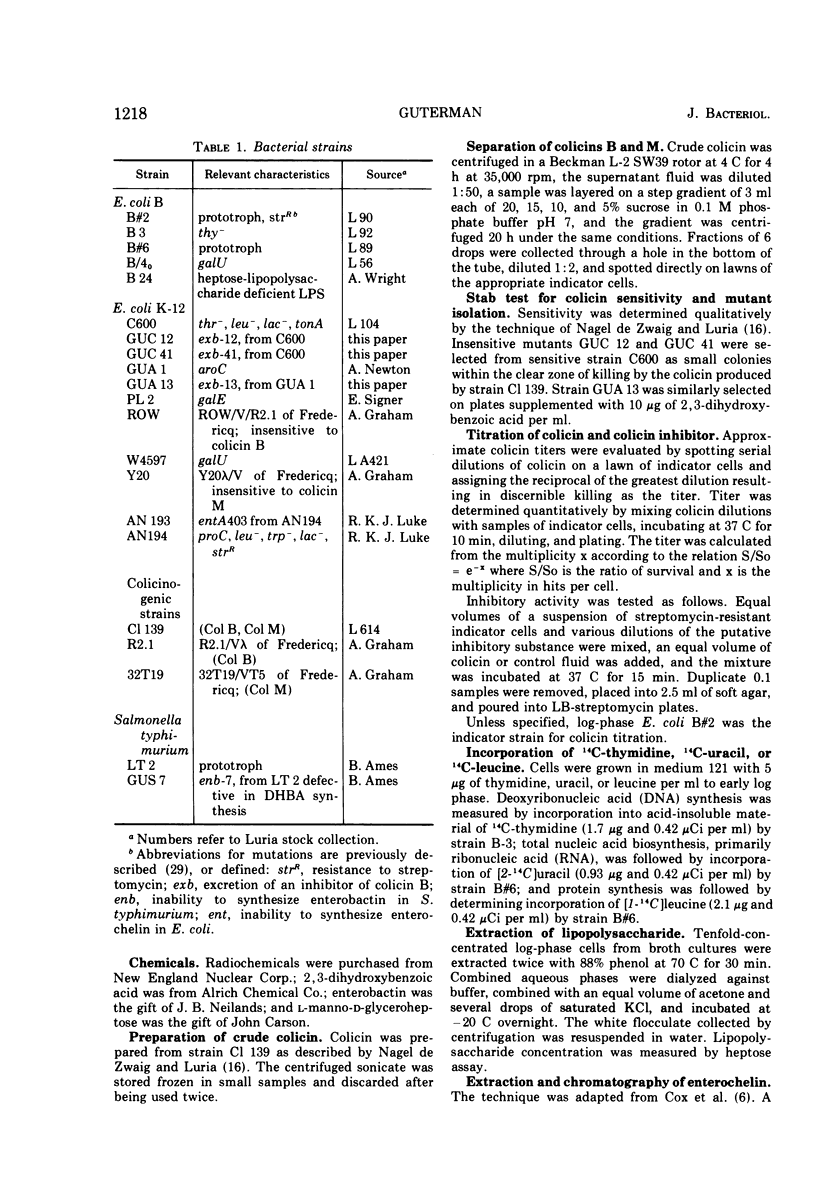
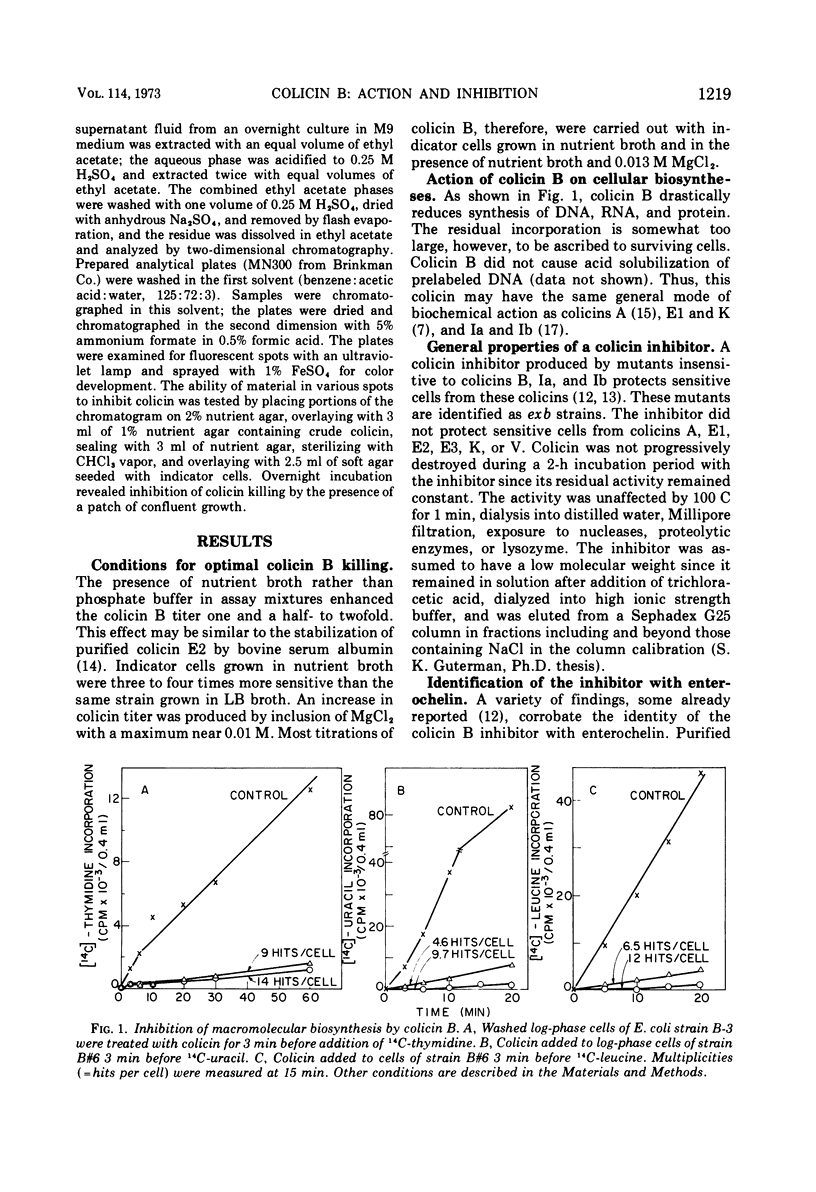
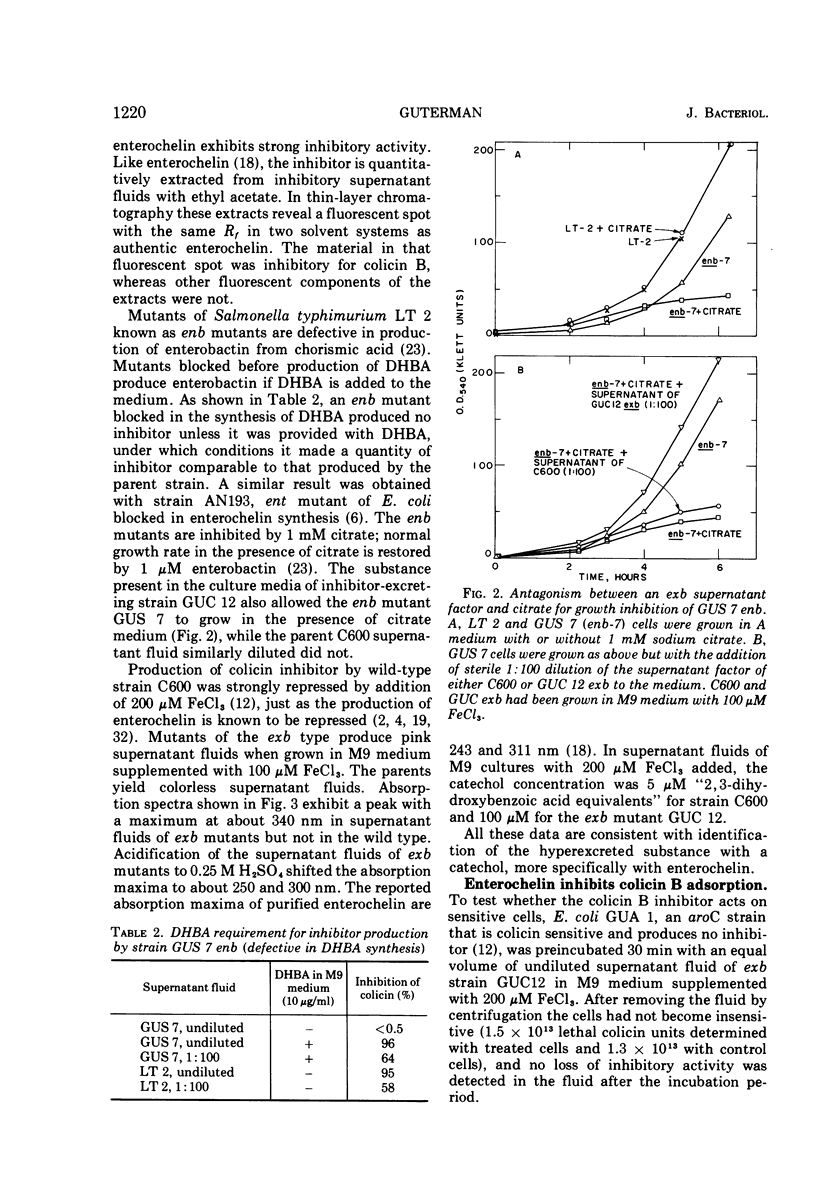
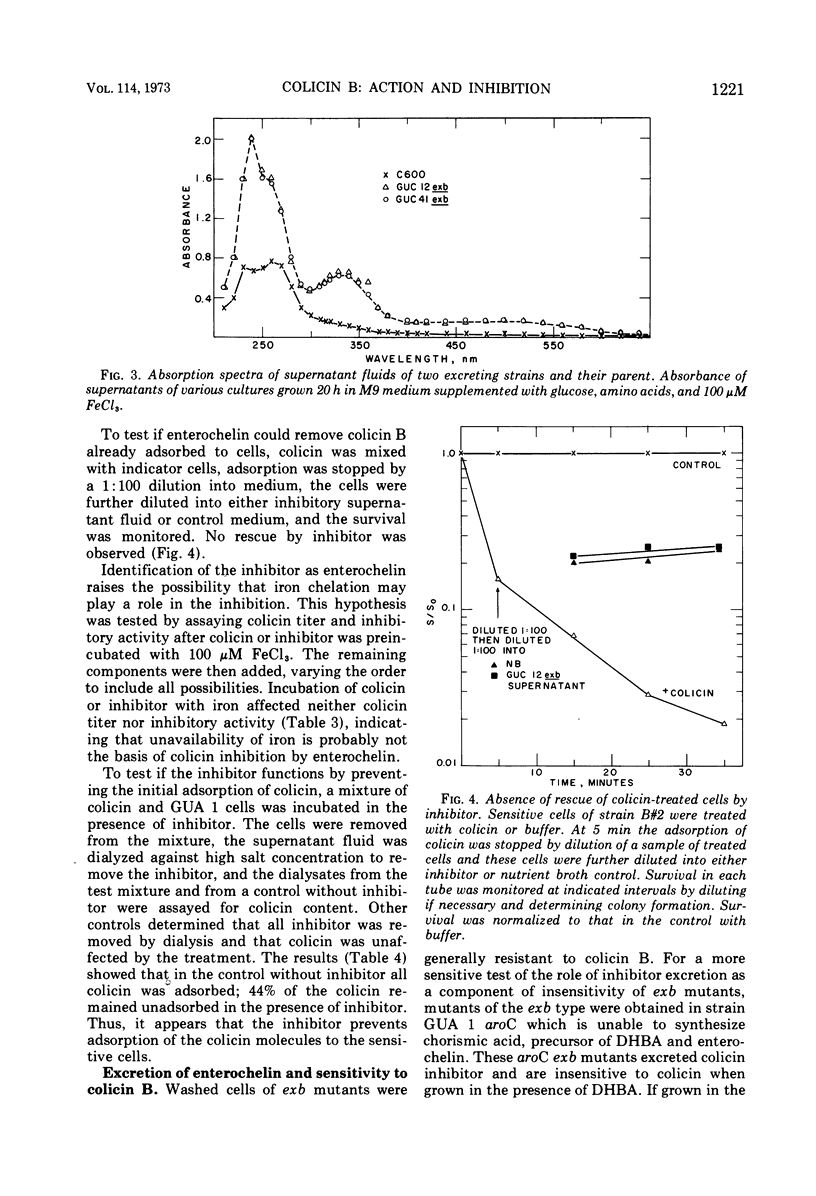
Adsorption of colicin B to a sensitive strain of Escherichia coli results in rapid cessation of deoxyribonucleic acid, ribonucleic acid, and protein synthesis. Some classes of mutants insensitive to colicin B hyperexcrete a colicin inhibitor into their growth medium. This inhibitor functions by preventing adsorption of colicin B and does not rescue cells to which colicin has already adsorbed. The inhibitor is insensitive to nucleases, proteolytic enzymes, and lysozyme and is not extracted into organic solvents. The inhibitory material has a low molecular weight, which rules out identification as lipopolysaccharide, although purified lipopolysaccharide has some inhibitory activity. Evidence is presented that the inhibitor is enterochelin, an iron chelator which is the cyclic trimer of 2,3-dihydroxybenzoylserine. Enterochelin does not inhibit colicin M, a colicin that is produced by many strains colicinogenic for colicin B.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot N., Goodwin J., Fales H. In vivo and in vitro formation of 2,3-dihydroxybenzoylserine by Escherichia coli K12. Biochem Biophys Res Commun. 1966 Nov 22;25(4):454–461. doi: 10.1016/0006-291x(66)90227-0. [DOI] [PubMed] [Google Scholar]
- Bryce G. F., Brot N. Iron transport in Escherichia coli and its relation to the repression of 2,3-dihydroxy-N-benzoyl-L-serine synthetase. Arch Biochem Biophys. 1971 Feb;142(2):399–406. doi: 10.1016/0003-9861(71)90503-0. [DOI] [PubMed] [Google Scholar]
- Chang Y. Y., Hager L. P. Inhibition of colicin e2 activity by bacterial lipopolysaccharide. J Bacteriol. 1970 Dec;104(3):1106–1109. doi: 10.1128/jb.104.3.1106-1109.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., Luke R. K., Newton N. A., O'Brien I. G., Rosenberg H. Mutations affecting iron transport in Escherichia coli. J Bacteriol. 1970 Oct;104(1):219–226. doi: 10.1128/jb.104.1.219-226.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDERICQ P. Colicins. Annu Rev Microbiol. 1957;11:7–22. doi: 10.1146/annurev.mi.11.100157.000255. [DOI] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on cellular metabolism. J Bacteriol. 1969 Jan;97(1):64–77. doi: 10.1128/jb.97.1.64-77.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. D., Shemin D. Concomitant synthesis of bacteriocin and bacteriocin inactivator from Serratia marcescens. J Bacteriol. 1969 Sep;99(3):661–666. doi: 10.1128/jb.99.3.661-666.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericq P., Smarda J. Complexité du facteur colicinogène B. Ann Inst Pasteur (Paris) 1970 Jun;118(6):767–774. [PubMed] [Google Scholar]
- GRATIA J. P. R'ESISTANCE 'A LA COLICINE B CHEZ E. COLI. RELATIONS DE SP'ECIFICIT'E ENTRE COLICINES B, I ET V ET PHAGE T-4. ETUDE G'EN'ETIQUE. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:151. [PubMed] [Google Scholar]
- Guterman S. K. Inhibition of colicin B by enterochelin. Biochem Biophys Res Commun. 1971 Sep;44(5):1149–1155. doi: 10.1016/s0006-291x(71)80206-1. [DOI] [PubMed] [Google Scholar]
- Guterman S. K., Luria S. E. Escherichia coli: strains that excrete an inhibitor of colicin B. Science. 1969 Jun 20;164(3886):1414–1414. doi: 10.1126/science.164.3886.1414. [DOI] [PubMed] [Google Scholar]
- Mitsui E., Mizuno D. Stabilization of colicin E2 by bovine serum albumin. J Bacteriol. 1969 Nov;100(2):1136–1137. doi: 10.1128/jb.100.2.1136-1137.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel de Zwaig R. Mode of action of colicin A. J Bacteriol. 1969 Sep;99(3):913–914. doi: 10.1128/jb.99.3.913-914.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- O'Brien I. G., Cox G. B., Gibson F. Biologically active compounds containing 2,3-dihydroxybenzoic acid and serine formed by Escherichia coli. Biochim Biophys Acta. 1970 Mar 24;201(3):453–460. doi: 10.1016/0304-4165(70)90165-0. [DOI] [PubMed] [Google Scholar]
- O'Brien I. G., Cox G. B., Gibson F. Enterochelin hydrolysis and iron metabolism in Escherichia coli. Biochim Biophys Acta. 1971 Jun 22;237(3):537–549. doi: 10.1016/0304-4165(71)90274-1. [DOI] [PubMed] [Google Scholar]
- O'Brien I. G., Gibson F. The structure of enterochelin and related 2,3-dihydroxy-N-benzoylserine conjugates from Escherichia coli. Biochim Biophys Acta. 1970 Aug 14;215(2):393–402. doi: 10.1016/0304-4165(70)90038-3. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi T., Yanagase Y., Higashi Y., Inoue K., Amano T. A specific inhibitor of megacin A from Bacillus megaterium producing megacin A. Biken J. 1970 Jun;13(2):63–76. [PubMed] [Google Scholar]
- Pollack J. R., Ames B. N., Neilands J. B. Iron transport in Salmonella typhimurium: mutants blocked in the biosynthesis of enterobactin. J Bacteriol. 1970 Nov;104(2):635–639. doi: 10.1128/jb.104.2.635-639.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. R., Neilands J. B. Enterobactin, an iron transport compound from Salmonella typhimurium. Biochem Biophys Res Commun. 1970 Mar 12;38(5):989–992. doi: 10.1016/0006-291x(70)90819-3. [DOI] [PubMed] [Google Scholar]
- REEVES P. THE BACTERIOCINS. Bacteriol Rev. 1965 Mar;29:24–45. doi: 10.1128/br.29.1.24-45.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Romeo D. Role of lipids in the biosynthesis of the bacterial cell envelope. Bacteriol Rev. 1971 Mar;35(1):14–38. doi: 10.1128/br.35.1.14-38.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabet S. F., Schnaitman C. A. Localization and solubilization of colicin receptors. J Bacteriol. 1971 Oct;108(1):422–430. doi: 10.1128/jb.108.1.422-430.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Newton A. Iron transport in Escherichia coli: roles of energy-dependent uptake and 2,3-dihydroxybenzoylserine. J Bacteriol. 1969 Jun;98(3):1142–1150. doi: 10.1128/jb.98.3.1142-1150.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzien H. U., Jesaitis M. A. The nature of the cilicin K receptor of Escherichia coli Cullen. J Exp Med. 1971 Mar 1;133(3):534–553. doi: 10.1084/jem.133.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G., Gibson F. Regulation of the enzymes involved in the biosynthesis of 2,3-dihydroxybenzoic acid in Aerobacter aerogenes and Escherichia coli. Biochim Biophys Acta. 1969 May 6;177(3):401–411. doi: 10.1016/0304-4165(69)90302-x. [DOI] [PubMed] [Google Scholar]



