Abstract
The existence of multiple subtypes of HIV-1 worldwide has created new challenges to control HIV-1 infection and associated neuropathogenesis. Previous studies indicate a difference in neuropathogenic manifestations of HIV-1-associated neuroAIDS between clade B- and clade C-infected subjects with clade B being more neuropathogenic than clade C. However, the exact mechanism underlying the differences in the neuropathogenesis by both the subtypes remains elusive. Development of neuroAIDS is associated with a complex interplay between proinflammatory and antiinflammatory cytokines and chemokines. In the current study, we hypothesize that HIV-1 clade B and C Tat protein exert differential effects on human primary monocytes leading to differences in gene and protein expression of cytokines implicated in neuroAIDS. Primary human monocytes were treated with clade B and clade C Tat protein and quantitative real time PCR was performed to determine gene expression of proinflammatory cytokines (IL-6 and TNF-α) and antiinflammatory cytokines (IL-4 and IL-10). Further, cytokine secretion was measured in culture supernatants by ELISA, whereas intracellular cytokine expression was detected by flow cytometry. Results indicate that monocytes treated with Tat B showed significant upregulation of proinflammatory cytokines, IL-6 and TNF-α, as compared to Tat C-treated cultures. However, expression of antiinflammatory molecules and IL-4 and IL-10 was found to be higher in Tat C-treated compared to Tat B-treated cultures. Thus, our result shows for the first time that Tat B and Tat C differentially modulate expression of neuropathogenic molecules that may be correlated with the differences in neuroAIDS manifestation induced by clade-specific infections.
Introduction
Human immunodeficiency virus type-1 (HIV-1) displays extraordinary genetic variation leading phylogenetically to three distinct groups and several subgroups (A–K) across the globe. The predominant subtype found in the United States and in the western world is clade B, which differs significantly from subtype C, which exists in sub-Saharan Africa and Asia, including India and China. Estimates suggest that of a total of about 46 million people infected with HIV-1, more than 56% of the infection is with clade C alone.1–4 Moreover, HIV-1C infection is rapidly spreading to Europe and there is a good possibility that HIV-1C infection will be affecting the United States in the future.
HIV-1 infection eventually progresses to severe deficiency of various immunological functions and neurological abnormalities, especially during the later stage of the disease. The prevalence of neurological abnormalities among HIV-1B-infected subjects is estimated to be around 15–30% in the United States and Europe.5–7 Our current understanding of the pathophysiology and neuropathology of neuroAIDS emanates mainly from clade B, whereas very little information is available on the neuropathogenesis of the clade C subtype. Previous studies have shown that clade C envelop glycoproteins are significantly different from those of clade B and the incidence of neuroAIDS in HIV-1 clade C-infected subjects is reported to be very low (∼1–2%).8–10 In contrast, Gupta and co-workers11 have recently reported mild to moderate cognitive deficits in clade C-infected antiretroviral treatment-naive subjects and found that it was similar to clade B-infected subjects.
HIV-1 infection and virus replication are regulated by a complex interplay of cytokines secreted by a variety of cells. Some cytokines may be inhibitory [interferons, granulocyte-monocyte colony-stimulating factor (GM-CSF), interleukin (IL)-10, IL-13, IL-16, and β-chemokines] or stimulatory [IL-1, IL-6, tumor necrosis factor (TNF)-α, TNF-β, M-CSF] or bifunctional (IL-4) to HIV-1 replication.12 IL-6 and TNF-α are proinflammatory cytokines, which are known to significantly upregulate HIV-1 production in monocytes-derived macrophages (MDM) through the activation of NF-κB signaling.13 Activated macrophages are known to transmigrate across the blood–brain barrier (BBB) and exert neurotoxicity through secretion of several cytokines and chemokines. On the other hand, IL-10 exerts antiinflammatory activity by inhibiting macrophage activation and secretion of proinflammatory cytokines (IL-1, IL-6, IL-8, IL-12, TNF-α).12 In contrast, IL-4 exerts both stimulatory and inhibitory effects on HIV-1 replication depending on the stage of maturation of monocytes into macrophages.12
Since our initial report14 others have also demonstrated significant immunoregulatory activity and neurotoxic effects of HIV gene products: Tat and gp120.15–19 Tat is known to play a significant role in the neuropathogenesis of HIV infection, which includes direct neurotoxicity and modulation of cytokine and chemokine production by immune cells.20,21 In the current study, we hypothesized that HIV-1 clade B and C Tat proteins exert differential effects on primary human monocytes leading to differential gene expression and production of proinflammatory and antiinflammatory cytokines associated with HIV neuropathogenesis. We report that primary monocytes treated with clade-specific neurotoxic protein Tat in identical culture conditions differentially modulate the expression of neuropathogenic molecules IL-6, TNF-α, IL-4, and IL-10.
Materials And Methods
HIV-1 Tat recombinant proteins
HIV-1 clade B recombinant Tat protein was obtained from the NIH AIDS research and reference reagent program and HIV-1 clade C recombinant Tat protein was commercially purchased from Diatheva (Fano, Italy).
Preparation of human monocytes
Normal peripheral blood monocytes were isolated by a density gradient centrifugation process from HIV-1, HIV-2, and hepatitis B-seronegative donor leukopacks as described earlier.22 Briefly, leukopack was diluted by adding five volumes of phosphate-buffered saline (PBS) and overlayed over Ficoll-Hypaque (1.077 g/ml, H889, Sigma Aldrich, St. Louis, MO). The samples were centrifuged at 1200 × g for 20 min at room temperature. Peripheral blood mononuclear cells (PBMCs) were carefully retrieved from the interface and washed twice with PBS. PBMCs were subjected to plastic adherence at 37°C for 1 h. The nonadherent cells were removed and adherent monocytes were cultured in RPMI 1640 culture medium supplemented with 10% fetal bovine serum (FBS), penicillin 100 U/ml and streptomycin 100 mg/ml, and 2 mM l-glutamine (Gibco-BRL, Gaithersburg, MD). The purity of isolated monocytes was assessed by flow cytometry using a fluorochrome-conjugated CD14 antibody and the purity was found to be >90%.
HIV-1 Tat treatment of monocytes
Monocytes were treated with Tat B and Tat C proteins to perform gene expression studies. For time kinetics experiments, monocytes were treated with 100 ng/ml Tat protein for different time intervals, 2, 4, 8, and 24 h. Based on the time kinetics data, dose–response experiments were set up at 4 h with 25, 50, 100, and 200 ng/ml of Tat protein. Heat-inactivated clade B and C Tat protein served as controls.
Quantitative real time polymerase chain reaction (qRT-PCR)
RNA from cell pellets was extracted using the RNAeasy mini kit (Qiagen, GmbH, Germany) followed by cDNA synthesis using the high capacity reverse transcriptase cDNA kit (Applied Biosystems, Foster City, CA) to perform qRT-PCR using Taqman gene expression assays (Applied Biosystems, Foster City, CA) for IL-6 (Assay ID Hs00174131_m1), TNF-α (Assay ID, Hs00174128_m1), IL-4 (Assay ID, Hs00174122_m1), IL-10 (Assay ID, Hs00174086_m1), and β-actin (Assay ID, Hs99999903_m1). β-Actin served as an internal control. The relative abundance of each mRNA species was assessed using SYBR green master mix from Stratagene using an Mx3000P instrument that detects and plots the increase in fluorescence versus PCR cycle number to produce a continuous measure of PCR amplification. To provide precise quantification of initial target in each PCR reaction, the amplification plot is examined at a point during the early log phase of product accumulation. This is accomplished by assigning a fluorescence threshold above background and determining the time point at which each sample's amplification plot reaches threshold (defined as the threshold cycle number or CT). Differences in threshold cycle number are used to identify the relative amount of PCR target contained within each tube.23 Relative mRNA species expression was quantitated and expressed as the transcript accumulation index (TAI = 2−ΔΔCT), calculated using the comparative CT method.24
Enzyme- linked immunosorbent assay
Monocytes were treated with clade B and C Tat proteins at concentration 100 ng/ml for 8 h. Cell culture supernatants were analyzed for protein levels of IL-6, TNF-α, and IL-10 using commercially available ELISA kits as per the manufacturer's instructions (Biolegend, San Diego, CA). The minimum assay detection limit for IL-6 is 4 pg/ml, whereas for TNF-α and IL-10 it is 2 pg/ml as provided by the manufacturer (Biolegend, San Diego, CA).
Flow cytometry analysis
Flow cytometry using a FACS Calibur instrument (BD Biosciences, San Jose, CA) was used to identify and quantify proinflammatory cytokine IL-6 and antiinflammatory cytokine IL-4 expression by monocytes treated with 10 ng/ml of Tat B and Tat C for 6 h. Fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (mAb) against IL-6 and IL-4 and an appropriately matched isotype control were obtained from Biolegend (San Diego, CA). Stained cells were subject to light scatter analysis and a fixed population of cells was gated after quadrant markers were set based on the isotype control. Cells positive for specific marker were expressed as a percentage of the total cells gated.
Statistics
Experiments were performed at least three times and the values obtained were averaged. Data are represented as mean ± SE. Comparisons between two groups were conducted using Student's paired t-test. Differences were considered significant at p ≤ 0.05, with a two-tailed test. Data analysis was performed with the Statistical Program, Graphpad prism software (La Jolla, CA).
Results
Tat B and C differentially modulate cytokines by primary monocytes
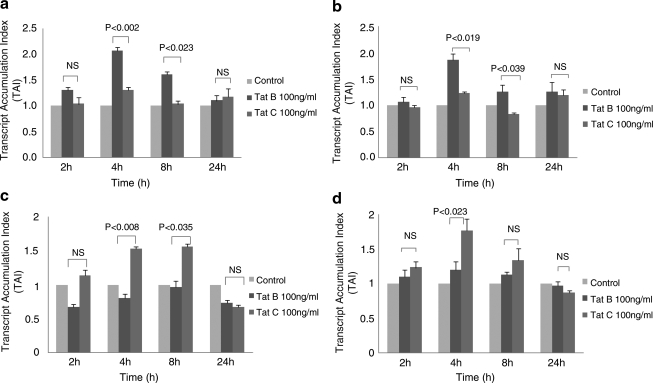
Since clade B- and clade C-infected subjects show differential neuropathogenic manifestations,8,11 we evaluated whether HIV-1 clade B and C Tat differentially modulate proinflammatory and antiinflammatory cytokine gene expression by primary monocytes. Data presented in Fig. 1 show the time kinetics of Tat B and Tat C effects on IL-6, TNF-α, IL-4, and IL-10 gene expression by monocytes at 2, 4, 8, and 24 h. Primary monocytes cultured with 100 ng/ml of Tat B demonstrated a significant increase in IL-6 gene expression at 4 h (TAI = 2.1 ± 0.07, p < 0.002) and 8 h (TAI = 1.6 ± 0.06, p < 0.023) compared to Tat C-treated cultures at 4 h (TAI = 1.3 ± 0.06) and 8 h (TAI = 1.0 ± 0.07) (Fig. 1a). Similarly, Tat B showed significant upregulation of TNF-α gene expression at 4 h (TAI = 1.9 ± 0.12, p < 0.019) and 8 h (TAI =1.3 ± 0.12, p < 0.039) compared to Tat C at 4 h (TAI = 1.2 ±0.03) and 8 h (TAI = 0.8 ± 0.03) (Fig. 1b). On the other hand, antiinflammatory cytokine IL-4 gene expression was found to be higher in Tat C-treated cultures at 4 h (TAI = 1.5 ± 0.03, p < 0.008) and 8 h (TAI = 1.6 ± 0.04, p < 0.035) compared to Tat B-treated cells at 4 h (TAI = 0.8 ± 0.06) and 8 h (TAI =1.0 ± 0.09) (Fig. 1c). Similarly, Tat C showed significant upregulation of another antiinflammatory cytokine, IL-10 (TAI = 1.8 ± 0.17, p < 0.023), compared to Tat B-treated monocytes (TAI = 1.2 ± 0.12) at 4 h (Fig. 1d).
FIG. 1.
Primary human monocytes (1 × 106 cells/ml) isolated from normal subjects were incubated with Tat B and Tat C (100 ng/ml) for 2, 4, 8, and 24 h and RNA was extracted and reverse transcribed followed by quantitative real time PCR for IL-6 (a), TNF-α (b), IL-4 (c), and IL-10 (d) genes. Relative expression of mRNA species was calculated using the comparative CT method. Data are means ± SE of three independent experiments. Statistical significance was calculated by Student's t test.
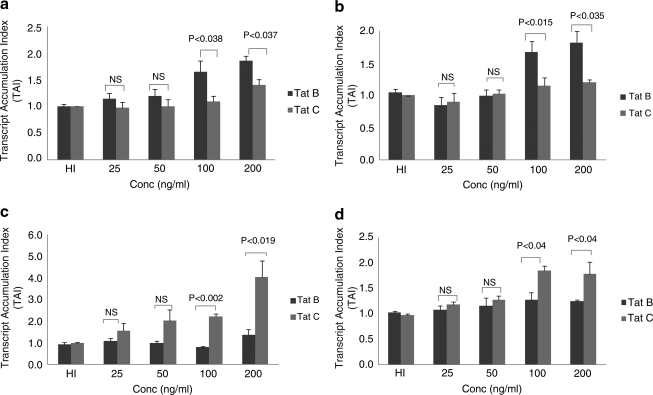
Based on the time kinetics results as presented in Fig. 1, a 4 h time point was selected to perform dose–response studies. Data presented in Fig. 2 show the dose–response effects of Tat B and Tat C on proinflammatory and antiinflammatory cytokine gene expression by primary monocytes. Results indicate that Tat B treatment showed a significant increase in IL-6 at 100 ng/ml (TAI = 1.7 ± 0.21, p < 0.038) and 200 ng/ml (TAI 1.9 ± 0.1, p < 0.037) compared to Tat C-treated cultures at similar concentrations (100 ng/ml, TAI = 1.1 ± 0.1; 200 ng/ml, TAI = 1.4 ± 0.12) (Fig. 2a). Similarly, Tat B showed significant upregulation of TNF-α gene expression at 100 ng/ml (TAI = 1.7 ± 0.17, p < 0.015) and 200 ng/ml (TAI = 1.8 ± 0.17, p < 0.035) compared to Tat C-treated cultures (100 ng/ml, TAI = 1.2 ± 0.13; 200 ng/ml, TAI = 1.2 ± 0.04) (Fig. 2b). In the case of the antiinflammatory cytokine, IL-4, Tat C produced significant upregulation at 100 ng/ml (TAI = 2.2 ± 0.12, p < 0.002) and 200 ng/ml (TAI = 4.0 ± 0.75, p < 0.019) compared to Tat B-treated cultures (100 ng/ml, TAI = 0.8 ± 0.05; 200 ng/ml, TAI = 1.4 ± 0.26) in a dose-dependent manner (Fig. 2c). Similarly, Tat C showed significant upregulation of another antiinflammatory cytokine IL-10 at 100 ng/ml (TAI = 1.9 ± 0.1, p < 0.04) and 200 ng/ml (TAI = 1.8 ± 0.24, p < 0.04) compared to Tat B-treated cultures at similar concentrations (100 ng/ml, TAI = 1.3 ± 0.14; 200 ng/ml, TAI =1.3 ± 0.03) (Fig. 2d).
FIG. 2.
Primary human monocytes (1 × 106 cells/ml) isolated from normal subjects were incubated with Tat B and Tat C (25, 50, 100, and 200 ng/ml) for 4 h; heat-inactivated (HI) Tat B and Tat C served as controls. RNA was extracted and reverse transcribed followed by quantitative real time PCR for IL-6 (a), TNF-α (b), IL-4 (c), and IL-10 (d) genes. Relative expression of mRNA species was calculated using the comparative CT method. Data are means ± SE of three independent experiments. Statistical significance was calculated by Student's t test.
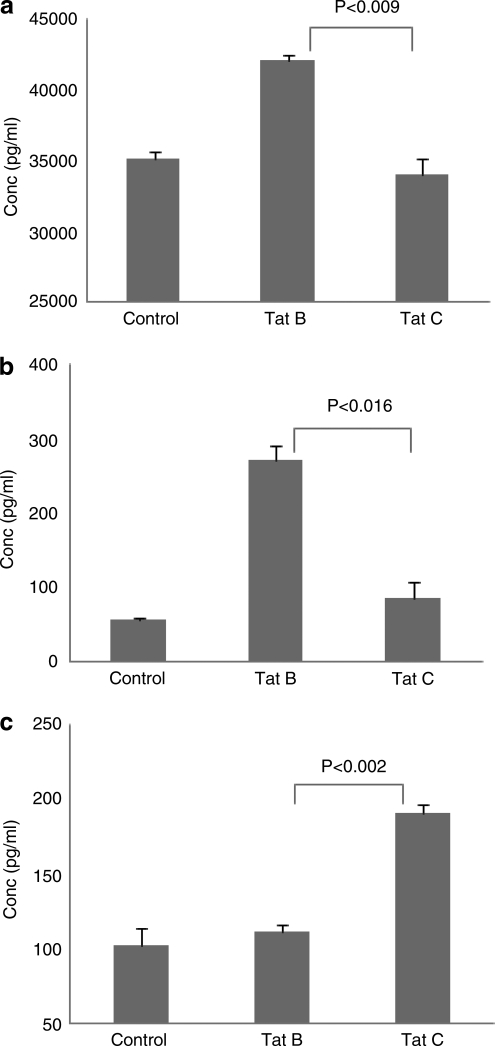
Data presented in Fig. 3 show the effect of Tat B and Tat C on IL-6, TNF-α, and IL-10 protein secretion as determined by ELISA. The results obtained indicate that cell culture supernatants from monocytes cultured with Tat B showed significantly higher levels of IL-6 (42,092 ± 446 pg/ml, p < 0.009) compared to Tat C (34,048 ± 1087 pg/ml) (Fig. 3a). Similarly, culture supernatants from Tat B also produced significant upregulation of TNF-α (271.4 ± 21 pg/ml, p < 0.016) compared to Tat C (87.4 ± 19.4 pg/ml) (Fig. 3b). Furthermore, a supernatant of monocytes treated with Tat C showed higher level of an antiinflammatory cytokine, IL-10 (190.9 ± 5 pg/ml, p < 0.002), compared to a supernatant from Tat B-treated cultures (112 ± 4.0 pg/ml) (Fig. 3c).
FIG. 3.
Primary human monocytes (3 × 106 cells) were incubated with Tat B and Tat C (100 ng/ml) separately for 8 h. After incubation, supernatants were collected and analyzed for IL-6 (a), TNF-α (b), and IL-10 (c) protein levels by ELISA. The data represent the means ± SE of three independent experiments. Statistical significance was calculated by Student's t test.
Tat B and Tat C differentially regulate intracellular cytokine expression by primary monocytes
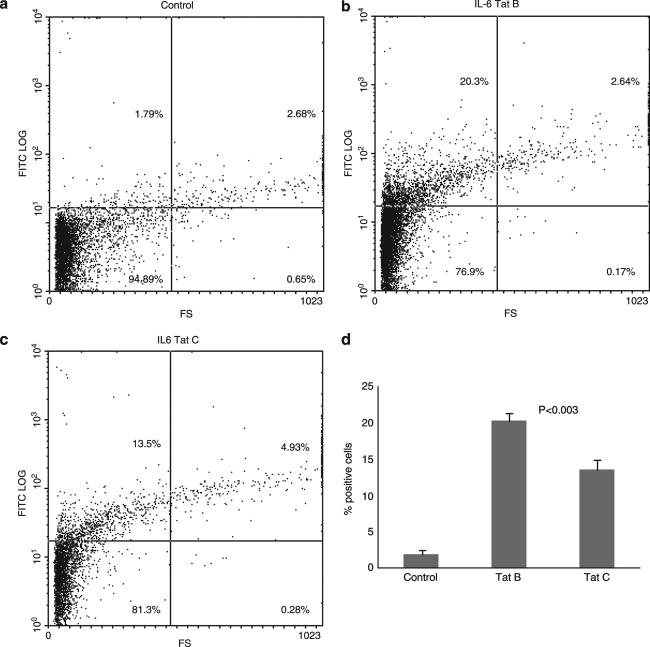
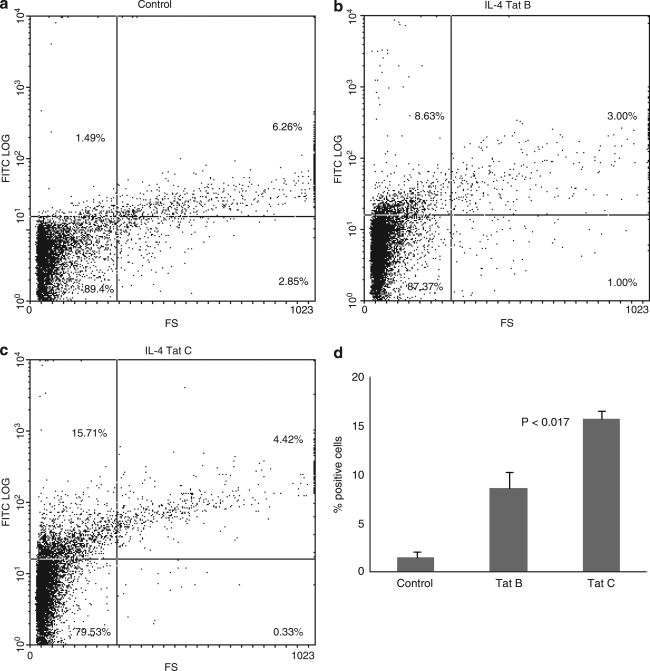
In addition to ELISA analysis for protein quantification, flow cytometry analysis using specific antibodies was performed to quantitate IL-6 and IL-4 intracellular protein expression by monocytes treated with Tat B and Tat C. Primary monocytes were incubated in the presence of either Tat B or Tat C proteins at concentration of 100 ng/ml for 6 h and then analyzed by flow cytometry. The results are expressed as the percentage of positive cells. The exposure of primary monocytes to Tat B produced significantly higher levels of IL-6 (20.3 ± 1.1%, p < 0.003) compared to Tat C-treated cultures (13.5 ± 1.45%) (Fig. 4). However, Tat C-treated monocytes resulted in significantly higher levels of IL-4-positive cells (15.7 ± 0.75%, p < 0.017) than Tat B-treated cultures (8.63 ±1.57%) (Fig. 5).
FIG. 4.
Tat protein modulates IL-6 expression by FACS analysis. Primary monocytes (1 × 106) were cultured with 100 ng/ml of Tat B and Tat C separately for 6 h in the presence of brefeldin A, 10 μg/ml. The number of IL-6 expressing cells was quantitated using FACS analysis. Stained cells were subjected to light scatter analysis and a fixed population of cells was gated after quadrant markers were set, based on isotype control. Cells positive for IL-6 were expressed as percentage of cells gated. Dot plot graphs of control (a), Tat B-treated (b), and Tat C-treated (c) cultures from a representative experiment are shown. Data presented in (d) show the bar graph representing mean values of three separate experiments performed.
FIG. 5.
Tat protein modulates IL-4 expression by FACS analysis. Primary monocytes (1 × 106) were cultured with 100 ng/ml of Tat B and Tat C separately for 6 h in the presence of brefeldin A, 10μg/ml. The number of IL-4 expressing cells was quantitated using FACS analysis. Stained cells were subjected to light scatter analysis and a fixed population of cells was gated after quadrant markers were set, based on isotype control. Cells positive for IL-4 were expressed as percentage of cells gated. Dot plot graphs of control (a), Tat B-treated (b), and Tat C-treated (c) cultures from a representative experiment are shown. Data presented in (d) show the bar graph representing mean values of three separate experiments performed.
Discussion
Neurological abnormalities in HIV-1-infected subjects are augmented by viral replication in mononuclear phagocytes (MP) that migrate across the BBB. These immune activated cells secrete or induce other cells to produce neurotoxic factors that lead to neuronal injury and cell death.25,26 Previous studies showed clade-specific differences in neuropathogenicity exist among clade B and clade C HIV-infected individuals. HIV-1 clade C-infected subjects show decreased or less severe neurocognitive impairments.9–11 Earlier reports show the effect of various HIV-1 clade B-derived viral proteins on the production of various proinflammatory and antiinflammatory cytokines, which are known to be involved in the neuropathogenesis of HIV-1B infections. However, no studies have been reported on the effects of HIV-1C proteins for their regulatory effects on proinflammatory and antiinflammatory cytokines in comparison with HIV-1B proteins.
Recent studies have demonstrated that the mutation of a cysteine to a serine at residue 31 of a Tat protein commonly found in subtype C variants significantly inhibits the monocytes chemotaxis by impeding Tat binding to chemokine receptor 2 (CCR2), which prevents induction of intracellular calcium flux, TNF-α, and CCL2 production.27 Furthermore, Rao and co-workers8 have investigated differential neuropathogenesis induced by HIV-1 clades in a SCID HIV-E mouse model and attributed the genotypic differences between virus subtypes to disease severity and the differences in neuropathogenesis. Results reported here demonstrate that clade B and C exert differential effects on primary monocytes leading to differential gene expression and production of proinflammatory and antiinflammatory cytokines associated with neuropathogenesis. Our studies showed that Tat B was more potent than Tat C in stimulating gene expression of proinflammatory cytokines TNF-α and IL-6 in a time- and dose-dependent manner by primary human monocyte cultures (Figs. 1 and 2). Additionally, maximum response for Tat treatment was found at 100 and 200 ng/ml, and these concentrations have been reported to be biologically active in previous studies.20,28,29 Earlier studies have reported that HIV-1B-infected individuals showed increased expression of TNF-α, which suggests a vital role of this cytokine in the development of neuroAIDS.27 Another important proinflammatory cytokine, IL-6, also has a wide spectrum of activities that includes B cell stimulation, monocyte differentiation, and induction of IL-4-producing cells.30 It also augments HIV-1 replication in MDMs as well as potentiates TNF-α-induced upregulation of HIV-1 production and transcription of NF-κB. Our results showed that clade B Tat significantly upregulates TNF-α and IL-6 gene expression by monocytes compared to HIV-1C Tat under identical experimental conditions, consistent with the reports that HIV-1B is more neuropathogenic. Conversely, antiinflammatory cytokine (IL-4 and IL-10) expression was upregulated by Tat C compared to Tat B. Previous studies31 suggest that IL-4 possesses a neuroprotective property by inhibiting inducible nitric oxide synthase (iNOS) in LPS-stimulated HIV-infected monocytes, which results in reduced NO synthesis and in turn reduced neuronal damage. It was proposed that failure of this neuroprotective mechanism is most likely associated with uncontrolled activation of macrophages resulting in development of neuroAIDS. In the current study, increased expression of IL-4 by monocytes with Tat C treatment may be possibly attributed to the neuroprotective effects exerted by IL-4 leading to reduced neuropathogenesis in HIV-1C-infected patients compared to HIV-1B patients.
IL-10, also called as the “cytokine synthesis inhibitory factor,” is known to inhibit inflammatory cytokine IL-1, TNF-α, and IL-6 production by activated macrophages and thereby prevents development of chronic inflammatory responses.32 Earlier studies have demonstrated higher IL-10 production in HIV-1-infected monocytic cells in vitro, which may be of significance because of its ability to inhibit HIV replication and limit viral entry by downregulating the expression of chemokine receptors on T cells.33 Thus, in the present study decreased expression of IL-6 and TNF-α by Tat C treatment could probably be associated with increased IL-10 production by Tat C.
Our results on gene expression data were further supported by protein expression studies in monocytes after Tat treatment by ELISA (Fig. 3) and FACS analysis (Figs. 4 and 5). Results were in agreement with qRT-PCR and showed modulation of proinflammatory and antiinflammatory cytokines in a similar manner, wherein Tat B upregulated proinflammatory cytokines, with a reciprocal upregulation of antiinflammatory cytokine expression by Tat C.
In summary, lower proinflammatory cytokine and higher antiinflammatory cytokine expression by Tat C compared to Tat B may be implicated in the lowering of neuropathogenesis in the case of clade C compared to clade B infections. Thus, these studies analyzing the differential effects of Tat B and Tat C open new avenues to design novel strategies to develop preventive and therapeutic global vaccines that can induce a cross-clade antiviral immune response against the multiclade or recombinant pandemic of HIV-1 infection that is currently facing the world, including the United States, where non-B subtypes have been recently reported in migrant populations in New York34 and among the military.35
Acknowledgments
This work was supported in part by National Institute on drug Abuse Grants RO1-DA012366, RO1-DA014218, RO1-DA015628, and RO1-DA021537.
Disclosure Statement
No competing financial interests exist.
References
- 1.Essex M. The human immunodeficiency virus (HIV) as a newly evolving pathogen. Southeast Asian J Trop Med Public Health. 1997;28(2):127–130. [PubMed] [Google Scholar]
- 2.Janssens W. Buve A. Nkengasong JN. The puzzle of HIV-1 subtypes in Africa. AIDS. 1997;11(6):705–712. doi: 10.1097/00002030-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Rodenburg CM. Li Y. Trask SA. Chen Y. Decker J, et al. Near full-length clones and reference sequences for subtype C isolates of HIV type 1 from three different continents. AIDS Res Hum Retroviruses. 2001;17(2):161–168. doi: 10.1089/08892220150217247. [DOI] [PubMed] [Google Scholar]
- 4.Geretti AM. HIV-1 subtypes: Epidemiology and significance for HIV management. Curr Opin Infect Dis. 2006;19:1–7. doi: 10.1097/01.qco.0000200293.45532.68. [DOI] [PubMed] [Google Scholar]
- 5.Heaton RK. Grant I. Butters N. White DA. Kirson D. Atkinson JH. McCutchan JA. Taylor MJ. Kelly MD. Ellis RJ, et al. The HNRC 500––neuropsychology of HIV infection at different disease stages. J Int Neuropsychol Soc. 1995;1(3):231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- 6.White DA. Heaton RK. Monsch AU. Neuropsychological studies of asymptomatic human immunodeficiency virus-type-1 infected individuals. J Int Neuropsychol Soc. 1995;1(3):304–315. doi: 10.1017/s1355617700000308. [DOI] [PubMed] [Google Scholar]
- 7.Wadia RS. Pujari SN. Kothari S. Udhar M. Kulkarni S. Bhagat S. Nanivadekar A. Neurological manifestations of HIV disease. J Assoc Physicians India. 2001;49:343–348. [PubMed] [Google Scholar]
- 8.Rao VR. Sas AR. Eugenin EA. Siddappa NB. Nelson HB. Berman JW. Ranga U. Tyor WR. Prasad VR. HIV-1 clade specific differences in the induction of neuropathogenesis. J Neurosci. 2008;28(40):10010–10016. doi: 10.1523/JNEUROSCI.2955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satishchandra P. Nalini A. Gourie-Devi M. Khanna N. Santosh V, et al. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989–1996) Indian J Med Res. 2000;111:14–23. [PubMed] [Google Scholar]
- 10.Riedel D. Ghate M. Nen M. Paranjape R. Mehendale S. Bollinger R. Sacktor N. McArthur J. Nath A. Screening of human immunodeficiency virus (HIV) dementia in HIV clade C-infected population in India. J Neurovirol. 2006;12:34–38. doi: 10.1080/13550280500516500. [DOI] [PubMed] [Google Scholar]
- 11.Gupta SK. Mather KJ. Agarwal R. Saha CK. Considine RV. Dube MP. Proteinuria and endothelial dysfunction in stable HIV-infected patients: A pilot study. J Acquir Immune Defic Syndr. 2007;45(5):596–598. doi: 10.1097/QAI.0b013e318061d2fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kedzierska K. Crowe SM. Turville S. Cunningham AL. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med Virol. 2003;13:39–56. doi: 10.1002/rmv.369. [DOI] [PubMed] [Google Scholar]
- 13.Herbein G. Gordon S. 55- and 75-kilodalton tumor necrosis factor receptors mediate distinct actions in regard to human immunodeficiency virus type 1 replication in primary human macrophages. J Virol. 1997;71:4150–4156. doi: 10.1128/jvi.71.5.4150-4156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair MP. Pottathil R. Heimer EP. Schwartz SA. Immunoregulatory activities of human immunodeficiency virus (HIV) proteins: Effect of HIV recombinant and synthetic peptides on immunoglobulin synthesis and proliferative responses by normal lymphocytes. Proc Natl Acad Sci USA. 1988;85(17):6498–6502. doi: 10.1073/pnas.85.17.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M. Singh MK. Balachandran R. Gupta P. Isolation and characterization of two divergent infectious molecular clones of HIV type 1 longitudinally obtained from a seropositive patient by a progressive amplification procedure. AIDS Res Hum Retroviruses. 1997;13(9):743–750. doi: 10.1089/aid.1997.13.743. [DOI] [PubMed] [Google Scholar]
- 16.Mayne M. Bratanich AC. Chen P. Rana F. Nath A. Power C. HIV-1 tat molecular diversity and induction of TNF-alpha: Implications for HIV-induced neurological disease. Neuroimmunomodulation. 1998;5(3–4):184–192. doi: 10.1159/000026336. [DOI] [PubMed] [Google Scholar]
- 17.Bolognesi DP. Perspectives on the development of anti-HIV vaccines. Curr Opin Biotechnol. 1991;2(6):893–896. doi: 10.1016/s0958-1669(05)80127-2. [DOI] [PubMed] [Google Scholar]
- 18.Robinson WE. Gorny MK. Xu JY. Mitchell WM. Zolla-Pazner S. Two immunodominant domains of gp41 bind antibodies which enhance human immunodeficiency virus type 1 infection in vitro. J Virol. 1991;65(8):4169–4176. doi: 10.1128/jvi.65.8.4169-4176.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyring SK. Cauda R. Tumbarello M. Ortona L. Kennedy RC. Chanh TC. Kanda P. Synthetic peptides corresponding to sequences in HIV envelope gp41 and gp120 enhance in vitro production of interleukin-1 and tumor necrosis factor but depress production of interferon-alpha, interferon-gamma and interleukin-2. Viral Immunol. 1991;4(1):33–42. doi: 10.1089/vim.1991.4.33. [DOI] [PubMed] [Google Scholar]
- 20.Nath A. Conant K. Chen P. Scott C. Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- 21.Huang L. Bosch I. Hofmann W. Sodroski J. Pardee AB. Tat protein induces human immunodeficiency virus type 1 (HIV-1) coreceptors and promotes infection with both macrophage- tropic and T-lymphotropic HIV-1 strains. J Virol. 1998;72:8952–8960. doi: 10.1128/jvi.72.11.8952-8960.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair MPN. Saiyed ZM. Nair N. Gandhi N. Rodriguez JW. Boukli N. Vasquez E. Malow RM. Burbano MJM. Methamphetamine enhances HIV-1 infectivity in monocyte derived dendritic cells. J Neuroimmune Pharmacol. 2009;4(1):129–139. doi: 10.1007/s11481-008-9128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shively L. Chang L. LeBon JM. Liu Q. Riggs AD. Singer-Sam J. Real-time PCR assay for quantitative mismatch detection. Biotechniques. 2003;34:498–502. doi: 10.2144/03343st01. [DOI] [PubMed] [Google Scholar]
- 24.Mahajan S. Schwartz S. Nair MP. Immunological assays for chemokine detection in in vitro culture of CNS cells. Biol Proced Online. 2003;5:90–102. doi: 10.1251/bpo50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Persidsky Y. Gendelman HE. Mononuclear phagocyte immunity and the europathogenesis of HIV-1 infection. J Leukoc Biol. 2003;74:691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- 26.Gendelman HE. Neural immunity: Friend or foe? J. Neurovirol. 2002;8:474–479. doi: 10.1080/13550280290168631. [DOI] [PubMed] [Google Scholar]
- 27.Campbell GR. Watkins JD. Singh KK. Loret EP. Spector SA. Human immunodeficiency virus type 1 subtype C Tat fails to induce intracellular calcium flux and induces reduced tumor necrosis factor production from monocytes. J Virol. 2007;81(11):5919–5928. doi: 10.1128/JVI.01938-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li JB. Lee DC. Cheung KW. Lau AS. Mechanisms of HIV Tat upregulation of IL-10 and other cytokine expression: Kinase signaling and PKR-mediated immune response. FEBS Lett. 2005;579:3055–3062. doi: 10.1016/j.febslet.2005.04.060. [DOI] [PubMed] [Google Scholar]
- 29.Siddappa NB. Venkatramanan M. Venkatesh P. Janki MV. Jayasuryan N. Desai A. Ravi V. Ranga U. Transactivation and signaling functions of Tat are not correlated: Biological and immunological characterization of HIV-1 subtype C Tat protein. Retrovirology. 2006;3:53. doi: 10.1186/1742-4690-3-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wesselingh SL. Tyor WR. Griffin DE. Cytokines, HIV associated dementia. In: Ransohoff RM, editor; Benveniste EN, editor. Cytokines and CNS. CRC Press; Boca Raton, FL: 1996. pp. 287–303. [Google Scholar]
- 31.Bukrinsky MI. Nottet HS. Schmidtmayerova H. Dubrovsky L. Flanagan CR. Mullins ME. Lipton SA. Gendelman HE. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: Implications for HIV-associated neurological disease. J Exp Med. 1995;181:735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breen EC. Pro- and anti-inflammatory cytokines in human immunodeficiency virus infection and acquired immunodeficiency syndrome. Pharmacol Ther. 2002;95:295–304. doi: 10.1016/s0163-7258(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 33.Kumar A. Angel JB. Daftarian MP. Parato K. Cameron WD. Filion L. Diaz-Mitoma F. Differential production of IL-10 by T cells and monocytes of HIV-infected individuals: Association of IL-10 production with CD28-mediated immune responsiveness. Clin Exp Immunol. 1998;114:78–86. doi: 10.1046/j.1365-2249.1998.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin HH. Gaschen BK. Collie M. El-Fishaway M. Chen Z. Korber BT. Beatrice ST. Zhang L. Genetic characterization of diverse HIV-1 strains in an immigrant population living in New York City. J Acquir Immune Defic Syndr. 2006;41(4):399–404. doi: 10.1097/01.qai.0000200663.47838.f1. [DOI] [PubMed] [Google Scholar]
- 35.Tovanabutra S. Brodine SK. Mascola JR. Sankale JL. Sanders-Buell E. Kim B. Birx DL. McCutchan FE. Characterization of complete HIV type 1 genomes from non-B subtype infections in U.S. military personnel. AIDS Res Hum Retroviruses. 2005;21(5):424–429. doi: 10.1089/aid.2005.21.424. [DOI] [PubMed] [Google Scholar]