Abstract
Histone deacetylases (HDACs) act on histones within the nucleosome-bound promoter of human immunodeficiency virus type 1 (HIV-1) to maintain proviral latency. HDAC inhibition leads to promoter expression and the escape of HIV from latency. We evaluated the ability of the potent inhibitor recently licensed for use in oncology, suberoylanilide hydroxamic acid (SAHA; Vorinostat), selective for Class I HDACs, to induce HIV promoter expression in cell lines and virus production from the resting CD4+ T cells of antiretroviral-treated, aviremic HIV-infected patients. In J89, a Jurkat T cell line infected with a single HIV genome encoding the enhanced green fluorescence protein (EGFP) within the HIV genome, SAHA induced changes at nucleosome 1 of the HIV promoter in chromatin immunoprecipitation (ChIP) assays in concert with EGFP expression. In the resting CD4+ T cells of antiretroviral-treated, aviremic HIV-infected patients clinically achievable exposures to SAHA induced virus outgrowth ex vivo. These results suggest that potent, selective HDAC inhibitors may allow improved targeting of persistent proviral HIV infection, and define parameters for in vivo studies using SAHA.
Introduction
Upon human immunodeficiency virus type 1 (HIV-1) infection, a latently infected pool of resting CD4+ T cells is established, making eradication of HIV infection impractical with current antiretroviral therapy (ART).1–3 Following integration of viral DNA into the cellular genome, the HIV long terminal repeat (LTR) promoter exists in a nucleosome-bound conformation and is transcriptionally silent without stimulation.4–6 Transcriptional activation of the HIV LTR involves complex interactions between HIV regulatory proteins and cellular transcription factors.
Eukaryotic DNA is condensed into chromatin of which the basic unit, the nucleosome, consists of DNA wrapped around an octamer of histone proteins. Post-translational modifications of histones are crucial for the regulation of gene expression. The amino acid tails of histones can be acetylated, sumoylated, and ubiquitylated on lysine residues, methylated on lysine or arginine residues, and phosphorylated on serine/threonine residues.7–14 These distinct modifications serve to recruit specific regulatory complexes to DNA, which in turn upregulate or downregulate gene expression.
Several studies provide evidence that histone deacetylases (HDAC) are critical regulators of HIV latency. Human transcription factors recruit the class I HDAC histone deacetylase 1 to the LTR. HDAC1 mediates chromatin remodeling resulting in repression of LTR promoter expression and viral production. The action of HDAC1 at the HIV-1 is required to maintain proviral quiescence as HDAC inhibition leads to LTR activation.15–18
HDAC1 can be recruited to the LTR by at least four mechanisms: by a complex containing the transcription factors YY1 and LSF, by a complex containing c-Myc and Sp1, by CBF-1, or by the NF-κB p50 homodimer.17–21 Disruption of HDAC1 recruitment to the LTR or inhibition of this enzyme with the weak HDAC inhibitor valproic acid (VPA) induces viral outgrowth from resting CD4+ T cell of aviremic HIV-infected individuals on ART.22–24
These observations led to the exploration of HDAC inhibitors as a potential therapeutic modality to disrupt latent HIV infection. The administration of valproic acid with intensified ART to aviremic, ART-treated HIV-infected individuals led to a depletion of resting cell infection (RCI) in three of four patients.25 However, further studies by our group and others26–28 found infrequent and modest depletion of RCI in patients on standard ART treated with valproic acid. As valproic acid is a weak and nonspecific HDAC inhibitor, we explored the potential of a more potent and selective HDAC inhibitor for use as a therapeutic tool to target persistent HIV infection.
Suberoylanilide hydroxamic acid (SAHA; vorinostat) is a member of the hydroxamic acid class of HDAC inhibitors.29 This potent HDAC inhibitor was recently approved by the FDA for the treatment of cutaneous T cell lymphoma.30,31 SAHA is selective for the Class 1 HDACs 1, 2, 3, and 8, has some activity against the Class II HDACs 6, 10, and 11, but no activity at clinically achievable concentrations against the Class II HDACs 4, 5, 7, and 9.
Class I HDACs regulate HIV LTR expression.17–21,32 Therefore SAHA may offer the possibility of modulating HIV gene expression, but with fewer effects on host genes than would be induced by global, nonselective HDAC inhibition. We evaluated the ability of SAHA to remodel chromatin about the HIV promoter, induce HIV promoter expression in cell lines, and allow virion recovery from the resting CD4+ T cells of aviremic HIV-infected individuals. Our findings provide a rationale for the study of the effect of SAHA in combination with ART on persistent HIV infection in aviremic HIV-infected patients.
Materials and Methods
Cell culture and chromatin immunoprecipitation (ChIP)
J89 cells33 and peripheral blood mononuclear cells (PBMCs) were cultured as described.27,34 Four million J89 cells were washed with phosphate-buffered saline (PBS) and incubated with 3 mM VPA (Sigma, St. Louis MO) or 500 nM SAHA (gift of Merck Research Laboratories, West Point, PA) or in media alone for 4 h. ChIP was performed as described34 with the following modifications. Sonicated cell lysates were centrifuged and 80–100 μg of soluble chromatin was incubated overnight with 5 μl of anti-acetyl histone 3 or anti-acetyl histone 4 (Ac-H3, Ac-H4, Milipore) or 5 μl of anti-HDAC-1 (Abcam, Cambridge, MA) or rabbit preimmune immunoglobulin G (Sigma). PCR was carried out with the following primers: LTR-109F (5′-TACAAGGGACTTTCCGCTGG-3′) and LTR+82R (5′-AGCTTTATTGAGGCTTAAGC-3′). A quantitative real-time PCR assay of the products of ChIP20 was performed to verify the significance of changes in the occupancy of HDAC1 and acetylated histones at the HIV-1 LTR region using the following primers: LTRrt9 forward (5′-AGCCCTCAGATGCTACATATAAGCA-3′) and LTRrt8 (5′-TAGCCAGAGAGCTCCCAGGCTCAGA-3′). The percent of input HIV LTR DNA was determined by comparing the cycle threshold values of each reaction to a standard curve generated from input DNA. Fold change in occupancy of acetylated histones and HDAC1 at the LTR relative to untreated control was calculated after subtracting background immunoprecipitation measured with nonspecific IgG.
LTR-driven GFP mRNA measurements
J89 cells were incubated with selected drug concentrations for 4–5 h. Cells were washed and snap frozen on an ethanol dry ice bath and stored at −80°C until use. Cells were thawed on ice and RNA was isolated using the RNeasy RNA isolation kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. cDNA was synthesized using Superscript II (Invitrogen). Semiquantitative PCR for EGFP was performed using a set of primers as previously reported.35 Endogenous GAPDH was measured on the same cDNA samples using the following primers: GAPDH-RT-FOR, 5′-CCATGGAGAAGGCTGGGG-3′ and GAPDH-RT-REV, 5′-CAAAGTTGTCATGGATGACC-3′. PCR products were resolved in an 8% agarose gel, visualized by ethidium bromide staining, and analyzed by GeneTools. Fold GFP enrichment was determined by normalizing to GAPDH and differences (n-fold) relative to untreated control were calculated.
To measure proliferation and viability of PBMCs and J89 cells in the presence of drugs, cells were subjected to an MTT assay using a cell proliferation kit following the manufacturer's instructions (Roche Applied Sciences, Indianapolis, IN). The percentage of cells proliferating was calculated from cells cultured in drug-free medium.
Limiting dilution cultures of latently infected CD4+ T cells from HIV-infected donors
Lymphocytes were obtained by continuous-flow leukopheresis. Isolation of resting CD4+ T cells and recovery and quantification of replication-competent virus were performed. Briefly, PBMCs were isolated from the apheresis product by Ficoll gradient and resting CD4+ T cells were purified by negative selection as previously described.23,25,27 Purity of isolated resting T cells was determined by flow cytometry; cells were routinely >98% CD4+, and less than 0.5% expressed activation markers.
Following purification, resting CD4+ T cells were incubated for 2 days with the HIV integrase inhibitor L-870812 and either efavirenz or abacavir as described.27 Cells were then plated in replicate dilutions of 2.5 million, 0.5 million, and 0.1 million to allow estimation of the frequency of RCI. Maximal mitogen stimulation has been widely used to quantitate the frequency of resting cell infection, and so cells were stimulated with 1 μg/ ml PHA-L (Remel), a 5-fold excess of allogeneic irradiated PBMCs from a seronegative donor, and 100 U/ml IL-2. To measure the frequency of outgrowth after HDAC inhibition, cells were exposed to either 40 μM VPA or 250–335 nM SAHA. The concentration of SAHA and VPA was selected after measuring free drug concentration in tissue culture conditions (data not shown) choosing concentrations comparable to free drug concentrations achievable in patients.
Further, to ensure that outgrowth was not attributed to rare nonresting infected cells that might exist within such a large number of cells, cells were cultured in parallel in the presence of 20 U/ml of IL-2. If the frequency of outgrowth in the presence of this “survival” concentration of IL-2 was within 0.3 log10 of the frequency of outgrowth in the presence of VPA or SAHA, outgrowth in the presence of HDAC inhibitor was considered nonspecific and nonsignificant. This occurred only rarely, and only in patients in which the frequency of resting cell infection was very low. As previously,27 HIV seronegative PBMCs screened for adequate CCR5 expression following PHA stimulation were collected by leukopheresis and stored in aliquots, and assays were performed with matched patient sample-donor aliquot pairs to reduce interassay variation. A maximum likelihood method was used to calculate the infectious units per billion of resting CD4+ T cells after exposure to PHA or SAHA.36
Results
SAHA induces chromatin changes at the HIV LTR leading to LTR expression
As HIV integrants are too rare in patients' cells to study chromatin, we studied modification induced by SAHA at the LTR in J89 cells, a latently infected Jurkat T cell line with a complete integrated proviral genome.33 The viral genome also encodes EGFP as a marker for HIV-LTR expression. We compared the ability of SAHA and VPA to induce acetylation of Nuc 1 and to alter HDAC1 recruitment to the Nuc-1 region as measured by ChIP. Cells were assayed after only 4 h of treatment to avoid assaying effects due to host cell cycling or secondary changes in host gene expression.
J89 cells were harvested for ChIP after 4 h in media, 3 mM VPA, or 500 nM to 1 μM SAHA and DNA products were quantitated by real-time PCR. A representative assay is shown in Fig. 1. Both SAHA and VPA induced an increase in acetylation of histone 3 at the HIV-LTR Nuc-1 (10.1-fold for SAHA). Similarly, occupancy of HDAC1 was decreased (31.3-fold for SAHA) as we have previously described after VPA exposure.18 Similar results were obtained when the acetylation of histone 4 was measured (data not shown).
FIG. 1.
Like VPA, SAHA increases acetylation of Nuc 1 and decreases HDAC-1 occupancy at the HIV-1 LTR. J89 cell treated with media, 3 mM VPA, or 500 nM SAHA for 4 h were assayed by chromatin immunoprecipitation with anti-acetylated H3, anti-HDAC-1. To demonstrate that the apparent quantitative changes are significant in this semi-quantitative assay, DNA products of ChIP were quantitated in triplicate by real-time PCR. Assays are representative of 3 independent experiments, and real-time quantitation of the fold change relative to untreated control is shown.
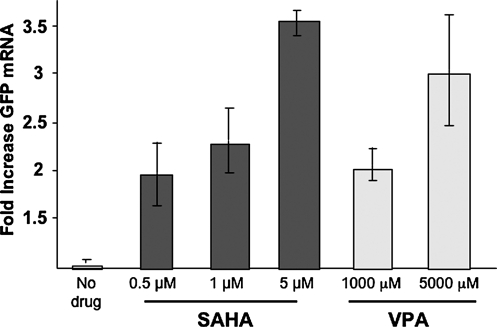
We then compared LTR-driven GFP mRNA expression in J89 cells treated with SAHA and VPA. Again cells were assayed after only 4 h of treatment. Total RNA was extracted and GFP RNA expression was measured by PCR. As shown in Fig. 2, after only 4 h of exposure, both SAHA and VPA induced LTR-driven GFP mRNA expression. Consistent with the greater potency of SAHA, GFP was induced by nanomolar concentrations of SAHA, as compared to millimolar concentrations of VPA. Similarly, both SAHA and VPA induced LTR-driven GFP protein expression (not shown).
FIG. 2.
SAHA activates HIV LTR transcription. J89 cells were treated with the indicated concentrations of SAHA or VPA for 4hrs. Semi-quantitative RT PCR was performed on total RNA isolated from treated and untreated cells as described in methods. The data presented is the mean ± SE of 3 independent experiments.
SAHA induces virus outgrowth from resting CD4+ T cells of aviremic HIV-infected individuals
Lymphocytes were obtained by leukopheresis from five aviremic HIV-infected patients with CD4 T cell counts ranging from 529 to 960 cells/μl. Using a rigorous assay to quantitate resting CD4+ cell HIV infection, we tested the ability of SAHA to induce HIV expression in patients' resting T cells.23
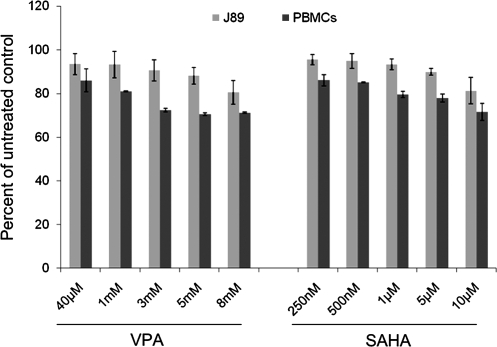
SAHA is primarily bound to albumin. In human plasma, 71% of SAHA is protein bound. When measured in tissue culture media with 10% FCS, only 5% of VPA is protein bound (data not shown). Although no such data exist for SAHA in culture media, we have conservatively assumed a similar degree of protein binding. Protein-unbound peak concentrations of SAHA in patients given a 400 mg dose are estimated to be 340 nM (Vorinostat Package Insert, Merck & Co., 2006). To approximate this exposure in vitro, we tested 250–335 nM SAHA in tissue culture (238–318 nM unbound drug concentration). These concentrations do not significantly affect J89 cell or PBMC proliferation (Fig. 3). Micromolar concentrations of SAHA are generally required to induce tumor cell apoptosis in vitro.37
FIG. 3.
Toxicity of SAHA in seronegative donor PBMC and J89 cells. PBMC or J89 cells were cultured in the absence or presence of SAHA or VPA at the indicated concentrations. MTT assays were performed as described in methods. The percentage of proliferating cells was calculated compared to cells cultured in standard media. The data represent the mean ± SE of 3 independent experiments.
Under these conditions, SAHA induced virus outgrowth in four of five patients assayed, although not always at the frequency seen after maximal mitogen stimulation (Table 1). The frequency of RCI in patients 1 and 3 was measured at two separate time points a month or more apart, and virus was recovered with comparable frequency in separate assays. Given the 0.3 log variance of our assay,27 recovery of HIV after exposure to SAHA was similar to that following 24 h of maximal stimulation in patients 2–4, and somewhat less frequent in patient 1. In a patient with low RCI (patient 5) as measured by maximal mitogen stimulation, virus recovery was not above background levels seen in the presence of the concentration (20 U/ml) of IL-2 used to promote cell survival.
Table 1.
Recovery of HIV from Resting CD4+ T Cells Isolated from Aviremic, ART-Treated, HIV-Infected Individuals after Exposure to SAHA, VPA, or Maximal Mitogen Activation
|
Infected units per billion of HIV+ donor resting CD4+ T cells expressing HIV after exposure to HDAC inhibitor or mitogen | |||
|---|---|---|---|
| Patients | SAHA (250–335 nM) | VPA (40 μM) | Maximal mitogena |
| 1visit A | 3560 | >3560 | 17950 |
| 1visit B | 4540 | 3220 | 11340 |
| 2 | 543 | 703 | 1243 |
| 3visit A | 561 | Not done | 487 |
| 3visit B | 410 | 223 | 440 |
| 4 | 280 | 179b | 426 |
| 5 | Not recoveredc | Not recovered | 45 |
PHA 1 μg/ml, IL2 100 U/ml, 5-fold excess irradiated allogeneic cells.
Frequency of HIV outgrowth not above background (IL-2 only control).
Patients 1 and 2: 335 nM; patients 3–5: 250 nM.
Further, to model the effect that a clinical dose of SAHA might have in vivo, we measured the effect of ex vivo exposure to the drug. SAHA has a short half-life in vivo, and when measured in cancer patients receiving multiple doses of 400 mg/day in the fed state was 2 h (Vorinostat Package Insert, Merck & Co., 2006). To model the effect of a clinical exposure to SAHA, we measured the frequency of recoverable virus after a similar, pulsatile exposure of resting CD4+ cells to the HDAC inhibitor. Resting cells from two additional patients were exposed to SAHA for 3 or 6 h. Cells were then washed and cocultured with CD8-depleted PBMCs as described above. Virus outgrowth after pulsatile exposure was compared to viral recovery from cells continuously exposed to mitogen stimulation for 24 h as per standard assay conditions (Table 2). In both patient samples, pulsatile exposure to SAHA simulating clinical dosing allowed recovery of HIV from resting CD4+ T cells. As fewer cells were available from these patients, we cannot quantitatively compare the frequency of RCI; its point estimate is less accurate. Nevertheless, the frequency of virus recovery after a brief exposure to SAHA was above that of background IL-2.
Table 2.
Recovery of HIV from Resting CD4+ T Cells Isolated from Aviremic, ART-Treated, HIV-Infected Individuals after a 3- or 6-h Pulsed Exposure to SAHA Compared to 24 h Exposure to Maximal Mitogen Activation
|
Infected units per billion resting CD4+ T cells | ||
|---|---|---|
| Patients | SAHAa | Maximal mitogenb |
| 6 | 162 (3 h) | 1160 (24 h) |
| 7 | 295 (6 h) | 360 (24 h) |
Patient 6 250 nM SAHA; patient 7 335 nM SAHA.
PHA 1 μg/ml, IL-2 100 U/ml, 5-fold excess irradiated allogeneic cells.
Ideally, a drug employed to reduce persistent HIV infection in patients must not induce the expression of activation markers or upregulate the expression of HIV entry coreceptors on uninfected cells, as this would render these cells more susceptible to de novo infection. To determine the effect of SAHA on cell surface activation markers, PBMCs were exposed to concentrations of SAHA used in resting cell assay in the presence of IL-2, and cell surface markers were assayed. Results were compared to cells stimulated with mitogen and IL-2 or IL-2 alone. Exposure of PBMCs to 335 nM SAHA did not significantly alter the expression of CXCR4, CCR5, CD69, and Ki67 when compared to cells exposed to control IL-2 and mitogen (data not shown). Finally, there was no difference in HIV p24 antigen output in either activated or resting cells that were infected in the presence or absence of SAHA (data not shown).
Discussion
A long-lived latently infected population of resting memory T cells, unaffected by both the immune response and antiretrovirals, is established early during infection. Therapies that target this persistent reservoir are necessary if HIV infection is to be eradicated. One mechanism that maintains proviral quiescence in resting CD4+ T cells is the activity of HDACs at the viral promoter.
We investigated the ability of SAHA, a potent HDAC inhibitor, to induce LTR expression in a cell line model of latency and to induce virus from CD4+ resting T cells of aviremic HIV-infected individuals. Our results illustrate that SAHA exposure leads to chromatin acetylation at nucleosome 1 and decreased HDAC1 occupancy. This is consistent with the known counterregulatory interactions of HDACs and histone acetyltransferases,38 and results in HIV transcription and virus production in a chronically infected, transcriptionally quiescent cell line. Ideally, it would be desirable to measure chromatin changes in infected resting CD4+ T cells from aviremic HIV-infected individuals. However, given the low frequency of such cells, such an assay is not technically feasible.
Clinically relevant concentrations of SAHA induce virus outgrowth ex vivo from resting T cells of aviremic HIV+ patients. Indeed, given the short half-life of SAHA it is notable that even a brief exposure to the HDAC inhibitor is sufficient to allow recovery of replication-competent HIV from patients' cells. The effect of SAHA on histone acetylation and virus outgrowth is achieved using nanomolar concentrations of the drug that are durably achieved in the clinical administration of the drug.
SAHA is known to alter the expression of 2–10% of the genome in transformed cell lines.29 However, the effect of SAHA on the expression of surface activation markers in cells of hematopoietic lineage was not known. The ideal drug used to purge persistent HIV infection should not render bystander cells more susceptible to de novo infection by inducing expression of cell surface activation markers. Exposures to SAHA do not upregulate either surface markers of activation in PBMCs or receptors for HIV infection. Further, virion production following de novo infection was not affected by SAHA.
In cells from some patients, but not all, recovery of HIV after SAHA exposure was comparable to recovery after maximal mitogen stimulation. These studies cannot yet address whether additional signals, such as those that activate NF-κB signaling, will be required to fully purge infection in resting CD4 cells.
Clearance of HIV infection should be a therapeutic goal, although it may be a distant one. Chronic suppressive antiviral therapy is complex and fraught with toxicities and costs. Selective induction of latent HIV might allow antiretrovirals and the immune response to clear HIV infection. Therapies with potent and selective HDAC inhibitors such as SAHA in combination with intensified ART may allow efficient clearance of persistent HIV infection.
Acknowledgments
We are grateful to A. Duff and N. Cheng, L. Ngo for study coordination, A. Kashuba, S. Fiscus, M. Kerkau, F. Ashton, and the UNC CFAR Pharmacology, Virology, Immunology, and Clinical Core facilities, and to the dedicated staff of the UNC Blood Bank. Funding for the study was provided by National Institutes of Health Grants AI064074 to D.M.M., R00046 to the UNC GCRC, U54RR024383 to the UNC CTSA, AI50410 to the UNC CFAR, and NIH T32AIO7151 grant to N.M.A. Most importantly, this effort would not have been possible without the selfless contribution of study volunteers.
Disclosure Statement
No competing financial interests exist.
References
- 1.Wong JK. Hezareh M. Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 2.Finzi D. Hermankova M. Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 3.Chun TW. Stuyver L. Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pazin MJ. Sheridan PL. Cannon K, et al. NF-kappa B-mediated chromatin reconfiguration and transcriptional activation of the HIV-1 enhancer in vitro. Genes Dev. 1996;10:37–49. doi: 10.1101/gad.10.1.37. [DOI] [PubMed] [Google Scholar]
- 5.Van Lint C. Emiliani S. Ott M. Verdin E. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 1996;15:1112–1120. [PMC free article] [PubMed] [Google Scholar]
- 6.El Kharroubi A. Piras G. Zensen R. Martin MA. Transcriptional activation of the integrated chromatin-associated human immunodeficiency virus type 1 promoter. Mol Cell Biol. 1998;18:2535–2544. doi: 10.1128/mcb.18.5.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenuwein T. Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 8.Strahl BD. Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 9.Gregory PD. Wagner K. Horz W. Histone acetylation and chromatin remodeling. Exp Cell Res. 2001;265:195–202. doi: 10.1006/excr.2001.5187. [DOI] [PubMed] [Google Scholar]
- 10.Davie JR. Covalent modifications of histones: Expression from chromatin templates. Curr Opin Genet Dev. 1998;8:173–178. doi: 10.1016/s0959-437x(98)80138-x. [DOI] [PubMed] [Google Scholar]
- 11.Turner BM. Histone acetylation and an epigenetic code. BioEssays. 2000;22:836–845. doi: 10.1002/1521-1878(200009)22:9<836::AID-BIES9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 12.Turner BM. Histone acetylation as an epigenetic determinant of long-term transcriptional competence. Cell Mol Life Sci. 1998;54:21–31. doi: 10.1007/s000180050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mai A. Massa S. Rotili D, et al. Histone deacetylation in epigenetics: An attractive target for anticancer therapy. Med Res Rev. 2005;25:261–309. doi: 10.1002/med.20024. [DOI] [PubMed] [Google Scholar]
- 14.Norton VG. Imai BS. Yau P. Bradbury EM. Histone acetylation reduces nucleosome core particle linking number change. Cell. 1989;57:449–457. doi: 10.1016/0092-8674(89)90920-3. [DOI] [PubMed] [Google Scholar]
- 15.Margolis DM. Somasundaran M. Green MR. Human transcription factor YY1 represses human immunodeficiency virus type 1 transcription and virion production. J Virol. 1994;68:905–910. doi: 10.1128/jvi.68.2.905-910.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romerio F. Gabriel MN. Margolis DM. Repression of human immunodeficiency virus type 1 through the novel cooperation of human factors YY1 and LSF. J Virol. 1997;71:9375–9382. doi: 10.1128/jvi.71.12.9375-9382.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coull JJ. Romerio F. Sun JM, et al. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J Virol. 2000;74:6790–6799. doi: 10.1128/jvi.74.15.6790-6799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He G. Margolis DM. Counterregulation of chromatin deacetylation and histone deacetylase occupancy at the integrated promoter of human immunodeficiency virus type 1 (HIV-1) by the HIV-1 repressor YY1 and HIV-1 activator tat. Mol Cell Biol. 2002;22:2965–2973. doi: 10.1128/MCB.22.9.2965-2973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams SA. Chen LF. Kwon H. Ruiz-Jarabo CM. Verdin E. Greene WC. NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25:139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang G. Espeseth A. Hazuda DJ. Margolis DM. c-myc and Sp1 contribute to proviral latency by recruiting histone deacetylase 1 to the human immunodeficiency virus type 1 promoter. J Virol. 2007;81:10914–10923. doi: 10.1128/JVI.01208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyagi M. Karn J. CBF-1 promotes transcriptional silencing during the establishment of HIV-1 latency. EMBO J. 2007;26:4985–4995. doi: 10.1038/sj.emboj.7601928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ylisastigui L. Coull JJ. Rucker VC, et al. Polyamides reveal a role for repression in latency within resting T cells of HIV-infected donors. J Infect Dis. 2004;190:1429–1437. doi: 10.1086/423822. [DOI] [PubMed] [Google Scholar]
- 23.Ylisastigui L. Archin NM. Lehrman G. Bosch RJ. Margolis DM. Coaxing HIV-1 from resting CD4 T cells: Histone deacetylase inhibition allows latent viral expression. AIDS. 2004;18:1101–1108. doi: 10.1097/00002030-200405210-00003. [DOI] [PubMed] [Google Scholar]
- 24.Quivy V. Adam E. Collette Y, et al. Synergistic activation of human immunodeficiency virus type 1 promoter activity by NF-kappaB and inhibitors of deacetylases: Potential perspectives for the development of therapeutic strategies. J Virol. 2002;76:11091–11103. doi: 10.1128/JVI.76.21.11091-11103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehrman G. Hogue IB. Palmer S, et al. Depletion of latent HIV-1 infection in vivo: A proof-of-concept study. Lancet. 2005;366:549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siliciano JD. Lai J. Callender M, et al. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J Infect Dis. 2007;195:833–836. doi: 10.1086/511823. [DOI] [PubMed] [Google Scholar]
- 27.Archin NM. Eron JJ. Palmer S, et al. Valproic acid without intensified antiviral therapy has limited impact on persistent HIV infection of resting CD4+ T cells. AIDS. 2008;22:1131–1135. doi: 10.1097/QAD.0b013e3282fd6df4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sagot-Lerolle N. Lamine A. Chaix ML, et al. ANRS EP39 study. Prolonged valproic acid treatment does not reduce the size of latent HIV reservoir. AIDS. 2008;22:1125–1129. doi: 10.1097/QAD.0b013e3282fd6ddc. [DOI] [PubMed] [Google Scholar]
- 29.Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–1356. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 30.Marks PA. Breslow R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- 31.Mann BS. Johnson JR. Cohen MH. Justice R. Pazdur R. FDA approval summary: Vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12:1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 32.Marban C. Suzanne S. Dequiedt F. de Walque S. Redel L. Van Lint C. Aunis D. Rohr O. Recruitment of chromatin-modifying enzymes by CTIP2 promotes HIV-1 transcriptional silencing. EMBO J. 2007;26:412–423. doi: 10.1038/sj.emboj.7601516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kutsch O. Benveniste EN. Shaw GM. Levy DN. Direct and quantitative single-cell analysis of human immunodeficiency virus type 1 reactivation from latency. J Virol. 2002;76:8776–8786. doi: 10.1128/JVI.76.17.8776-8786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klichko V. Archin N. Kaur R. Lehrman G. Margolis D. Hexamethylbisacetamide remodels the human immunodeficiency virus type 1 (HIV-1) promoter and induces Tat-independent HIV-1 expression but blunts cell activation. J Virol. 200;80:4570–4579. doi: 10.1128/JVI.80.9.4570-4579.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan A. Defechereux P. Verdin E. The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to tat transactivation. EMBO J. 2001;20:1726–1738. doi: 10.1093/emboj/20.7.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers LE. McQuay LJ. Hollinger FB. Dilution assay statistics. J Clin Microbiol. 1994;32:732–739. doi: 10.1128/jcm.32.3.732-739.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butler LM. Agus DB. Scher HI, et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;60:5165–5170. [PubMed] [Google Scholar]
- 38.Yamagoe S. Kanno T. Kanno Y, et al. Interaction of histone acetylases and deacetylases in vivo. Mol Cell Biol. 2003;23:1025–1033. doi: 10.1128/MCB.23.3.1025-1033.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]