Abstract
Darunavir is the most recently licensed protease inhibitor currently used in treatment-experienced HIV-infected individuals. Our objective was to determine darunavir concentrations in cerebrospinal fluid (CSF) and plasma in subjects receiving antiretroviral treatment regimens containing ritonavir-boosted darunavir. Darunavir concentrations were determined by liquid chromatography tandem mass spectrometry in 14 paired CSF and plasma samples from eight HIV-1-infected individuals. The lower limit of quantification was 5.0 ng/ml. All of the 14 CSF samples had detectable darunavir concentrations with a median darunavir concentration of 34.2 ng/ml (range 15.9–212.0 ng/ml). The median (range) plasma darunavir concentration was 3930 (1800–12900) ng/ml. All CSF samples had detectable darunavir concentrations. Most of them exceeded or were in the same range as levels needed to inhibit replication of wild type virus, making it probable that darunavir, at least to some extent, contributes to the suppression of HIV replication in the central nervous system.
Introduction
Highly active antiretroviral therapy (HAART) has considerably reduced the incidence of HIV-associated morbidity and mortality. Antiretroviral treatment has also had a tremendous impact on the occurrence of neurological complications, including not only central nervous system (CNS) opportunistic infections but also the AIDS dementia complex related to more direct infection of the CNS.1 This is most probably attributable to improvements in immune function alongside a reduction of the viral burden and inflammatory processes in the CNS resulting from effective treatment.
For an antiretroviral drug to effectively inhibit viral replication in the CNS, it must be able to penetrate the blood–brain barrier (BBB). The capacity of a drug to enter the CNS depends on a number of factors: molecular size, lipophilicity, degree of ionization and plasma protein binding, and whether or not the drug is a substrate for transmembrane transporters.
The protease inhibitors (PIs) are in general large molecules highly bound to plasma proteins such as α1-acidic glycoprotein (AAG) and albumin. In addition, they are to varying extent substrates for P-glycoprotein, a transport pump located on brain endothelial cells, making PIs more or less susceptible to active efflux from the CNS.2 All these characteristics make it more difficult for PIs to traverse the BBB, and as a consequence, CSF concentrations of most PIs are low or undetectable.3–6 However, as PIs are very potent drugs, only small concentrations may be required to inhibit viral replication within a sanctuary site such as the CNS.
Darunavir is the most recently licensed PI. The coadministration of darunavir with low-dose ritonavir leads to a several-fold increase of the systemic exposure to darunavir.7 It has high in vitro activity against many viral strains resistant to other PIs,8 and has clinically been shown to be effective in both treatment-naive as well as heavily treatment-experienced subjects.9–11 It is currently licensed for use in treatment-experienced patients. Like most other PIs, it is highly bound to plasma proteins (95%), with a small fraction (5%) of unbound drug available to penetrate the CNS. The objective of this study was to determine if darunavir, when administered with low-dose ritonavir (darunavir/r), could traverse the BBB and achieve sufficient concentrations in CSF.
Materials And Methods
Patients
A total of eight HIV-1-infected subjects were included from Gothenburg, Sweden (n = 5), San Francisco, CA (n = 2), and Dublin, Ireland (n = 1). Patient characteristics are presented in Table 1. All patients were on HAART containing darunavir/ritonavir 600/100 mg twice daily. Two of the patients had neurological complications; one was diagnosed with HIV-encephalitis and the other with atypical mycobacterial infection involving the CNS. The other six were neurologically asymptomatic and underwent a lumbar puncture in the context of longitudinal cohort studies.12 Seven of the patients were male. The median (range) age was 50 (22–61) years. The CD4+ T cell counts ranged from 0 to 430 ×106 cells/liter, with a median value of 140 × 106 cells/liter.
Table 1.
Patient Characteristics Including Plasma and CSF Darunavir Concentrations for Each Subjecta
| |
|
|
|
|
|
|
|
|
Drv concentrations (ng/ml) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Patient number | Visit number | Sex | Age | CD4 cell count(×106/liter) | Plasma VL (log10 copies/ml) | CSF VL (log10 copies/ml) | Antiretroviral treatment in addition to drv/r | Time on ART(weeks) | Plasma | CSF |
| 1 | 1 | m | 57 | 320 | 2.51 | <1.30 | zdv + 3TC + abc + T20 + ral | 13.0 | 2680 | 34.9 |
| 2 | m | 57 | 110 | <1.30 | <1.30 | zdv + 3TC + abc + ral | 43.5 | 8550 | 96.5 | |
| 2 | 1 | m | 46 | 160 | <1.30 | <1.30 | zdv + 3TC + abc + ral | 16.0 | 3870 | 25.6 |
| 2 | m | 46 | 190 | <1.30 | <1.30 | zdv + 3TC + abc + ral | 66.0 | 1800 | 19.6 | |
| 3 | 1 | m | 61 | 430 | 3.73 | <1.30 | zdv + 3TC + abc + T20 | 12.0 | 3210 | 33.5 |
| 2 | m | 61 | 370 | 1.82 | <1.30 | zdv + 3TC + abc + T20 | 50.0 | 6740 | 53.8 | |
| 4 | 1 | m | 43 | 80 | <1.30 | <1.30 | zdv + 3TC + abc + ral | 20.5 | 4060 | 35.5 |
| 2 | m | 43 | 140 | <1.30 | <1.30 | zdv + 3TC + abc + ral | 56.0 | 3990 | 23.5 | |
| 5 | 1 | f | 22 | 0 | 5.20 | 5.66 | zdv + 3TC + abc + ral | 0.5 | 12900 | 212.0 |
| 6 | 1 | m | 49 | 38 | 4.05 | <1.30 | zdv + 3TC + tdf + ral | 0.9 | 9120b | 43.9b |
| 2 | m | 49 | 92 | 2.66 | <1.30 | zdv + 3TC + tdf + ral | 4.0 | 3770 | 28.7 | |
| 7 | 1 | m | 50 | 133 | 4.10 | 4.00 | tdf + ftc + ral | 1.0 | 4590 | 15.9 |
| 2 | m | 50 | 140 | 2.35 | 1.32 | tdf + ftc + ral | 4.9 | 2820 | 20.1 | |
| 8 | 1 | m | 54 | 168 | 2.38 | <1.30 | zdv + 3TC + abc | 2.5 | 3550 | 64.8 |
VL, viral load; ART, antiretroviral treatment; m, male; f, female; zdv, zidovudine; 3TC, lamivudine; abc, abacavir; drv/r, darunavir/ritonavir; T20, enfuvirtide; ral, raltegravir; tdf; tenofovir; ftc, emtricitabine.
Sampling time unknown.
This study was approved by the ethics committees of the University's of each center.
Methods
Lumbar punctures were conducted in a standardized manner, and CSF and plasma specimens were immediately stored at −70°C until analysis. CSF levels of darunavir were determined in the laboratories of Johnson and Johnson Pharmaceutical Research and Development, Beerse, Belgium. Total (i.e., bound + unbound) plasma and CSF levels of darunavir were determined using a liquid chromatography tandem mass spectrometry (LC-MS/MS) method. After matrix matching with 50-μl aliquots of blank human CSF or plasma, respectively, the 14 paired plasma and CSF samples from the eight patients (50-μl aliquots) were spiked with 50 μl of internal standard solution in acetonitrile. Calibration curves (Cal) and independent quality control samples (QC) (at three concentration levels in duplicate) were prepared in the same matrix as the study samples. These Cal and QC samples were analyzed together with the study samples. Precipitation was done by the addition of 0.5 ml acetonitrile. The samples were centrifuged and 10 μl of the supernatant was injected onto a reversed-phase column (3 cm × 4.6 mm, packed with 3.5 μm Xbridge C18, Waters) for final separation and quantification. LC-MS/MS analysis was carried out on an API-4000 triple quadruple mass spectrometer (Applied Biosystems), which was coupled to a high-performance liquid chromatography pump (Agilent) and autosampler (Shimadzu). The quantification limit was 5.0 ng/mL. Linearity of the Cal ranged from 5.0 to 10,000.0 ng/ml. Acceptance criteria of the bioanalytical run were in line with current Food and Drug Administration (FDA) guidance on bioanalysis with respect to accuracy and precision on Cal and QC samples.
HIV-1 RNA was quantified in CSF and plasma with COBAS Amplicor HIV-1 Monitor Test, version 1.5 (Roche AB, Basel, Switzerland) and run according to the manufacturer's protocol. The assay has a dynamic range down to 50 copies/ml (1.70 log10 copies/ml) and a lower detection limit of 20 copies/ml (1.30 log10 copies/ml). All HIV-1 RNA values <20 copies/ml were estimated to 19 copies/ml (1.28 log10 copies/ml). The peripheral blood CD4+ T cell count was measured by direct immunofluorescence in a flow cytometer.
Statistical analysis
Nonparametric methods were used for group descriptives and for darunavir concentrations (median and range).
Results
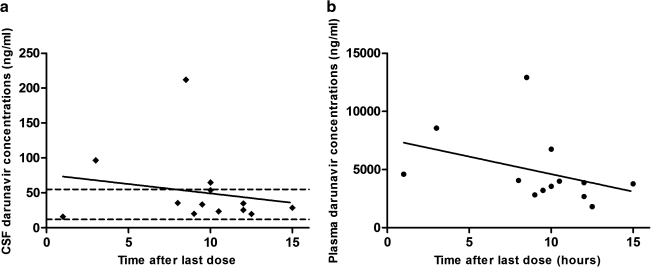
Total CSF and plasma darunavir concentrations are presented in Fig. 1 and Table 1. At the time of sampling patients had been receiving darunavir for a median (range) of 12.5 (0.5–66.0) weeks. All 14 CSF samples had detectable darunavir concentrations ranging from 15.9 to 212.0 ng/ml (median 34.2 ng/ml). The median (range) plasma darunavir concentration was 3930 (1800–12900) ng/ml. The CSF-plasma ratios ranged from 0.3 to 1.8% (median 0.9%). Darunavir concentrations in all 14 CSF samples were in the range of or above the protein-adjusted IC50 (drug concentration needed to inhibit 50% of viral replication) of 12–55 ng/ml.8 Eleven out of 14 CSF samples had HIV RNA levels <1.28 log10 copies/ml, and all patients except subject number 5, with atypical mycobacterial infection in the CNS, had higher viral loads in plasma than in CSF. Among the patients with detectable CSF viral loads, two had been on treatment for only 1 week or less. The third subject had received antiretroviral therapy with tenofovir, emtricitabine, and raltegravir in addition to darunavir/ritonavir for almost 5 weeks.
FIG. 1.
Darunavir cerebrospinal fluid (CSF) (a) and plasma (b) concentrations by postdose time for the 13 paired specimens with known sampling time. The solid lines are linear regression lines for CSF and plasma. The dotted lines in (a) indicate the estimated range of the IC50 in CSF (12–55 ng/ml).
Discussion
The PIs have a number of unfavorable characteristics, which make it more difficult for them to traverse the BBB. Most of them, including darunavir, are highly bound to plasma proteins. Darunavir was detected in all 14 CSF samples, but the drug concentrations in CSF were only around 0.9% of plasma concentrations. This could be due to active transport out of CSF or limited entry into CSF.
The IC50 value for darunavir of 12–55 ng/ml used in this study was obtained in vitro from acutely infected MT-4 cells, peripheral blood mononuclear cells, and in macrophages in the presence of 50% human serum.8 The addition of human serum leads to a substantial increase of the IC50 for most PIs, for darunavir about 20-fold. The CSF contains far less proteins than plasma, but we do not know the degree of drug binding to proteins in CSF, and therefore it is difficult to determine an IC50 adjusted for this compartment. Probably, the IC50 for CSF is somewhere between the IC50 without and with 50% human serum. All CSF samples had darunavir levels exceeding 12 ng/ml, suggesting that darunavir, at least to some extent, might contribute to the inhibition of wild-type HIV replication in the CNS. It should, however, be kept in mind that drug concentrations in CSF do not directly correlate with drug levels reached within the brain parenchyma or to antiviral effect. In contrast, lopinavir and indinavir are PIs that have previously been shown to penetrate into the CNS with CSF levels exceeding the IC50 several-fold.13–15 Saquinavir/ritonavir, nelfinavir, and atazanvir lead to undetectable or very low CSF concentrations, and do not fully suppress viral replication in CSF when used as monotherapy.6,16,17
The highest CSF darunavir concentration was observed in patient number 5 who had an atypical mycobacterial infection involving the CNS at the time of sampling. An ongoing infection in the CNS compromises the BBB and might theoretically lead to increased drug distribution into the CNS and higher CSF drug concentrations. This patient, however, also had the highest darunavir level in plasma, which is probably the explanation for the high darunavir level in the CSF. The second patient with neurological complications, patient number 8 with HIV-encephalitis, had a CSF darunavir concentration of 64.8 ng/ml.
We cannot draw far-reaching conclusions about the efficacy, or lack of efficacy, of darunavir/r in CNS from this study because all patients were on concomitant treatment with at least three other antiretroviral drugs. Subject number 7 had detectable CSF HIV RNA despite almost 5 weeks on treatment. There are no published human data on the CNS distribution for two of the drugs in his regimen (raltegravir and emtricitabine), and tenofovir reaches only low concentrations in the CSF.18 The majority of the patients with suppressed CSF viral loads were on triple-NRTI treatment with zidovudine, abacavir, and lamivudine; these drugs are known to penetrate the BBB.19–21 In addition, two of the patients (numbers 5 and 6) had received treatment for less than 1 week, making it somewhat uncertain whether they had reached steady-state concentrations of darunavir in the CSF.
The effectiveness of antiretroviral drugs and drug combinations in the CNS has been a subject of interest for almost two decades. Results thus far suggest that most combinations previously used are also effective in the CNS,13,22,23 although some antiretroviral drugs, such as atazanavir, saquinavir, and nelfinavir, do not treat HIV inside the CNS as well as in the periphery.6,16,17 Most HIV-infected individuals initiating HAART will have a marked reduction of their CSF viral load,24–26 and a larger number of them will reach HIV RNA levels <2.5 copies/ml in CSF than in plasma.27,28 This could probably, at least in part, be explained by the fact that most subjects have lower pretreatment viral loads in CSF than in plasma. Differences in the predominant infected cell types in each compartment might also contribute.29–31 In contrast, CSF levels of neopterin, a marker of the cell-mediated immune activation, will remain elevated in a large number of patients despite many years of suppressive therapy.32 We do not know the long-term consequences, if any, of having an activated immune system inside the CNS for a prolonged time. Our previous findings suggest that this persisting intrathecal immune activation observed in treated patients is mainly driven by residual viral replication within the CNS. By suppressing the viral replication in the CNS to a maximum, it should be possible to further diminish the immune activation in the CNS.33 If this turns out to be the case it would seem even more important to start patients on regimens with a high degree of CNS penetration.
Virological failure and drug resistance are problems seen in some of HAART-treated individuals. Increased viral replication and immune activation may also take place inside the CNS, rendering such patients at increased risk for neurological complications and neurocognitive deficits. In this category of patients it could be important to consider the neuroeffectiveness of the antiretroviral drugs when putting together a regimen. This issue might become significant in patients receiving novel or not commonly used antiretroviral drug combinations, such as NRTI-sparing regimens. The use of darunavir as part of HAART is increasing around many parts of the world, mainly in experienced patients. All patients in this study had detectable levels of darunavir in CSF with concentrations in the range of, or exceeding the IC50 for wild-type virus making it probable that darunavir, at least to some extent, contributes to the suppression of HIV replication in the CNS.
Acknowledgments
This study was supported by grants from the National Institutes of Health (NS43103, MH62701, and RR024131), the Medical faculty of the University of Gothenburg (ALFGBG-2874), the Gothenburg Medical Society, and an unconditional grant from Tibotec.
Disclosure Statement
No competing financial interests exist.
References
- 1.d'Arminio Monforte A. Cinque P. Mocroft A. Goebel FD. Antunes F. Katlama C. Justesen US. Vella S. Kirk O. Lundgren J. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann Neurol. 2004;55:320–328. doi: 10.1002/ana.10827. [DOI] [PubMed] [Google Scholar]
- 2.Huisman MT. Smit JW. Schinkel AH. Significance of P-glycoprotein for the pharmacology and clinical use of HIV protease inhibitors. AIDS. 2000;14:237–242. doi: 10.1097/00002030-200002180-00005. [DOI] [PubMed] [Google Scholar]
- 3.Aweeka F. Jayewardene A. Staprans S. Bellibas SE. Kearney B. Lizak P. Novakovic-Agopian T. Price RW. Failure to detect nelfinavir in the cerebrospinal fluid of HIV-1-infected patients with and without AIDS dementia complex. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:39–43. doi: 10.1097/00042560-199901010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Kravcik S. Gallicano K. Roth V. Cassol S. Hawley-Foss N. Badley A. Cameron DW. Cerebrospinal fluid HIV RNA and drug levels with combination ritonavir and saquinavir. J Acquir Immune Defic Syndr. 1999;21:371–375. [PubMed] [Google Scholar]
- 5.Solas C. Lafeuillade A. Halfon P. Chadapaud S. Hittinger G. Lacarelle B. Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2003;47:238–243. doi: 10.1128/AAC.47.1.238-243.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vernazza P. Daneel S. Schiffer V. Decosterd L. Fierz W. Klimkait T. Hoffmann M. Hirschel B. The role of compartment penetration in PI-monotherapy: The Atazanavir-Ritonavir Monomaintenance (ATARITMO) Trial. AIDS. 2007;21:1309–1315. doi: 10.1097/QAD.0b013e32814e6b1c. [DOI] [PubMed] [Google Scholar]
- 7.Prezista SPC. Tibotec 2007. http://www.emea.europa.eu/humandocs/PDFs/EPAR/prezista/H-707-PI-en.pdf http://www.emea.europa.eu/humandocs/PDFs/EPAR/prezista/H-707-PI-en.pdf
- 8.De Meyer S. Azijn H. Surleraux D. Jochmans D. Tahri A. Pauwels R. Wigerinck P. de Bethune MP. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob Agents Chemother. 2005;49:2314–2321. doi: 10.1128/AAC.49.6.2314-2321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clotet B. Bellos N. Molina JM. Cooper D. Goffard JC. Lazzarin A. Wohrmann A. Katlama C. Wilkin T. Haubrich R, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: A pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369:1169–1178. doi: 10.1016/S0140-6736(07)60497-8. [DOI] [PubMed] [Google Scholar]
- 10.Madruga JV. Berger D. McMurchie M. Suter F. Banhegyi D. Ruxrungtham K. Norris D. Lefebvre E. de Bethune MP. Tomaka F, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: A randomised controlled phase III trial. Lancet. 2007;370:49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- 11.De Jesus E. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, IL. Sep 17–20;; 2007. Abstract H-718b. [PubMed] [Google Scholar]
- 12.Gisslén M. Hagberg L. Brew BJ. Cinque P. Price RW. Rosengren L. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis. 2007;195:1774–1778. doi: 10.1086/518043. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz A. Ståhle L. Hagberg L. Svennerholm B. Fuchs D. Gisslén M. Cerebrospinal fluid and plasma HIV-1 RNA levels and lopinavir concentrations following lopinavir/ritonavir regimen. Scand J Infect Dis. 2004;36:823–828. doi: 10.1080/00365540410025320. [DOI] [PubMed] [Google Scholar]
- 14.Capparelli EV. Holland D. Okamoto C. Gragg B. Durelle J. Marquie-Beck J. van den Brande G. Ellis R. Letendre S. Lopinavir concentrations in cerebrospinal fluid exceed the 50% inhibitory concentration for HIV. AIDS. 2005;19:949–952. doi: 10.1097/01.aids.0000171409.38490.48. [DOI] [PubMed] [Google Scholar]
- 15.Tashima KT. Caliendo AM. Ahmad M. Gormley JM. Fiske WD. Brennan JM. Flanigan TP. Cerebrospinal fluid human immunodeficiency virus type 1 (HIV-1) suppression and efavirenz drug concentrations in HIV-1-infected patients receiving combination therapy. J Infect Dis. 1999;180:862–864. doi: 10.1086/314945. [DOI] [PubMed] [Google Scholar]
- 16.Lafeuillade A. Solas C. Halfon P. Chadapaud S. Hittinger G. Lacarelle B. Differences in the detection of three HIV-1 protease inhibitors in non-blood compartments: Clinical correlations. HIV Clin Trials. 2002;3:27–35. doi: 10.1310/WMWL-6W9Y-PXV2-X148. [DOI] [PubMed] [Google Scholar]
- 17.Gisolf EH. Enting RH. Jurriaans S. de Wolf F. van der Ende ME. Hoetelmans RM. Portegies P. Danner SA. Cerebrospinal fluid HIV-1 RNA during treatment with ritonavir/saquinavir or ritonavir/saquinavir/stavudine. AIDS. 2000;14:1583–1589. doi: 10.1097/00002030-200007280-00014. [DOI] [PubMed] [Google Scholar]
- 18.Best B. Letendre S. Koopmans P. Clifford D. Collier AC. Gelman BB. McArthur JC. Simpson D. Capparelli E. Ellis R. Low tenofovir concentrations in cerebrospinal fluid. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. Feb 3–6;; 2008. Abstract no. 131. [Google Scholar]
- 19.Foudraine NA. Hoetelmans RM. Lange JM. de Wolf F. van Benthem BH. Maas JJ. Keet IP. Portegies P. Cerebrospinal-fluid HIV-1 RNA and drug concentrations after treatment with lamivudine plus zidovudine or stavudine. Lancet. 1998;351:1547–1551. doi: 10.1016/S0140-6736(98)07333-4. [DOI] [PubMed] [Google Scholar]
- 20.Burger DM. Kraaijeveld CL. Meenhorst PL. Mulder JW. Koks CH. Bult A. Beijnen JH. Penetration of zidovudine into the cerebrospinal fluid of patients infected with HIV. AIDS. 1993;7:1581–1587. doi: 10.1097/00002030-199312000-00006. [DOI] [PubMed] [Google Scholar]
- 21.McDowell JA. Chittick GE. Ravitch JR. Polk RE. Kerkering TM. Stein DS. Pharmacokinetics of [(14)C]abacavir, a human immunodeficiency virus type 1 (HIV-1) reverse transcriptase inhibitor, administered in a single oral dose to HIV-1-infected adults: A mass balance study. Antimicrob Agents Chemother. 1999;43:2855–2861. doi: 10.1128/aac.43.12.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gisslén M. Svennerholm B. Norkrans G. Franzen C. Säll C. Svensson R. Öberg S. Hagberg L. Cerebrospinal fluid and plasma viral load in HIV-1-infected patients with various anti-retroviral treatment regimens. Scand J Infect Dis. 2000;32:365–369. doi: 10.1080/003655400750044926. [DOI] [PubMed] [Google Scholar]
- 23.Eggers C. Hertogs K. Sturenburg HJ. van Lunzen J. Stellbrink HJ. Delayed central nervous system virus suppression during highly active antiretroviral therapy is associated with HIV encephalopathy, but not with viral drug resistance or poor central nervous system drug penetration. AIDS. 2003;17:1897–1906. doi: 10.1097/00002030-200309050-00008. [DOI] [PubMed] [Google Scholar]
- 24.Mellgren Å. Antinori A. Cinque P. Price RW. Eggers C. Hagberg L. Gisslein M. Cerebrospinal fluid HIV-1 infection usually responds well to antiretroviral treatment. Antiviral Ther. 2005;10:701–707. [PubMed] [Google Scholar]
- 25.Polis MA. Suzman DL. Yoder CP. Shen JM. Mican JM. Dewar RL. Metcalf JA. Falloon J. Davey RT., Jr Kovacs JA, et al. Suppression of cerebrospinal fluid HIV burden in antiretroviral naive patients on a potent four-drug antiretroviral regimen. AIDS. 2003;17:1167–1172. doi: 10.1097/00002030-200305230-00008. [DOI] [PubMed] [Google Scholar]
- 26.Stellbrink HJ. Eggers C. van Lunzen J. Albrecht H. Greten H. Rapid decay of HIV RNA in the cerebrospinal fluid during antiretroviral combination therapy. AIDS. 1997;11:1655–1657. [PubMed] [Google Scholar]
- 27.Yilmaz A. Svennerholm B. Hagberg L. Gisslein M. Cerebrospinal fluid viral loads reach less than 2 copies/ml in HIV-1-infected patients with effective antiretroviral therapy. Antiviral Ther. 2006;11:833–837. [PubMed] [Google Scholar]
- 28.Spudich S. Lollo N. Liegler T. Deeks SG. Price RW. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J Infect Dis. 2006;194:1686–1696. doi: 10.1086/508750. [DOI] [PubMed] [Google Scholar]
- 29.Gisslén M. Fuchs D. Svennerholm B. Hagberg L. Cerebrospinal fluid viral load, intrathecal immunoactivation, and cerebrospinal fluid monocytic cell count in HIV-1 infection. J Acquir Immune Defic Syndr. 1999;21:271–276. doi: 10.1097/00126334-199908010-00003. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi K. Wesselingh SL. Griffin DE. McArthur JC. Johnson RT. Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- 31.Ho DD. Perspectives series: Host/pathogen interactions. Dynamics of HIV-1 replication in vivo. J Clin Invest. 1997;99:2565–2567. doi: 10.1172/JCI119443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edén A. Price RW. Spudich S. Fuchs D. Hagberg L. Gisslén M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007;196:1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz A. Price RW. Spudich S. Fuchs D. Hagberg L. Gisslén M. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:168–173. doi: 10.1097/QAI.0b013e31815ace97. [DOI] [PMC free article] [PubMed] [Google Scholar]