Abstract
This was a proof-of-principle study to evaluate the impact of short cycle therapy (SCT; 4 days on/3 days off) in adolescents and young adults with good viral suppression on a protease inhibitor-based antiretroviral regimen. Subjects were recruited by the Adolescent Trials Network for HIV/AIDS Interventions and the Pediatric AIDS Clinical Trials Group. Subjects were infected either through perinatal/early childhood transmission or later via risk behaviors. All subjects were required to have at least 6 months of documented viral suppression below 400 copies/ml plus a preentry value below 200 copies/ml and an entry CD4+ T cell count above 350 cells/mm3. Of the 32 subjects enrolled, 12 (37.5%) had confirmed viral load rebound >400 copies, with 18 subjects (56%) coming off for any reason. The majority of subjects resuppressed when placed back onto continuous therapy using the same agents. Although no difference was found in virologic rebound rates between the early and later transmission groups, those infected early in life had higher rates of coming off SCT for any reason. There was no impact of SCT on the CD4+ T cell counts in those who remained on study or those who came off SCT for any reason. Subjects demonstrated good adherence to the SCT regimen. This study suggests that further evaluation of SCT may be warranted in some groups of adolescents and young adults infected with HIV.
Introduction
The use of antiretroviral therapy (ART) has made a significant impact on disease progression in individuals infected with the human immunodeficiency virus (HIV).1 The impact of HIV/AIDS in adolescents and young adults is significant. The World Health Organization estimates that 11.8 million young people, 15 to 24 years of age, worldwide are infected with HIV, with 2 million infected each year.2 Research on the impact of highly active antiretroviral therapy (HAART), especially management strategies, is limited in this population.3–5 A number of management strategies have been tried to limit antiretroviral exposure, enhance adherence, limit medication-associated toxicity, and improve quality of life. These strategies include CD4-guided treatment interruptions, structure treatment interruptions, and short-cycle therapy.6,7 Studies evaluating CD4-guided treatment interruption and structured treatment interruptions have had mixed results and have not demonstrated consistent benefits to subjects.8–13
Results of studies of short-cycle therapy, the interruption of therapy for 2 to 7 days only, have demonstrated mixed results. Dybul and colleagues demonstrated good initial results of a study in adults of 7 days on/7 days off therapy with sustained viral control and stable CD4+ T cell counts.14 However, Ananworanich and colleagues showed less favorable results with the 7 on/7 off approach, with high rates of viral rebound.15 Cohen and colleagues studied a strategy of five days on- two days off (FOTO) strategy with overall good viral control in 89.6% of subjects with 100% virologic control in subjects on an efavirenz-based HAART regimen.16 As adolescents and young adults face a lifetime of HAART once meeting guidelines, a short-cycle management approach is particularly appealing in this population to limit ART exposure as well as potentially impact on long-term adherence. The Adolescent Trials Network 015 study was a proof of concept study of a 4-day on/3-day off short-cycle therapy in adolescents and young adults, on a protease inhibitor-based HAART regimen, who demonstrated good viral control for at least 6 months and CD4+ T cells above 350 cells/mm3 at study entry.
Materials and Methods
This was a multicenter, prospective, proof-of-concept study designed to assess the impact of switching from continuous HAART with a protease inhibitor (PI) to short-cycle therapy (SCT) consisting of HAART 4 days/week (Monday through Thursday) and no antiretroviral therapy (ARV) 3 days/week (Friday through Sunday). The study enrolled subjects between June 24, 2003 and February 13, 2006 and planned to accrue up to 40 subjects within a 2-year period. This number was adjusted from 30 subjects after a decision to include subjects infected before age 9 years. After additional consideration, the decision was made to stop enrollments at 32 as continued enrollments up to a total of 40 was not required to assess the primary outcome in this proof-of-principle study.
Participants
Subjects enrolled were HIV-positive adolescents and young adults between 12 and 24 years of age with either vertical or horizontal HIV acquisition, currently on a HAART regimen that included a PI but excluded nonnucleoside reverse transcriptase inhibitors (NNRTIs) and abacavir, and documented viral suppression of HIV-1 RNA viral load (VL) to <400 copies/ml (c/ml) for at least 6 months before enrollment. Preentry requirements were VL < 200 c/ml, CD4+ T cell (CD4) count > 350 cells/μl, and no ≥grade 3 clinical or laboratory toxicities as defined by the Division of AIDS, NIH toxicity tables. During the study, inclusion criteria were modified to allow grade 3 indirect bilirubin elevation at enrollment and during the study if the subject was receiving atazanavir and had no other evidence of liver pathology. Each subject provided written informed consent or assent and all subjects <18 years of age also had a parent/guardian provide written, informed permission for the subject's participation in the study. The study protocol and consent documents were reviewed and approved by the IRB at each participating site. Female subjects could not be pregnant and were required to use protocol-specified methods of birth control. Participants were classified as either having become HIV infected through perinatal transmission or contaminated blood products in infancy or after 9 years of age via risk behaviors. These two groups are referred to as “nonbehaviorally infected” and those infected through “risk behavior” based on the most likely route of infection for youth in each of the groups as determined by site investigators. Analyses were included for the entire cohort in addition to comparison analyses between the two groups. Although these two groups were similar in age, those infected earlier in age had a greater duration of infection and therefore were believed to have the potential for different outcomes from such a management approach.
Study management
Once enrolled, each subject initiated SCT and continued the cycle weekly. Adherence was monitored every 2 weeks for the first 6 months and every 4 weeks for the remainder of the study, either by phone or during an in-person visit. Study participants were asked about the number of medications they currently take and then the number that were missed over the previous 3 days. Adherence was alternately assessed on Mondays (when subjects on SCT should have missed all doses) and Thursdays (when all subjects should have taken all doses). The percent adherence measure was then calculated as the doses the patient actually took over the previous 3 days divided by the total doses the subject should have taken. VL was measured monthly for the first 6 months, and then every 8 weeks thereafter and was always measured on a Monday so as to have the greatest chance to detect low-level viral breakthrough. If any VL was >400 c/ml, the subject was asked to return immediately for confirmatory testing and if confirmed to be >400 c/ml, the subject resumed the continuous HAART regimen with or without a change in specific medications (determined by the site investigator/clinical care provider) and had no further treatment interruptions. Hematology, chemistries, and CD4 count were monitored at every study visit; fasting glucose, cholesterol, and triglycerides were measured at baseline and at weeks 24 and 48.
Laboratory
VL was measured in real time with the Roche ultrasensitive assay (version 2.0) in a Pediatric AIDS Clinical Trials Group (PACTG)-supported virology laboratory. All participants with VL > 1000 c/ml, even if only at a single time point, had genotypic resistance testing performed by the commercial laboratory used by the PACTG virology laboratory performing the viral load assays. All other laboratory measures were performed in the clinical laboratory at each site.
Safety monitoring and adverse event reporting
All study laboratory results were reviewed and managed as clinically necessary by clinicians at each research site. For events considered grade 1 or 2, subjects were managed at the discretion of the site care team and were permitted to remain on SCT. If events were ≥grade 3, confirmation within 72 hr was required. If confirmed, grade 3 events required that subjects discontinue SCT but they could resume SCT if the toxicity improved to ≤grade 2 within 2 weeks; whereas grade 4 events required permanent discontinuation of SCT. For subjects on atazanavir, no dose modification or treatment interruption was required for grade 3 or 4 increases in indirect bilirubin, as long as the other liver enzymes remained normal. Grade 3 or 4 elevated cholesterol and triglycerides were similarly exempt from the requirements for ARV modification.
Treatment (SCT) and/or study discontinuation
Subjects were required to stop SCT and resume HAART but were encouraged to stay on study and continue all follow-up evaluations, if they met any of the following criteria: confirmed VL rebound to >400 c/ml; pregnancy; decrease in CD4 count by 30% or more from their entry CD4; toxicity as noted previously.
Statistical analysis
Baseline data on demographics and HIV-related characteristics were summarized with simple descriptive statistics and compared between participants infected before or after age 9 years by χ2 and Wilcoxon tests. Time to the primary outcome (VL rebound) was analyzed using Kaplan–Meier curves; log-rank tests were used to evaluate the significance of differences between survival times for various subgroups. Proportional hazards models were used to evaluate predictors of time to VL rebound. For those with VL rebound, the time to resuppression of viral load was analyzed by similar methodologies. Temporary increases in viral load were counted as “blips” and were evaluated as predictive measures of later VL rebound; specifically, a blip was defined as a single VL between 50 and 400 c/ml, or above 400 c/ml but below 400 c/ml on repeat testing within 7 days of the first measure. Change over time in CD4 count and percent was assessed by the slope of a regression line fit to the sequence of CD4 measures from study entry to either stopping SCT or going off-study. These slopes were then used as predictors in generalized linear models and in analysis of survival curves. Significance tests for comparison of mean values were done using t tests, while frequency data were compared using χ2 tests. Exact tests were performed as necessary due to small sample sizes. All analyses were done using SAS procedures (SAS Institute).
Results
Study population
A total of 32 subjects were enrolled from Adolescent Trials Network for HIV/AIDS Interventions (ATN) and Pediatric AIDS Clinical Trials Group (PACTG) sites. Seventeen behaviorally infected youths and 15 nonbehaviorally infected youths or youths infected during early childhood (2 subjects infected through blood products, 11 confirmed perinatal infection, and 2 suspected perinatal infection) were enrolled. Subjects in the behavioral group were significantly older, with 15 (88%) compared with only 4 (26.7%) 20- to 24-year-olds in the nonbehavioral group (p = 0.007) whereas the gender distribution was similar for both groups. Significantly more youth identified as African American (n = 17, 53.1%) compared with white or other (p = 0.008).
The clinical characteristics of the cohort at baseline are presented in Table 1. The mean CD4 count at preentry was similar in the nonbehavioral and behavioral groups (909 vs. 706 cells/mm3; p = 0.146). There were no significant differences in the numbers of subjects with a CDC category C condition. Although subjects in the nonbehavioral group were exposed to a greater number of antiretroviral agents, this did not reach significance. Members of the nonbehavioral group were on their current regimen for significantly more time than the behavioral group (>24 months: 100 vs. 52.9%; p = 0.003).
Table 1.
Baseline Characteristics by Age Known to Acquire HIV-1 Infection
| Total (n = 32) | Nonbehavioral (n = 15) | Behavioral (n = 17) | p Value | |
|---|---|---|---|---|
| HIV-related characteristics | ||||
| Age of first documented HIV-1 positive test (year) | ||||
| n | 32 | 15 | 17 | <.0001 |
| Mean | 12.95 | 6.77 | 18.40 | |
| HIV-related diagnosis at preentry | ||||
| Subjects with at least one CDC category C diagnosis | ||||
| Yes | 11 (36.7) | 6 (46.2) | 5 (29.4) | 0.4539 |
| No | 19 (63.3) | 7 (53.8) | 12 (70.6) | |
| Retrospective antiretroviral exposure history at preentry | ||||
| Number of ART regimen | ||||
| 2 | 5 (15.6) | 0 (0.0) | 5 (29.4) | 0.0644 |
| 3 | 2 (6.3) | 1 (6.7) | 1 (5.9) | |
| 4 | 3 (9.4) | 1 (6.7) | 2 (11.8) | |
| 5+ | 22 (68.8) | 13 (86.7) | 9 (52.9) | |
| Total length of time exposed to current ART prior to study entry | ||||
| >6 but ≤24 months | 8 (25.0) | 0 (0.0) | 8 (47.1) | 0.0029 |
| >24 months | 24 (75.0) | 15 (100) | 9 (52.9) | |
| CD4 T cell percent at preentry (%) | ||||
| n | 32 | 15 | 17 | 0.6915 |
| Mean | 33.60 | 34.26 | 33.02 | |
| CD4 T cell count at preentry (cells/μl) | ||||
| n | 32 | 15 | 17 | 0.1460 |
| Mean | 801.13 | 908.93 | 706.00 | |
| Std. Dev. | 328.20 | 408.16 | 205.95 |
Longitudinal viral load and CD4 T cell dynamics
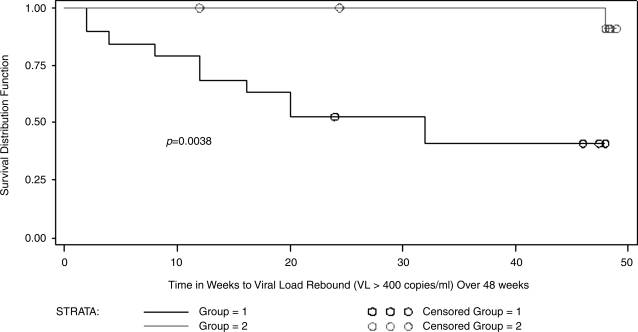
The primary outcome of the study was a confirmed VL above 400 c/ml. Of the 32 subjects enrolled, 12 (37.5%) had confirmed VL rebound with 18 subjects (56%) coming off for any reason. In the nonbehavioral group, viral rebound was confirmed in 7 of 15 compared with 5 of 17 in the behavioral group in intention to treat analysis (p = 0.467). One subject in the behavioral group had come off SCT for mental health issues before a confirmed VL rebound. Significantly more nonbehavioral subjects came off SCT for either VL rebound, a 30% drop in the CD4 count, or other reasons (pregnancy in two subjects) compared with behavioral subjects (12 of 15 vs. 6 of 17; p = 0.016). Among the 12 subjects with VL rebound, we evaluated rates of resuppression on continuous HAART utilizing the same agents. Overall, the rates were six of seven (85.7%) resuppressing in the nonbehavioral group compared with three of five (60%) in the behavioral group. No significant difference was found in the time to resuppression between the two groups. However, subjects with a CDC category C condition and/or nadir CD4 count <200 cells/mm3 had a significantly earlier time to VL rebound compared with those without either of these characteristics (Fig. 1).
FIG. 1.
Time to viral load rebound (>400 copies/ml) over 48 weeks by nadir CD4 count < 200 at preentry and/or CDC classification for HIV-related and AIDS-defining events of “C” (group 2) versus preentry nadir CD4 count : 200 and CDC classification of A or B (group 1).
Overall, there were no significant differences in the CD4 counts at the time of VL rebound, at the time coming off SCT, or at the end of the study when compared with baseline values. This held true when examined individually for each transmission group.
The unadjusted relationship of baseline characteristics to time of VL rebound was examined and two were associated with time to VL rebound by week 48: older age (HR, 0.85; CI, 0.73–0.98; p = 0.025) and racial background black/African American (HR, 0.21; CI, 0.06–0.77; p = 0.018). Gender, age at HIV transmission, CD4 at entry, adherence :90% or <90%, and use of a PI-boosted regimen were not found to be associated with time to VL rebound. CD4 count over time as a continuous variable was also found not to be associated with time to VL rebound (p = 0.294). Finally, the impact of VL blips was evaluated. Viral load blips were not found to be significantly associated with VL rebound at either 24 or 48 weeks (p = 0.147 and 0.452, respectively); this held true regardless of the number of blips reported.
Adherence
Adherence to SCT was evaluated during days on HAART and was overall good. The mean adherence for subjects with VL rebound was 95% compared with 96% for subjects without VL rebound (p = 0.893). When evaluated as a categorical variable (:90% vs. <90%), no significant difference was found between those with and those without VL rebound (p = 0.620).
Regimens and resistance genotyping
Of the 33 subjects, 23 were on a boosted PI regimen while 10 were on a nonboosted regimen. Of those on a nonboosted regimen, one was on nonboosted atazanavir while two were on ritonavir and seven were on nelfinavir. The following NRTIs were used: zidovudine (13), lamivudine (16), tenofovir (9), stavudine (7), didanosine (8), and emtricitabine (4). There was one subject also on T-20. Genotypic resistance was evaluated for all subjects with rebound VL of 1000 c/ml or greater (Table 2). No pretherapy genotypes were available for comparison as all subjects came into the study fully suppressed. Overall, the nonbehavioral group demonstrated greater numbers of resistance mutations when compared with the behavioral group. The majority of mutations occurred in reverse transcriptase and reflects both current regimens as well as mutations most likely related to previous HAART regimens.
Table 2.
Resistance Mutations in Subjects with Confirmed Virologic Rebound
| Transmission | Current ART | RT mutations | PI mutations |
|---|---|---|---|
| Behavioral | |||
| Subject 1 | Viral load not high enough | ||
| Subject 2 | FTC, TDF, ATVr | K103N, V108I, P237H | None |
| Subject 3 | DDI, TDF, ATVr | K103N | None |
| Subject 4 | AZT, 3TC, NLF | M184V | V77I |
| Subject 5 | AZT, DDI, LPVr | None | None |
| Nonbehavioral | |||
| Subject 1 | FTC, D4T, NLF | M41L, D63N, K70R, K103R, M184V, R211K, T215F, K218G | L63P |
| Subject 2 | 3TC, TDF, LPVr | M46L, M184V, T215V | L63P |
| Subject 3 | AZT, 3TC, RTV | L74V | None |
| Subject 4 | 3TC, D4T, NLF | M184V | L10V, K70R, M36I |
| Subject 5 | DDI, D4T, INDr | K70R, K219E, T215F, M184V, V118I/V | None |
| Subject 6 | DDI, D4T, LPVr | D67N, K70R, K219Q, M184V | A71T, D30N, K20N, L63P, M36I |
| Subject 7 | DDI, FTC, ENF, LPVr | M41L, T215IY, M184V | A71T, L63P, L90M, M46I |
Safety monitoring
Only one adverse event, worsening depression, was thought to be possibly related to SCT based on the potential impact of decreasing ART on the pharmacokinetics of SSRI therapy.
Discussion
We report here on a 48-week multicenter, prospective, proof-of-concept study designed to assess the impact of switching from continuous HAART with a protease inhibitor (PI) to short-cycle therapy (SCT) consisting of HAART 4 days/week (Monday through Thursday) and no ARV 3 days/week (Friday through Sunday). For our primary outcome of confirmed virologic breakthrough above 400 copies/ml, we found higher but not significant differences between subjects infected through risk behaviors after age 9 years compared with those infected through perinatal or early childhood transmission. However, those infected early in life had significantly greater risk of coming off SCT for any reason compared with those infected after age 9 years. Compared with previous studies of SCT, our findings are the first to examine this in adolescents and young adults. In one of the earliest pilot studies of SCT, Dybul and colleagues reported on 10 subjects on indinavir-based HAART who switched to a 7 on/7 off cycle. In their report, subjects maintained suppression from 32 to 68 weeks14 with no impact on CD4 T cell counts or proviral DNA. In a subsequent SCT study using both protease inhibitor and efavirenz-based HAART regimen, seven of eight subjects maintained viral suppression for 60 to 84 weeks.17 There were no notable increases in low-level viral replication for those for whom adequate samples were available.
The rates of viral rebound in our study were higher than those reported in either study by Dybul and colleagues.15,18 However, our rates were either lower or similar to those reported in other studies. In a randomized study of continuous versus CD4 guided versus SCT (1 week on/1 week off), those in the SCT arm had a virologic rebound rate of 46%.15,18 This rate is similar to that seen in the nonbehavioral infection group in our study but higher than that seen in the behavioral group. In a study of SCT utilizing a 5 on/2 off strategy, virologic suppression was maintained in 89.6% of subjects, including 100% on efavirenz-based HAART, which is slightly greater than that seen in our behaviorally infected group.15 A major difficulty in comparing rates of virologic failure in these different studies is differences in the definition of virologic failure. Not all studies have required confirmation and those that have do not have a standard time for doing so. We immediately confirmed all virologic breakthroughs. It has been shown that viral blips from transient activation can last longer than 5 to 7 days, which may explain our higher rate of virologic failure compared with some studies.19,20 Those studies not confirming virologic failure with second tests would be more likely to report higher levels of breakthrough based on either the precision of the test or on very transient increases in viral load.
The difference in rates of virologic rebound and coming off SCT for any reason between the two populations of subjects in our study deserves comment. Both populations met the same entry criteria. However, there are two important differences in the populations. First, those infected through perinatal or early childhood infection were infected significantly longer than those infected through behaviors. Second, those infected early in life had exposure to more antiretroviral agents and longer exposure to ART. Finally, in those subjects with documented VL breakthroughs, there did appear to be a greater number of resistance mutations in the nonbehavioral group. Thus, the two populations had some important differences at baseline that could have impacted on the outcome of SCT.
We found no impact of SCT on CD4+ T cells in either those with or without virologic rebound. A number of studies of SCT have also found no impact of SCT on CD4+ T cells.14,17,18,21 The impact of management strategies must assess the impact of the strategy on the immunologic health of the subject. When we evaluated CD4+ T cells in subjects who came off SCT for any reason, including a 30% or great drop in CD4+ T cells from preentry, we again found overall no significant changes. Most of our subjects resuppressed when placed back onto continuous therapy with the same HAART regimen. Thus, from our small number of subjects, there does not appear to be any deleterious effects of HAART on CD4+ T cell counts.
Adherence is a major predictor of virologic suppression in adolescents and young adults placed on HAART.3–5 Data from the REACH Project demonstrated that long-term adherence is a particular challenge for adolescents.22 We found very high rates of adherence in subjects on our study placed onto SCT. As our subjects had to have demonstrated high rates of viral suppression for 6 to 12 months before initiating SCT, we had a select population with demonstrated good adherence.
The majority of resistance mutations we found in our subjects with viral rebound above 1000 copies were in reverse transcriptase, with higher numbers in those infected early in life. As noted, we do not have pretherapy genotypes on subjects for comparison. As many of these resistance mutations are to agents not in the current regimen, it is likely that these mutations developed during previous regimens and were maintained in archived virus. The development of resistance was an issue in studies of structured treatment interruption.6,23,24 This was not consistent in all studies, however.25 The majority of subjects in our study with virologic rebound resuppressed on the same continuous HAART regimen. The variable half-lives of different PIs, both boosted and unboosted, as well as of various NRTIs, could have impacted viral suppression in our study. We excluded efavirenz from our study because of the absence of adult data on the use of this agent in short-cycle therapy at the time of study conception and also because of concerns about the use of efavirenz, with its low genetic barrier to resistance, in youths. More recent data in adults suggests that efavirenz may be an effective agent for this management strategy.16,26 In a select population of youth, agents with long half-lives such as NNRTIs, and potentially some of the newer NRTIs, may indeed show more promise for maintaining viral suppression in short-cycle therapy.
Our study has a number of important limitations. First, this was a proof-of-concept, nonrandomized trial. Thus, there are significant limitations to its conclusions compared with large, randomized trials. Second, subjects were on multiple PI-based HAART regimens including some nonboosted regimens. Finally, the evaluation of the emergence of resistance mutations was limited by the lack of pre-HAART genotypes. Studies are planned to evaluate resistance in archived virus to better evaluate the impact of SCT on resistance mutations in our subjects. Our study suggests that short-cycle therapy may be a viable management strategy for some adolescents. However, for subjects with long-standing HIV infection treated with multiple regimens, caution should be taken with this approach.
Acknowledgments
This work was supported by the Adolescent Trials Network for HIV/AIDS Interventions (ATN) from the National Institutes of Health (U01 HD 040533 and U01 HD 040474) through the National Institute of Child Health and Human Development (B. Kapogiannis, L. Serchuck), with supplemental funding from the National Institutes on Drug Abuse (N. Borek) and Mental Health (P. Brouwers, S. Allison). The study was scientifically reviewed by the ATN's Therapeutic Leadership Group. Network, scientific, and logistical support was provided by the ATN Coordinating Center (C. Wilson, C. Partlow) at the University of Alabama at Birmingham. Network operations and analytic support were provided by the ATN Data and Operations Center at Westat, Inc. (J. Korelitz, B. Driver). Additional support was provided by the Pediatric AIDS Clinical Trials Group funded by grant no. U01-A141089. The following GCRC/CTRC grants provided support: Children's National Medical Center (5-MO1-RR-020359-04) and the Children's Hospital of Philadelphia (UL1-RR-024134). The team would like to thank Kelly Lannutti for assistance with manuscript preparation.
Author Disclosure Statement
No competing financial interests exist.
ATN Sites Participating in This Study
Children's Hospital National Medical Center (D'Angelo, Trexler, Crane), Children's Hospital of Philadelphia (Rudy, Tanney, DiBenedetto), John H. Stroger Jr. Hospital and the CORE Center (Martinez, Bojan, Jackson, Henry-Reid), University of Puerto Rico (Febo, Ayala, Flores, Santos), Montefiore Medical Center (Futterman, Enriquez-Bruce, Campos, Myerson), Mount Sinai Medical Center (Levin-Carmine, Geiger, Lee), Tulane University Health Sciences Center (Abdalian, Kozina, Baker), University of Miami School of Medicine (Friedman, Maturo, Major-Wilson), Children's Diagnostic and Treatment Center (Puga, Leonard, Eysallanne, Inman). The following PACTG sites participated in the study: St. Jude's Children's Research Hospital (Flynn, Patel, Utech, Donohoe), Children's Memorial Hospital (Yogev, Heald, Kershaw), and University of New York, Syracuse (Weiner, Holz, Contello, Famiglietti).
References
- 1.Pallela FJ. Delaney KM. Moorman AC, et al. Declining morbidity, mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Ross D, editor; Dick B, editor; Ferguson J, editor. World Health Organization: Preventing HIV/AIDS in young people: a systematic review of the evidence from developing countries. WHO Technical Report Series. World Health Organization; Geneva: 2006. [PubMed] [Google Scholar]
- 3.Flynn P. Rudy B. Douglas S, et al. Virologic and immunologic outcomes after 24 weeks in HIV type 1-infected adolescents receiving highly active antiretroviral therapy. J Infect Dis. 2004;190:271–279. doi: 10.1086/421521. [DOI] [PubMed] [Google Scholar]
- 4.Rudy B. Lindsey J. Flynn P, et al. Immune reconstitution and predictors of virologic failure in adolescents infected through risk behaviors and initiating HAART: Week 60 results from the PACTG 381 cohort. AIDS Res Hum Retroviruses. 2006;22:213–221. doi: 10.1089/aid.2006.22.213. [DOI] [PubMed] [Google Scholar]
- 5.Flynn P. Rudy B. Lindsey J, et al. Long-term observation of adolescents initiating HAART therapy: Three-year follow-up. AIDS Res Hum Retroviruses. 2007;22:213–221. doi: 10.1089/aid.2006.0290. [DOI] [PubMed] [Google Scholar]
- 6.Pai NP. Tulsky JP. Lawrence J. Colford JM., Jr Reingold AL. Structured treatment interruptions (STI) in chronic suppressed HIV infection in adults. Cochrane Database Syst Rev. 2005;4:CD005482. doi: 10.1002/14651858.CD005482. [DOI] [PubMed] [Google Scholar]
- 7.Pai NP. Lawrence J. Reingold AL. Tulsky JP. Structured treatment interruptions (STI) in chronic unsuppressed HIV infection in adults. Cochrane Database Syst Rev. 2006;3:CD006148. doi: 10.1002/14651858.CD006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Sadr WM. Lundgren JD. Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence J. Hullsiek KH. Thackeray LM, et al. Disadvantages of structured treatment interruption persist in patients with multidrug-resistant HIV-1: Final results of the CPCRA 064 study. J Acquir Immune Defic Syndr. 2006;43:169–178. doi: 10.1097/01.qai.0000242450.74779.ee. [DOI] [PubMed] [Google Scholar]
- 10.Papasavvas E. Kostman JR. Mounzer K, et al. Randomized, controlled trial of therapy interruption in chronic HIV-1 infection. PLoS Med. 2004;1:e64. doi: 10.1371/journal.pmed.0010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danel C. Moh R. Minga A, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in West Africa (Trivacan ANRS 1269 trial): A randomised trial. Lancet. 2006;367:1981–1989. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence J. Mayers DL. Hullsiek KH, et al. Structured treatment interruption in patients with multidrug-resistant human immunodeficiency virus. N Engl J Med. 2003;349:837–846. doi: 10.1056/NEJMoa035103. [DOI] [PubMed] [Google Scholar]
- 13.Walmsley SL. Thorne A. Loutfy MR, et al. A prospective randomized controlled trial of structured treatment interruption in HIV-infected patients failing highly active antiretroviral therapy (Canadian HIV Trials Network Study 164) J Acquir Immune Defic Syndr. 2007;45:418–425. doi: 10.1097/QAI.0b013e318061b611. [DOI] [PubMed] [Google Scholar]
- 14.Dybul M. Chun TW. Yoder C, et al. Short-cycle structured intermittent treatment of chronic HIV infection with highly active antiretroviral therapy: Effects on virologic, immunologic, and toxicity parameters. Proc Natl Acad Sci USA. 2001;98:15161–15166. doi: 10.1073/pnas.261568398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ananworanich J. Siangphoe U. Hill A, et al. Highly active antiretroviral therapy (HAART) retreatment in patients on CD4-guided therapy achieved similar virologic suppression compared with patients on continuous HAART: The HIV Netherlands Australia Thailand Research Collaboration 001.4 study. J Acquir Immune Defic Syndr. 2005;39:523–529. [PubMed] [Google Scholar]
- 16.Cohen CJ. Colson AE. Sheble-Hall AG. McLaughlin KA. Morse GD. Pilot study of a novel short-cycle antiretroviral treatment interruption strategy: 48-week results of the five-days-on, two-days-off (FOTO) study. HIV Clin Trials. 2007;8:19–23. doi: 10.1310/hct0801-19. [DOI] [PubMed] [Google Scholar]
- 17.Dybul M. Nies-Kraske E. Dewar R, et al. A proof-of-concept study of short-cycle intermittent antiretroviral therapy with a once-daily regimen of didanosine, lamivudine, and efavirenz for the treatment of chronic HIV infection. J Infect Dis. 2004;189:1974–1982. doi: 10.1086/386344. [DOI] [PubMed] [Google Scholar]
- 18.Cardiello PG. Hassink E. Ananworanich J, et al. A prospective, randomized trial of structured treatment interruption for patients with chronic HIV type 1 infection. Clin Infect Dis. 2005;40:594–600. doi: 10.1086/427695. [DOI] [PubMed] [Google Scholar]
- 19.Staprans S. Hamilton B. Follansbee S, et al. Activation of virus replication after vaccination of HIV-1-infected individuals. J Exp Med. 1995;182:1727–1737. doi: 10.1084/jem.182.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nettles R. Kieffer T. Kwon P, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293:817–829. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 21.Casseb J. Da Silva Duarte AJ. Structured intermittent therapy with seven-day cycles of HAART for chronic HIV infection: A pilot study in São Paulo, Brazil. AIDS Patient Care STDS. 2005;19:425–428. doi: 10.1089/apc.2005.19.425. [DOI] [PubMed] [Google Scholar]
- 22.Murphy D. Belzer M. Durako S, et al. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2005;159:764–770. doi: 10.1001/archpedi.159.8.764. [DOI] [PubMed] [Google Scholar]
- 23.Arnedo-Valero M. Garcia F. Gil C, et al. Risk of selecting de novo drug-resistance mutations during structured treatment interruptions in patients with chronic HIV infection. Clin Infect Dis. 2005;41:883–890. doi: 10.1086/432881. [DOI] [PubMed] [Google Scholar]
- 24.Wang YM. Dyer WB. Workman C, et al. Drug resistance and viral evolution in plasma and peripheral blood cells during structured treatment interruption (STI) and non-interrupted HAART. Curr HIV Res. 2007;5:235–250. doi: 10.2174/157016207780077039. [DOI] [PubMed] [Google Scholar]
- 25.Nuesch R. Ananworanich J. Sirivichayakul S, et al. Development of HIV with drug resistance after CD4 cell count-guided structured treatment interruptions in patients treated with highly active antiretroviral therapy after dual-nucleoside analogue treatment. Clin Infect Dis. 2005;40:728–734. doi: 10.1086/427878. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds SJ. Kityo C. Ssali F, et al. A randomized, controlled, non-inferiority trial of two short cycle intermittent regimens compared with continuous antiretroviral therapy for the treatment of chronic HIV infection in Uganda. ICCAA/IDSA. 2008 doi: 10.1371/journal.pone.0010307. abstract H-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]