Abstract
Resistance to chemotherapy is a major problem facing breast cancer patients. Cisplatin, a highly effective DNA-damaging drug, has shown only little success in breast cancer treatment. We are reporting that low nanomolar doses of bisphenol A (BPA) or estradiol antagonize cisplatin cytotoxicity in breast cancer cells, with their effects not mediated via classical estrogen receptors. Although both compounds increase the expression of Bcl-2, a Bcl-2 inhibitor completely blocked the protective effects of BPA while only partially affecting those of estradiol. Blockade of BPA and E2 actions should sensitize ER-negative breast tumors to anti-cancer drugs and allow for the inclusion of cisplatin in treatment regimens.
Keywords: breast cancer cells, cisplatin, bisphenol A, estradiol, estrogen receptors, Bcl-2
1. Introduction
Chemotherapy is the mainstay treatment for breast cancer patients with advanced or metastatic disease. However, many patients exhibit resistance to chemotherapy that is either inherent or acquired during treatment. Cisplatin is a prototypical DNA-damaging agent which forms adducts and intra-strand crosslinks in DNA, leading to cell cycle arrest and cell death [1]. Although cisplatin is highly effective against many types of cancer, it has shown little success in the treatment of breast cancer [2]. Resistance to chemotherapy has been attributed to many mechanisms, including efflux by transporters, inactivation by detoxification enzymes, or increased DNA-repair [3;4]. In addition, alterations in pro/anti-apoptotic proteins play a role in chemoresistance. Central players in the apoptotic pathways include the anti-apoptotic proteins Bcl-2 and Bcl-xL and the pro-apoptotic protein Bax which regulate apoptosis at the level of the mitochondria [5]. A balance between pro-apoptotic and survival proteins determines the fate of the cell in response to many drugs.
Much evidence supports a role for estradiol (E2) in breast cancer initiation and progression [6;7]. Consequently, anti-estrogen therapy is widely used in the treatment of patients with ER+ tumors [8]. The effects of E2 are generally attributed to its binding to the classical estrogen receptors, ERα and ERβ, or to G-protein coupled receptor 30 (GPR30) [9;10]. In addition to its role as a mitogen, E2 also acts as survival factor. For example, in immune deficient mice, xenografts derived from ER+ MCF-7 cells decrease in size following E2 removal [11]. Under in vitro conditions, MCF-7 cells undergo apoptosis in the absence of serum growth factors, an effect attributed to the ability of E2 to stimulate Myc expression [12]. E2 antagonizes taxol- and doxorubicin-induced cytotoxicity in breast cancer cells [13–15], but it is unknown whether it also decreases the responsiveness of breast cancer cells to cisplatin.
Bisphenol A (BPA) is a monomer of polycarbonate plastics used in many consumer products, including water and baby bottles, dental fillings and the lining of metal food cans [16]. Small amounts of BPA can be liberated from incompletely polymerized polycarbonates or via partial hydrolysis, especially upon heating [17]. Early exposure of rodents to BPA caused increased susceptibility to both mammary and prostate tumorigenesis [18;19]. BPA at 0.2–5 ng/ml has been detected in serum of most adults examined in the USA, Europe and Japan [16]. The effects of BPA on breast cancer cells have generated conflicting results, largely due to the micromolar concentrations of BPA utilized by most studies [20–23]. The mechanism by which BPA exerts its biological actions is unclear, given that its binding affinity to ERα and ERβ is significantly lower than that of E2 [24]. However, there is evidence that BPA also binds to non-classical ERs, such as GPR30 or members of the estrogen related receptors (ERR) family [25;26]. We recently reported that BPA at low nanomolar concentrations antagonized the cytotoxic effects of doxorubicin in breast cancer cells [27]. In the present investigation, we expanded on these findings by comparing the effects of BPA with those of E2 on a molar basis and by focusing on the mechanism by which either compound antagonizes cisplatin cytotoxicity.
The specific objectives were to: 1) compare the effects of low doses of BPA and E2 on cisplatin-induced alterations in cell viability, proliferation and apoptosis in T47D breast cancer cells, 2) determine the effects of an ERα antagonist (ICI) and an ERβ-specific antagonist (PHTPP) on the ability of BPA or E2 to protect cells from cisplatin cytotoxicity, 3) examine the protective effects of these compounds in ERα- negative MDA-MB-468 cells and ERβ-knockdown T47D cells, and 3) determine whether antagonism of cisplatin cytotoxicity by these compounds involves the pro/anti-apoptotic proteins.
2. Materials & Methods
2.1. Drug and Inhibitors
Cisplatin (Sigma, St Louis, MO) was dissolved in water. ICI182780 (Tocris Bioscience, Ellisville, MO) and PHTPP (Tocris) were dissolved in DMSO or ethanol, respectively. HA14-1 (Biomol, Plymouth Meeting, PA), a Bcl-2 antagonist, was dissolved in ethanol. Drugs and inhibitors were diluted in culture medium immediately before treatment.
2.2. Cell lines and culture conditions
T47D and MDA-MB-468 cells were obtained from the American Type Culture Collection (Manassas, VA). T47D cells were maintained in RPMI (Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (FBS; Hyclone), 5µg/ml bovine insulin, 10 mM HEPES, 1 mM sodium pyruvate and 50 µg/ml normocin (Invivogen, San Diego, CA). MDA-MB-468 cells were cultured in low glucose DMEM (Hyclone) supplemented with 10% FBS and 50 µg/ml normocin. For all experiments, T47D cells were plated in phenol red-free RPMI with 5% charcoal stripped serum (CSS) and ITS+ supplement (1:200; BD biosciences, Bedford, MA) and were treated in RPMI with 2% CSS and ITS+. MDA-MB-468 cells were plated in phenol red-free DMEM supplemented with 3% CSS and treated in DMEM with 1% CSS.
2.3. Cytotoxicity assay
Cells were plated at a density of 6000 or 8000 cells/well in 96 well plates in plating medium. The following day, cells were incubated with BPA or E2 for 24 hrs in treatment medium. Inhibitors, ICI, PHTPP or HA14-1, were added 1 hr before BPA or E2. After 24 hrs, cisplatin was added for an additional 1 to 3 days. Cytotoxicity was determined by the 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl tetrazolium bromide (MTT) method. MTT was added at a final concentration of 0.5 mg/ml for 2 hrs. Following medium aspiration, the formazan dye was extracted with DMSO and absorbance was read at 570 nm using a plate reader (Bio-Tek, Winooski, VT).
2.4. BrdU incorporation
BrdU analysis was done using a cell proliferation ELISA kit (Roche, Indianapolis, IN) according to instructions. Briefly, cells were incubated with BrdU for 8 hrs, fixed and then incubated with an anti-BrdU antibody. Absorbance was read after addition of TMB substrate.
2.5. Flow cytometry
Cells were washed and re-suspended in buffer containing 5µl APC Annexin V (BD Pharmingen, San Jose, CA) and 1 µg/ml propodium iodide (PI). Samples were analyzed by flow cytometry at 488 nm and 633 nm using a BD LSRII (Becton Dickinson, San Jose, CA). Log fluorescence was collected for PI and Annexin IV using a 610/20 or 660/20 band pass filter, respectively. Ten thousand gated events per experiment were collected.
2.6. ERβ knockdown by siRNA
T47D cells were plated at a density of 200,000 cells/dish 24 hrs before transfection with siRNA targeted against ERβ and scrambled siRNA as a control (Dharmacon, Lafayette, CO). Both were transfected at 200 nM using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), according to manufacturer’s protocol. Cells were harvested 4 and 8 days after transfection for analysis of ERβ expression by RT-PCR.
2.7. RT-PCR
Total RNA was isolated using TRI Reagent (Molecular Research Center, Cincinnati, OH), and cDNA was synthesized as described [28]. PCR was performed on cDNA, using intron-spanning primers for ERβ: forward 5’-TGAAAAGGAAGGTTAGTGGGAACC-3’, reverse 5’-TGGTCAGGGACATCATCATGG-3’. GAPDH was used as a reference gene: forward 5’-CCACCCATGGCAAATTCCATGGCA-3’, reverse 5’-TCTAGACGGCAGGTCAGGTCCACC-3’. The cycle parameters were 30 seconds at 95°, 45 seconds at 60° and 45 seconds at 72° for 24 cycles (GAPDH) or 34 cycles (ERβ). Products were resolved on a 2% agarose gel containing ethidium bromide and photographed.
2.8. Platinum Determination by mass spectroscopy
DNA was isolated using the DNeasy tissue kit (Qiagen, Valencia, CA). Platinum content was determined with an Aligent 7500ce (Aligent Technologies, Santa Clara, CA). Platinum standards (SpexCertiPrep, Metuchen, NJ) were introduced at a flow rate of 1 ml/min using a 100µl injection volume. Instrument parameters were as follows: forward power of 1450 W; plasma gas flow rate of 15.0 L/min, auxiliary gas flow rate of 1.0 L/min, carrier gas flow rate of 0.97 L/min, makeup gas flow rate of 0.14 L/min, octopole bias of −18 V, quadrupole bias of −16 V and 194Pt and 195Pt monitored isotopes.
2.9. Western Blotting
Cells were homogenized in buffer (10nM Tris-HCl, 5mM EDTA, 50nM NaCl, 50mM sodium fluoride, 30mM sodium pyrophosphate, 1% Triton-X, 200µM sodium orthovanadate, 1mM phenylmethylsulfonyl, 1µg/ml pepstatin, µg/ml leupeptin, 5µg/ml aprotinin). Protein concentrations were determined by the Pierce BCA protein assay. Cell lysates (40µg proteins) were electrophoresed on 12% or 15% SDS-PAGE gels. After transfer to PVDF membranes, samples were blocked in 5% dry milk and incubated overnight with the following primary antibodies: Bcl-2, Bcl-xL, Bax (1:1000 each; Cell Signaling, Danvers, MA) or β-actin (1:10,000; Sigma). After incubation with horseradish peroxidase-conjugated secondary antibody (Amersham, Piscataway, NJ), products were developed on film using SuperSignal chemiluminescence reagents (Pierce, Rockford, IL).
2.10. Data Analysis
Statistical differences were determined by one-way ANOVA followed by Newman-Keuls post hoc analysis. P values <0.05 were considered significant. All experiments were done at least three times.
3. Results
3.1. BPA and E2 antagonize cisplatin-induced cytotoxicity
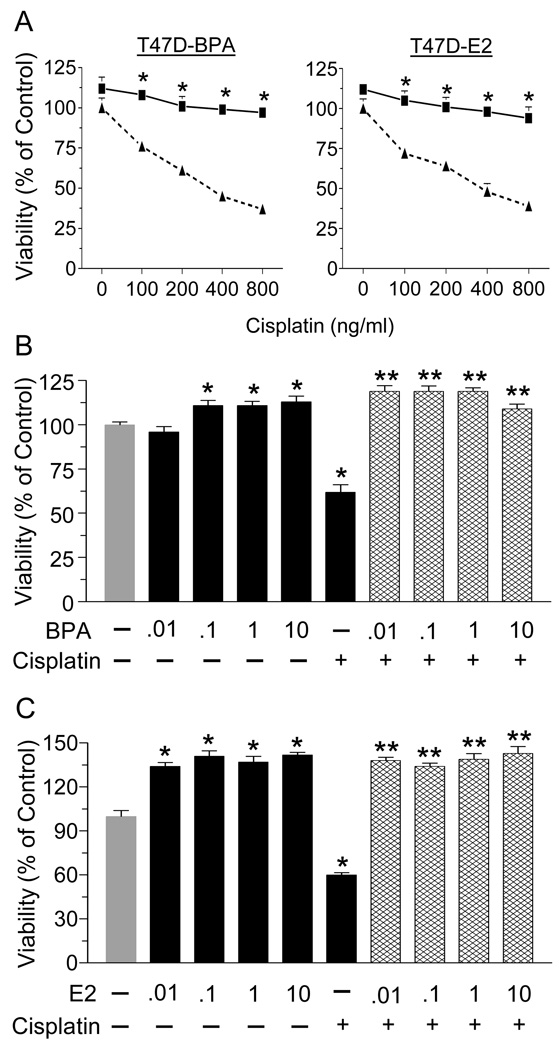
We first examined the sensitivity of the ER-positive T47D cells to increasing concentrations of cisplatin and determined protection from cytotoxicity by BPA or E2. As shown in Fig. 1A, cisplatin induced a dose-dependent decrease in cell viability, with the highest dose reducing survival by 60%. Pre-treatment with 1 nM of BPA (left panel) or E2 (right panel) completely antagonized cisplatin cytotoxicity. We next examined the effect of environmentally or physiologically relevant doses of BPA or E2, respectively, against 800 ng/ml of cisplatin. All doses (0.01–10nM) of BPA (Fig. 1B) or E2 (Fig. 1C) prevented cisplatin-induced cytotoxicity.
Fig. 1.
BPA and E2 protect T47D cells from cisplatin cytotoxicity. (A) Cells were treated with 1 nM BPA or E2 for 24 hrs, followed by incubation with increasing concentrations of cisplatin (0–800 ng/ml) for 72 hrs. (B) T47D cells were treated with increasing concentrations of BPA (0–10nM) for 24 hrs prior treatment with 800 ng/ml cisplatin for 24 hrs. (C) T47D cells were treated with increasing concentrations of E2 (0–10nM) for 24 hrs before treatment with 800 ng/ml cisplatin for 24 hrs. In all cases, cell viability was determined by the MTT assay. Each value is a mean±SEM of six replicates. In (A) * significant (p< 0.05) vs cisplatin alone. In (B&C) * significant vs control, and ** significant vs cisplatin.
3.2. BPA and E2 oppose the anti-proliferative and pro-apoptotic effects of cisplatin
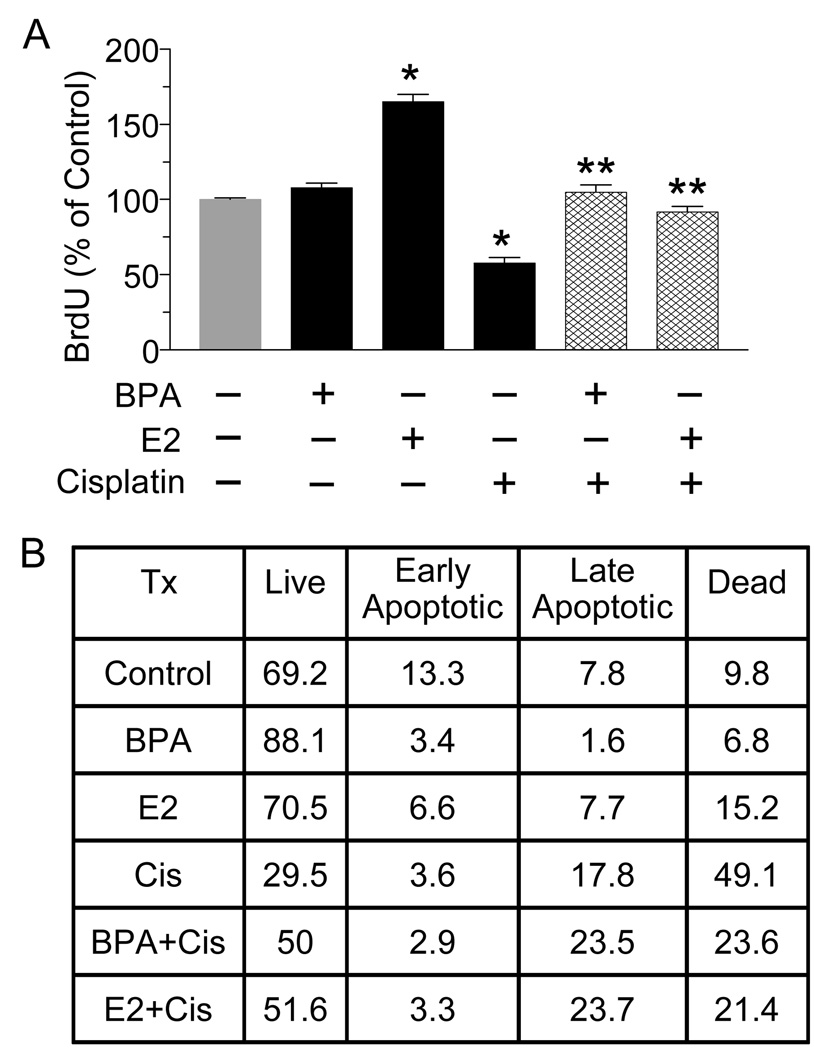
The next experiments examined the effects of these compounds on cisplatin-induced alterations in cell proliferation and apoptosis. As determined by BrdU incorporation, cisplatin caused a 50% decrease in cell proliferation (Fig. 2A), an effect which was completely abrogated by pre-treatment with 10nM of BPA or E2. Notably, a 50% increase in cell proliferation is observed with E2 alone but not with BPA alone. Apoptosis was then analyzed by flow cytometry following Annexin/PI staining (Fig. 2B). Exposure to BPA alone increased the percent of live cells from 69.2% to 88.1%, while E2 alone had no effect. A 24 hr exposure to cisplatin resulted in a 40% decrease in the percentage of live cells, which was accompanied by an increase in the number of cells that were either in late apoptosis/necrosis or dead. Both BPA and E2 partially antagonized cisplatin-induced apoptosis, as evident by fewer dead cells following combination treatments as compared to cisplatin alone.
Fig. 2.
BPA and E2 protect T47D cells from cisplatin-induced decreases in cell proliferation and increases in apoptosis. (A) T47D cells were treated with 10 nM BPA or E2 for 24 hrs followed by cisplatin (800 ng/ml) for 72 hrs. Cells were fixed and incubated with a BrdU antibody for 8 hrs. After adding substrate, the product was quantified by measuring absorbance. Each value is a mean±SEM of four replicates. * significant (p< 0.05) vs control. ** significant vs cisplatin. (B) T47D cells were treated as in (A), stained with Annexin V and propidium iodide (PI), and analyzed by flow cytommetry. The table depicts the percentage of cells in each treatment group that are alive (no stain), in early apoptosis (Annexin V-positive), late apoptosis/necrosis (Annexin V+PI-positive) or dead (PI- positive). Shown is a representative experiment repeated 3 times
3.3. The protective effects of BPA and E2 do not require classical ERs
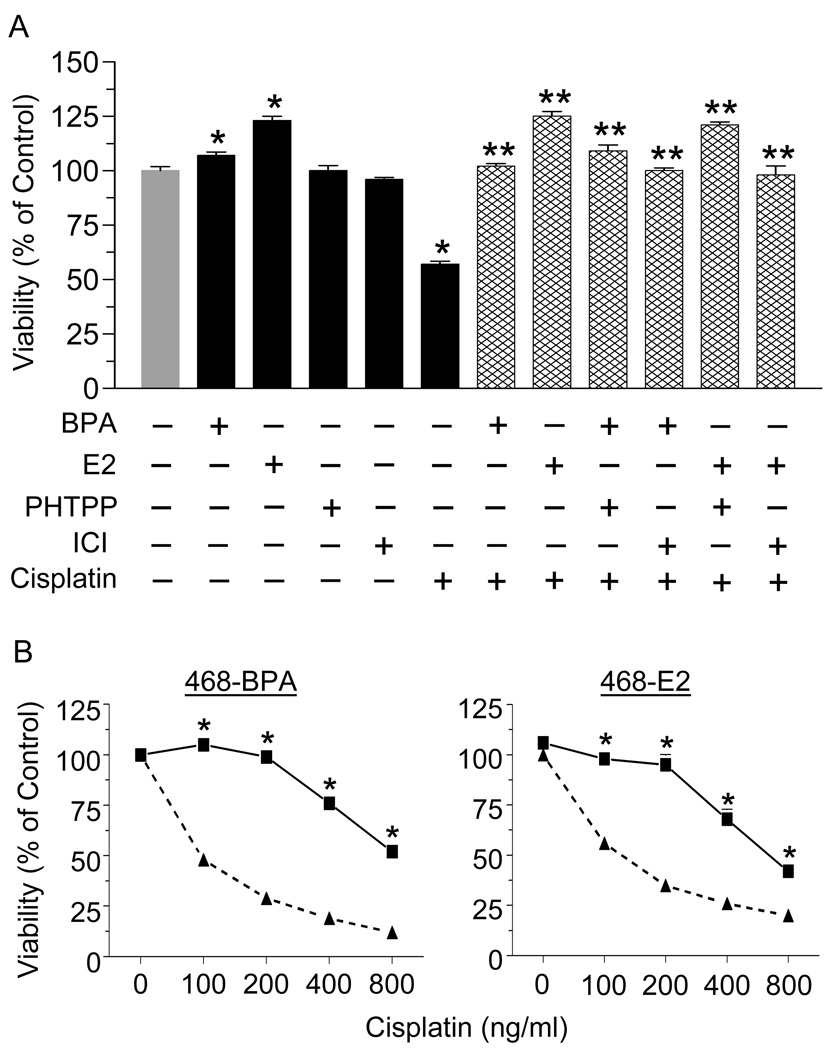
To determine if the protective effects of BPA or E2 involve ERα or ERβ, we used ICI, an antagonist of both receptors and PHTPP, a specific ERβ antagonist (Fig. 3A). BPA or E2 alone increased cell viability, ICI or PHTPP had no effect on their own, while cisplatin treatment caused a 40% decrease in cell viability. The ability of BPA or E2 to antagonize cisplatin-induced cytotoxicity was not altered by ICI or PHTPP. To further confirm that BPA and E2 act independent of ERα, we used the ERα-negative MDA-MB-468 cells. Fig. 3B shows that these cells are highly sensitive to cisplatin, with the highest dose of the drug inhibiting cell viability by more than 80%. Importantly, BPA or E2 completely, or partially, protected the cells from all doses of cisplatin in these ERα-negative cells.
Fig. 3.
BPA and E2 mediate their protective effects independent of classical estrogen receptors. (A) T47D cells were treated with 100 nM ICI or PHTPP for 1 hr before incubation with 10 nM BPA or E2 for 24 hrs. Cells were then exposed to 800 ng/ml cisplatin for 24 hrs and cell viability was determined by the MTT assay. Each value is a mean±SEM of six replicates. * significant (p <0.05) vs control, and ** significant vs cisplatin. (B) MDA-MB-468 cells were treated with 10 nM of BPA or E2 for 24 hrs followed by increasing doses of cisplatin for 24 hrs. Cytotoxicity was determined by the MTT assay. Each value is a mean±SEM of six replicates. * significant vs corresponding doses of cisplatin alone.
3.4. BPA and E2 antagonize cisplatin in the absence of ERβ
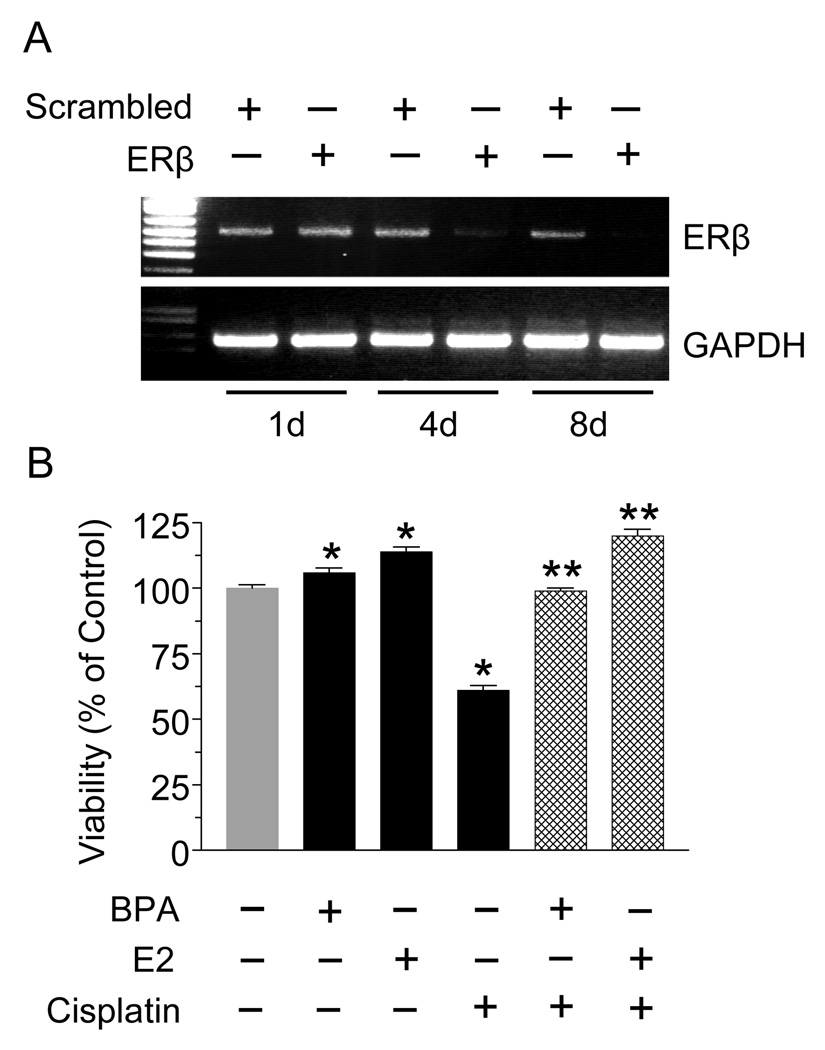
Next we used siRNA to downregulate ERβ expression in T47D cells. As evident by PCR, ERβ expression was barely detectable 4 days after siRNA transfection and was undetectable after 8 days (Fig. 4A). The effects of BPA and E2 against cisplatin were then examined in the ERβ-null T47D cells. To ensure complete knockdown of ERβ, the experiment was performed on days 5–7 after transfection. Fig. 4B clearly shows that both BPA and E2 conferred protection against cisplatin in the absence of ERβ.
Fig. 4.
BPA and E2 antagonize cisplatin in ERβ-knockdown T47D cells. (A) Cells were transfected with siRNA targeted towards ERβ or with scrambled siRNA. On days (d) 1, 4 and 8 post-transfection, ERβ expression was determined by RT-PCR; GAPDH was used as a control. (B) ERβ knockdown cells were incubated with 10 nM BPA or E2 for 24 hrs, followed by 800 ng/ml cisplatin for 24 hrs. Cytotoxicity was determined by the MTT assay. Each value is a mean±SEM of six replicates. * significant (p <0.05) vs control and ** significant vs cisplatin.
3.5. BPA likely promotes chemoresistance by increasing Bcl-2 expression
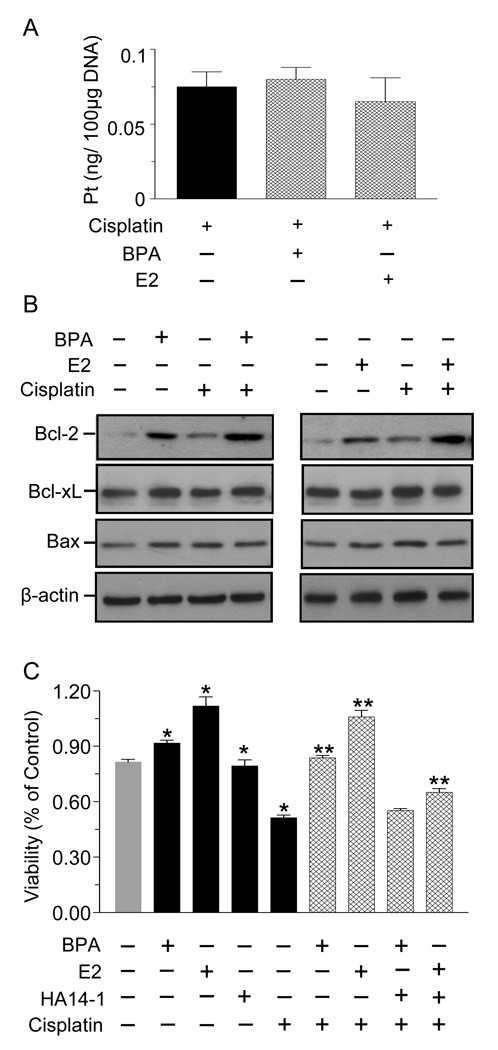
We next explored potential mechanisms by which BPA and E2 antagonize cisplatin. Since the primary target of cisplatin is DNA, we first used mass spectroscopy to examine if BPA or E2 prevent cisplatin from binding to DNA. Fig. 5A shows that the amount of cisplatin bound to DNA was not altered by treatment with E2 or BPA. The next experiment examined whether cisplatin, E2 and BPA, or their combinations alter the expression of pro- and anti-apoptotic proteins. Fig. 5B reveals a marked increase in Bcl-2 expression following 24 hr treatment with BPA or E2 in the presence and absence of cisplatin. Bcl-xL or Bax expression was unchanged regardless of treatment. Notably, Fig. 5C shows that HA14-1, a Bcl-2 inhibitor, completely abolished the protective effects of BPA, while that of E2 was only partially blocked.
Fig. 5.
BPA and E2 may confer chemoresistance by increasing Bcl-2 expression. (A) T47D cells were treated with 10 nM BPA or E2 for 24 hrs, followed by 800 ng/ml cisplatin for 24 hrs. DNA was isolated and platinum content was analyzed by inductively coupled plasma mass spectroscopy. (B) Cells were treated as in (A). Western blots were probed for Bcl-2, Bcl-xL and Bax; β-actin serves as a loading control. Shown are representative blots, repeated at least three times. (C) Cells were pre-treated for 1 hr with 10 µM HA14-1 and then with 10 nM BPA or E2 for 24 hrs. This was followed by exposure to 800 ng/ml cisplatin for 24 hrs. Cytotoxicity was determined by the MTT assay. Each value is a mean±SEM of six replicates. * significant (p < 0.05) vs control, and ** significant vs cisplatin.
4. Discussion
We are reporting that both estradiol and the endocrine disruptor BPA at very low concentrations confer resistance against cisplatin in breast cancer cells. Both compounds antagonize cisplatin cytotoxicity by increasing cell proliferation and viability and decreasing apoptosis. As judged by the use of ERα and ERβ specific antagonists, ERα-negative MDA-MB-468 cells, and ERβ-knockdown T47D cells, BPA and E2 do not appear to mediate their effects through the classical ERs. Whereas BPA and E2 do not affect the entry of cisplatin into the nucleus, they increase the expression of Bcl-2, suggesting that their action is exerted downstream of cisplatin binding to DNA. The fact that a Bcl-2 inhibitor completely blocked the protective effect of BPA while only partially antagonizing that of E2 suggests a somewhat different mechanism by which each compound confer chemoresistance.
Although the role of hormones in chemoresistance has not received considerable attention, several reports have shown that E2 opposes anti-cancer drugs in breast cancer cells. Based on viability and apoptotic endpoints, E2 at 1–100 nM protected cells from toxicity by taxol, a microtubule altering drug that is widely used in breast cancer treatment [13;15;29]. Additionally, Teixeira et al reported that MCF-7 breast cancer cells were less sensitive to doxorubicin when the medium was supplemented with estrogen [14]. We are the first to report that the survival effect of E2 extends to cisplatin, and that such protection is observed with as little as 0.01 nM E2. In spite of the growing health concerns attributed to endocrine disruptors, little has been done to address their potential role in chemoresistance. We recently reported that BPA opposed the actions of several anti-cancer drugs with a focus on doxorubicin [27]. We now extended these studies to cisplatin by finding that BPA is just as effective as E2 at antagonizing the drug. Importantly, BPA is effective at environmentally relevant concentrations of 0.01–10 nM.
Estradiol is well known as a mitogen for normal breast epithelial cells as well as breast cancer cells [30], while BPA activity as a mitogen has been controversial. Although BPA increased MCF-7 cell proliferation, its relative mitogenic activity was 60,000 times lower than that of E2 [31]. A confounding issue is the lack of linear dose-dependence of BPA, which often shows a ‘U’ or an inverted ‘U’ shaped curves [32]. Thus, extrapolation from an action, or a lack of action, of BPA at high doses to its presumed bioactivity at low doses is unwarranted. Indeed, Sameuelson et al. reported maximal MCF-7 cell proliferation in response to 1 µM BPA, an effect which was not observed at higher or lower doses [33]. For these reasons, our studies utilized only low doses of BPA. The finding that BPA and E2 opposed cisplatin cytotoxicity by increasing cell viability could be due to increased cell proliferation, decreased apoptosis, or both. As judged by both BrdU incorporation and flow cytometry, E2 alone increases cell proliferation, while BPA alone increases survival. This suggests that in antagonizing cisplatin, E2 may act primarily as a mitogen while BPA may act more as an anti-apoptotic factor.
Our next goal was to determine which receptor(s) mediate the protective effects of E2 and BPA. ERα and ERβ, often referred to as classic ERs, are well characterized in terms of their ability to transduce the actions of E2. The relative binding affinity of BPA to either receptor is 1,000 to 10,000 times lower than that of E2, suggesting that BPA would have to be at the µM range to activate these receptors [24]. Our studies clearly show that BPA and E2 exert their effects when ERα or ERβ were blocked by specific inhibitors. This was supported by the use of MDA-MB-468 cells, which express ERβ but not ERα [27], and ERβ-depleted T47D cells. Our results do not agree with Sui et al reporting that estradiol significantly reduced taxol cytotoxicity in breast cancer cells overexpressing ERα but has no effect on the ER-negative parental cells. Their study showed that ICI sensitized MCF-7 and T47D cells to taxol [29].
There are several non-classical ERs through which BPA and E2 can signal, including yet unidentified membrane-associated estrogen binding proteins, GPR30 and members of the ERR family. For example, E2 rapidly stimulates ERK1/2 phosphorylation in MCF-7 cells via GPR30 [34]. BPA binds to GPR30 with an IC50 of 630 nM, as compared to 17.8 nM for E2 [25]. BPA binds strongly to ERRγ with a Kd of 5.5 nM, while E2 presumably does not bind to this receptor [26]. ERRγ is expressed in 75% of breast tumors as compared to normal mammary epithelial cells [35]. We have previously shown by Real-Time PCR that both T47D and MDA-MB-468 cells express several of the non-classical receptors [27]. We postulate that non-classical ERs, or as yet an unidentified receptor, mediate the protective effects of these compounds. This issue is currently under investigation.
One of the mechanisms underlying resistance to chemotherapeutic agents is by conjugation of electrophilic drugs such as cisplatin to glutathione, thereby facilitating their extrusion from the cell and reducing their availability to enter the nucleus [36]. The use of inductively coupled mass spectroscopy which can measure platinum reveals that the amount of cisplatin bound to DNA was not affected by either E2 or BPA, suggesting that their protective actions occur downstream of DNA damage rather than by regulating detoxification enzymes or membrane transporters.
Teixeira et al reported that an E2-induced increase in Bcl-2 plays a role in antagonizing doxorubicin in MCF-7 cells [14]. This is supported by another study underscoring the importance of increased Bcl-2 to Bax ratio for protecting MCF-7 cells against taxol-mediated cytotoxicity [13]. Our data demonstrate that BPA and E2 alone or in combination with cisplatin dramatically increased Bcl-2 expression without altering Bcl-xL or Bax. The effects of the Bcl-2 inhibitor, which completely prevented BPA actions while only partially affecting E2, further supports the notion that the two compounds may activate somewhat different mechanisms, i.e., proliferation vs anti-apoptosis, in opposing cisplatin cytotoxicity.
In conclusion, we have demonstrated that BPA and E2 confer resistance against cisplatin, likely through their ability to increase Bcl-2 expression, thereby preventing drug-induced apoptosis. Since their actions appear to be independent of ERα and ERβ, patients with ER-negative tumors may show high resistance to cisplatin because of their endogenous estrogens. These studies also highlight an harmful effect of the endocrine disruptor BPA. Blockade of BPA and E2 actions should be beneficial for breast cancer patients by increasing the sensitivity of their tumors to anti-cancer drugs and allowing for the introduction of cisplatin into treatment regimens.
Acknowledgements
This work was supported by NIH grants ES012212 and CA096613, Department of Defense grant BC05725 and Susan G. Komen Breast Cancer Foundation grant BCRT87406 (NBJ), and NIH training grant 5T32ES007250 (to EWL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors have nothing to declare.
References
- 1.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 2.Decatris MP, Sundar S, O'byrne KJ. Platinum-based chemotherapy in metastatic breast cancer: current status. Cancer Treat. Rev. 2004;30:53–81. doi: 10.1016/S0305-7372(03)00139-7. [DOI] [PubMed] [Google Scholar]
- 3.Coley HM. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat. Rev. 2008;34:378–390. doi: 10.1016/j.ctrv.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat. Rev. Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 5.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 6.Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J. Steroid Biochem. Mol. Biol. 2006;102:89–96. doi: 10.1016/j.jsbmb.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Platet N, Cathiard AM, Gleizes M, Garcia M. Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev. Oncol. Hematol. 2004;51:55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol. Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 10.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu. Rev. Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 11.Kyprianou N, English HF, Davidson NE, Isaacs JT. Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res. 1991;51:162–166. [PubMed] [Google Scholar]
- 12.Rodrik V, Zheng Y, Harrow F, Chen Y, Foster DA. Survival signals generated by estrogen and phospholipase D in MCF-7 breast cancer cells are dependent on Myc. Mol. Cell Biol. 2005;25:7917–7925. doi: 10.1128/MCB.25.17.7917-7925.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Ray S, Reed JC, Ibrado AM, Tang C, Nawabi A, Bhalla K. Estrogen increases intracellular p26Bcl-2 to p21Bax ratios and inhibits taxol-induced apoptosis of human breast cancer MCF-7 cells. Breast Cancer Res. Treat. 1997;42:73–81. doi: 10.1023/a:1005777219997. [DOI] [PubMed] [Google Scholar]
- 14.Teixeira C, Reed JC, Pratt MA. Estrogen promotes chemotherapeutic drug resistance by a mechanism involving Bcl-2 proto-oncogene expression in human breast cancer cells. Cancer Res. 1995;55:3902–3907. [PubMed] [Google Scholar]
- 15.Razandi M, Pedram A, Levin ER. Plasma membrane estrogen receptors signal to antiapoptosis in breast cancer. Mol. Endocrinol. 2000;14:1434–1447. doi: 10.1210/mend.14.9.0526. [DOI] [PubMed] [Google Scholar]
- 16.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 17.Le HH, Carlson EM, Chua JP, Belcher SM. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol. Lett. 2008;176:149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soto AM, Vandenberg LN, Maffini MV, Sonnenschein C. Does breast cancer start in the womb? Basic Clin. Pharmacol. Toxicol. 2008;102:125–133. doi: 10.1111/j.1742-7843.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prins GS, Birch L, Tang WY, Ho SM. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod. Toxicol. 2007;23:374–382. doi: 10.1016/j.reprotox.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ. Health Perspect. 1995;103 Suppl 7:113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diel P, Olff S, Schmidt S, Michna H. Effects of the environmental estrogens bisphenol A, o,p'-DDT, p-tert-octylphenol and coumestrol on apoptosis induction, cell proliferation and the expression of estrogen sensitive molecular parameters in the human breast cancer cell line MCF-7. J. Steroid Biochem. Mol. Biol. 2002;80:61–70. doi: 10.1016/s0960-0760(01)00173-x. [DOI] [PubMed] [Google Scholar]
- 22.Singleton DW, Feng Y, Yang J, Puga A, Lee AV, Khan SA. Gene expression profiling reveals novel regulation by bisphenol-A in estrogen receptor-alpha-positive human cells. Environ. Res. 2006;100:86–92. doi: 10.1016/j.envres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Dairkee SH, Seok J, Champion S, Sayeed A, Mindrinos M, Xiao W, Davis RW, Goodson WH. Bisphenol A induces a profile of tumor aggressiveness in high-risk cells from breast cancer patients. Cancer Res. 2008;68:2076–2080. doi: 10.1158/0008-5472.CAN-07-6526. [DOI] [PubMed] [Google Scholar]
- 24.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der BB, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 25.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Okada H, Tokunaga T, Liu X, Takayanagi S, Matsushima A, Shimohigashi Y. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma. Environ. Health Perspect. 2008;116:32–38. doi: 10.1289/ehp.10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lapensee EW, Tuttle TR, Fox SR, Ben-Jonathan N. Bisphenol A at low nanomolar doses confers chemoresistance in estrogen receptor-alpha-positive and -negative breast cancer cells. Environ. Health Perspect. 2009;117:175–180. doi: 10.1289/ehp.11788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hugo ER, Brandebourg TD, Comstock CE, Gersin KS, Sussman JJ, Ben Jonathan N. LS14: a novel human adipocyte cell line that produces prolactin. Endocrinology. 2006;147:306–313. doi: 10.1210/en.2005-0989. [DOI] [PubMed] [Google Scholar]
- 29.Sui M, Huang Y, Park BH, Davidson NE, Fan W. Estrogen receptor alpha mediates breast cancer cell resistance to paclitaxel through inhibition of apoptotic cell death. Cancer Res. 2007;67:5337–5344. doi: 10.1158/0008-5472.CAN-06-4582. [DOI] [PubMed] [Google Scholar]
- 30.Russo J, Fernandez SV, Russo PA, Fernbaugh R, Sheriff FS, Lareef HM, Garber J, Russo IH. 17-Beta-estradiol induces transformation and tumorigenesis in human breast epithelial cells. FASEB J. 2006;20:1622–1634. doi: 10.1096/fj.05-5399com. [DOI] [PubMed] [Google Scholar]
- 31.Olsen CM, Meussen-Elholm ET, Samuelsen M, Holme JA, Hongslo JK. Effects of the environmental oestrogens bisphenol A, tetrachlorobisphenol A, tetrabromobisphenol A, 4-hydroxybiphenyl and 4,4'-dihydroxybiphenyl on oestrogen receptor binding, cell proliferation and regulation of oestrogen sensitive proteins in the human breast cancer cell line MCF-7. Pharmacol. Toxicol. 2003;92:180–188. doi: 10.1034/j.1600-0773.2003.920408.x. [DOI] [PubMed] [Google Scholar]
- 32.Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ. Health Perspect. 2008;116:1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuelsen M, Olsen C, Holme JA, Meussen-Elholm E, Bergmann A, Hongslo JK. Estrogen-like properties of brominated analogs of bisphenol A in the MCF-7 human breast cancer cell line. Cell Biol. Toxicol. 2001;17:139–151. doi: 10.1023/a:1011974012602. [DOI] [PubMed] [Google Scholar]
- 34.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 35.Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S, Kimura M, Shimohigashi Y. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma. J. Biochem. 2007;142:517–524. doi: 10.1093/jb/mvm158. [DOI] [PubMed] [Google Scholar]
- 36.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]