Abstract
Community-based cohorts of older persons may differ neuropathologically from clinic-based cohorts. This study investigated age-related pathologies in persons with and without dementia and included autopsied participants from two community-based cohorts, the Rush Religious Orders Study (n = 386) and the Memory and Aging Project (n = 195), and one clinic-based cohort, the Clinical Core of the Rush Alzheimer’s Disease Center (n = 392). Final clinical diagnoses included no cognitive impairment (n = 202), mild cognitive impairment (MCI) (n = 150), probable Alzheimer’s disease (AD) (n = 474), possible AD (n = 88), and other dementias (n = 59). Postmortem diagnoses included pathologic AD, cerebral infarcts, and Lewy body disease. Community-based persons with clinical AD had less severe AD pathology (p < 0.001) and had more cerebral infarcts (p < 0.001) compared to clinic-based persons. Additionally, community-based persons with MCI had more infarcts compared to clinic-based persons. Overall, there was a higher proportion of Lewy bodies and atypical pathologies in the clinic-based compared to the community-based cohorts (p < 0.001). Community-based persons with probable AD show less severe AD pathology and more often have infarcts and mixed pathologies; those with MCI more often have infarcts and mixed pathologies. Overall, clinic-based persons have more Lewy bodies and atypical pathologies. The spectrum of pathologies underlying cognitive impairment in clinic-based cohorts differs from community-based cohorts.
Keywords: Clinic, community, epidemiology, neuropathology, selection bias
INTRODUCTION
Persons who seek medical attention from specialty clinics may differ in important ways from persons living in the community [1–3]. This may be particularly true of older persons, who may have cognitive impairment, mobility disability, financial constraints, or other issues that may limit access or use of physicians and clinics. Nonetheless, much of the current knowledge regarding the pathology of cognitive impairment is founded on traditional clinical-pathologic studies from specialty clinics devoted to memory disorders and Alzheimer’s disease (AD). While these studies have been invaluable in laying a foundation of knowledge regarding the pathologic changes that underlie cognitive impairment in older persons, especially AD and related disorders, further studies are needed to determine whether these clinical-pathologic findings can be generalized to older persons living in the community. Recent studies of community-based persons have shown that dementia, including probable AD, is often the result of mixed pathologies, specifically AD pathology mixed with cerebral infarcts and/or Lewy bodies [5–9]. There is less information on the pathology of mild cognitive impairment (MCI), which has variably shown different levels of AD pathology and pathologic heterogeneity [10–14].
The current study compares the neuropathology underlying no cognitive impairment, MCI, and dementia (probable and possible AD, and other dementias) in older persons from two community-based cohorts compared to one clinic-based cohort. The objective was to investigate the differences in neuropathologic findings from persons with and without dementia in clinic versus community-based settings.
MATERIALS AND METHODS
Study populations
We included 581 subjects from community cohorts (386 from the Religious Orders Study and 195 from the Rush Memory and Aging Project) and 392 from a clinic cohort.
The subjects were consecutively deceased and autopsied subjects from the Religious Orders Study [15] and the Rush Memory and Aging Project [16], and the Clinical Core of the Rush Alzheimer’s Disease Center [17], all longitudinal clinical-pathologic studies of aging and dementia. The three studies were approved the by the Institutional Review Board at the Rush University Medical Center.
Community cohorts
Participants in the community cohorts were recruited through targeted recruitment strategies, including community presentations and seminars. After the presentation, attendees indicated their level of interest, and study personnel followed-up with interested subjects.Participants of the community-based studies are without known dementia at baseline, signed informed consent, and agreed to annual clinical evaluations and brain donation at the time of death. Participants were tested annually and at follow-up in their individual, community, or communal residences.
Religious orders study
Participants of the Religious Orders Study are older catholic clergy without known dementia at the time of enrollment and are from the Chicago area and about 40 additional sites throughout the country. Since January of 1994, more than 1,100 persons have enrolled in the study and annual follow-ups have exceeded 95% of survivors. Details of the study have been previously reported [15,18,19]. Through April 1, 2009, 472 participants died and 443 had undergone brain autopsy (93.9% autopsy rate). We included the first 386 consecutive autopsies for which there was complete neuropathologic data.
Memory and aging project
Participants of the Memory and Aging Project are older Chicago area residents without known dementia at the time of enrollment, which mostly come from about 40 retirement centers and senior subsidized housing facilities. Details of the study have been previously reported [16,20]. Since November of 1997, more than 1,200 persons enrolled and completed their baseline evaluation. The overall annual follow-up rate of survivors exceeds 90%. Through April 1, 2009, 350 participants died and 289 had undergone brain autopsy (82.6% autopsy rate). We included the first 195 consecutive autopsies for which there was complete neuropathologic data.
Clinic cohort
In contrast to the community subjects, the clinic patients presented to the Rush Memory Clinic, a specialty referral clinic at the Rush University Medical Center for evaluation of concerns regarding cognition. At the time of the initial visit, the patient and family, friend or caregiver, were presented with information regarding enrollment in the clinical core of our Rush Alzheimer’s Disease Center and signed informed consent at the clinic visit. The clinical core enrolled normal subjects and subjects with MCI, AD, and other dementias; there were no specific exclusion criteria. Controls in this setting are typically relatives or friends of the patients. Interviews and evaluations were conducted within the clinic unless the subject was too impaired to travel in which case the evaluation was performed in the community. Following death, next of kin provided consent for autopsy. Through April 1, 2009, 522 participants died and 438 had undergone brain autopsy (83.9% autopsy rate). We included the first 392 consecutive autopsies for which there was complete neuropathologic data
Clinical evaluations
Community cohorts
Both community studies included uniform and structured annual clinical evaluations with medical history questions, neurologic examination, and detailed cognitive testing [15,16,18–20]. Diagnostic classification followed a multi-step procedure as previously described [17]. Briefly, neuropsychologic tests encompassing a wide range of cognitive function were scored and adjusted for education by computer and reviewed by a neuropsychologist who rated the presence of cognitive impairment in multiple cognitive domains, including episodic memory. Participants were examined or records were reviewed by a clinician with expertise in the evaluation of older persons, and diagnostically classified using the recommendations of the joint working group of the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) [24]. The diagnosis of probable AD required a history of cognitive decline and evidence of impairment in memory and other cognitive abilities. Probable AD referred to persons meeting these criteria without a co-existing condition contributing to dementia, and possible AD referred to persons meeting these criteria who had a coexisting condition (e.g., stroke) contributing to dementia. The diagnosis of MCI referred to persons with cognitive impairment by the neuropsychologist but without a clinical diagnosis of dementia by the examining clinician [13,19]. Cognitive impairment related to stroke was made according to the National Institute of Neurological Disorders and Stroke/Association Internationale pour la Recherche et l’Enseignement in Neurosciences (NINDS/AIREN) criteria for vascular dementia [25]; parkinsonism and Parkinson’s disease according to the clinical criteria recommended by the Core Assessment Program for Intracerebral Transplantation (CAPIT) [26]; and major depression based on DSM--R criteria supported by the Hamilton rating scale for depression [27].
Clinic cohort
Subjects underwent uniform, structured, clinical evaluations, as previously reported [21–23]. Briefly, evaluations included a detailed medical history, neurologic examination, cognitive function testing, brief psychiatric evaluation, an interview with a knowledgeable informant, laboratory testing, and structural neuroimaging. Ancillary tests (e.g., examination of cerebrospinal fluid, positron emission tomography) were obtained when clinically indicated. The procedures were compatible with the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) [27], similar to those conducted by the Clinical Cores of other federally-funded AD Centers, and consistent with the current practice parameters for the diagnostic evaluation for dementia [28].
Cognitive function was assessed with a battery of neuropsychological tests administered by a research assistant during an approximately one hour session. The battery included 19 individual performance-based measures of orientation, attention, memory, language, and visual perception widely used in clinical evaluation of older persons. Detailed psychometric information on the test battery used from 1992 to 1999 is published elsewhere [21]. In 1999, the core battery was changed to be compatible with the cognitive testing being performed in the Religious Orders Study and Memory and Aging Project. Five tests were administered across all years.
For all three studies, at the time of death, all available clinical data were reviewed by a neurologist and a summary diagnostic opinion was rendered regarding the most likely clinical diagnosis at the time of death. Difficult cases were subjected to case conferencing with a second neurologist and a neuropsychologist.
Brain autopsy procedures
Brain autopsies were performed at Rush and 11 predetermined sites across the United States for nearly all cases, as previously described [13,16]. Brains were removed and weighed, and the brainstem and cerebellum were removed. The hemispheres were cut into 1 cm coronal slabs in a Plexiglas jig. All fresh slabs were photographed and examined for visible pathology. One hemisphere (without visualized pathology) was frozen. The other hemisphere and slabs with visible pathology were fixed for 3–21 days in 4% paraformaldehyde after which there was a macroscopic review (including assessment of macroscopic infarctions) and the dissection of diagnostic blocks (midfrontal, middle temporal, inferior parietal, anterior cingulate, entorhinal cortex, hippocampus, basal ganglia, thalamus, and midbrain with substantia nigra). Blocks were embedded in paraffin, cut into 6 µm sections, and mounted on glass slides.
Pathologic diagnoses
Neuropathologic diagnoses were made by a board-certified neuropathologist blinded to age and clinical data. For the pathologic diagnosis of AD, Bielschowsky silver stain was used to visualize neuritic plaques, diffuse plaques, and neurofibrillary tangles in the frontal, temporal, parietal, entorhinal, and hippocampal cortices, as previously described [13,29]. A neuropathologic diagnosis of “no AD,” “low likelihood AD,” “intermediate likelihood AD,” or “high likelihood AD” was rendered based on semiquantitative estimates of neuritic plaque density as recommended by CER-AD [30] and the Braak score [31] as recommended by the National Institute on Aging (NIA)-Reagan criteria [32]. Details of the pathologic diagnoses have been described previously [13,33]. A final pathologic diagnosis of AD required either intermediate or high likelihood AD by these criteria. The minimum criteria for a diagnosis of intermediate likelihood AD required both a Braak score of III and moderate plaques; the minimum criteria for a diagnosis of high likelihood AD required both a Braak score of V and frequent plaques.
Chronic macroscopic infarcts were summarized as present or absent as previously described [13,33]. We used H&E stain to document chronic microscopic infarcts on the diagnostic blocks (midfrontal, middle temporal, inferior parietal, anterior cingulate, entorhinal cortex, hippocampus, basal ganglia, thalamus, and midbrain with substantia nigra) [29]. We used α-synuclein immunohistochemistry (Zymed, 1:100) to detect Lewy bodies in the substantia nigra, entorhinal, cingulate, midfrontal, middle temporal, and inferior parietal cortex, as previously described [33]. Only neocortical Lewy body disease was included in the estimates of mixed pathology in both in the tables.
Data analysis
We first compared findings between the two community cohorts. We used t-tests for continuous data and chi-squares for categorical data to compare the proportion of persons in each group (no cognitive impairment, MCI, probable AD, possible AD, and other dementias) and pathologic diagnoses within each group. Because the findings were not significantly different across the community cohorts, we combined data from these two cohorts and then compared the means from the community cohorts to the clinic cohort. We again used t-tests for continuous data and chi-squares for categorical data to compare the proportion of persons in each group and pathologic diagnoses within each group. Because of the small number of clinic subjects in the no cognitive impairment and MCI groups, we did not include statistical comparisons of those subgroups. Data was programmed in SAS® software Version 9.1.3 of the SAS system for UNIX [34].
RESULTS
Community cohorts
We first investigated the clinical-pathologic findings from the two community cohorts (386 from the Religious Orders Study and 195 from the Rush Memory and Aging Project). On average, the age at death was only two years greater in the Memory and Aging cohort (88.0 years) compared to the Religious Orders Study cohort (86.2 years); this difference reached statistical significance (p = 0.002, Table 1). In addition, the Religious orders cohort had more years of education (p < 0.0001). The average Mini Mental Status Examination (MMSE) scores were similar in both cohorts, as was the proportion of each cohort with no cognitive impairment, MCI, probable AD, possible AD, and other dementias. Neuropathologic findings were comparable across the two community cohorts, with a similar overall proportion of persons with a pathologic diagnosis of AD, cerebral infarcts (gross and microscopic), neocortical Lewy bodies, and mixed pathologies (Table 1). Because we wanted to compare community to clinic cohorts and the neuropathology of the community cohorts was similar within each diagnostic group, we merged the data from the two cohorts to compare to the clinic cohort.
Table 1.
Demographics [mean (SD)] and distribution [number (%)] of pathology in two community and one clinic cohort
| Religious orders study |
Memory and aging project |
Clinical cohort | |
|---|---|---|---|
| Number | 386 | 195 | 392 |
| Age at death (yrs) | 86.2 (SD = 7.0) | 88.0 (SD = 5.7) | 78.6 (SD = 10.4) |
| Education (yrs) | 17.9 (SD = 3.6) | 14.7 (SD = 3.0) | 14.9 (SD = 12.2) |
| MMSE | 21.0 (SD = 9.1) | 21.9 (SD = 8.8) | 7.3 (SD = 9.3) |
| No cognitive impairment | 124 (32.1%) | 64 (32.8%) | 14 (3.6%) |
| Mild cognitive impairment | 87 (22.5%) | 54 (27.7%) | 9 (2.3%) |
| Probable AD | 130 (33.7%) | 64 (32.8%) | 280 (71.2%) |
| Possible AD | 33 (8.5%) | 9 (4.6%) | 46 (11.7%) |
| Other dementia | 12 (3.1%) | 4 (2.0%) | 43 (10.9%) |
| Pathologic diagnosis of AD (NIA-Reagan) | 234 (60.6%) | 115 (59.0%) | 323 (83.7%) |
| High | 70 (18.1%) | 30 (15.4%) | 232 (59.0%) |
| Intermediate | 164 (42.5%) | 85 (43.6%) | 91 (23.2%) |
| Infarct (any) | 189 (49.0%) | 89 (45.6%) | 109 (27.8%) |
| Macroscopic | 136 (35.2%) | 65 (33.3%) | 66 (16.8%) |
| Microscopic | 115 (29.8%) | 45 (23.1%) | 71 (18.1%) |
| Lewy bodies (any) | 79 (20.5%) | 29 (14.9%) | 99 (25.2%) |
| Neocortical | 39 (10.1%) | 13 (6.7%) | 73 (18.6%) |
| One pathology (AD, infarcts, or Lewy bodies) | 184 (47.7%) | 103 (52.8%) | 223 (56.7%) |
| Mixed pathology | 107 (27.7%) | 44 (22.6%) | 116 (29.5%) |
| FTLD or other atypical pathology | 1 (0.25%) | 2 (1.0%) | 36 (9.2%) |
Clinic cohort
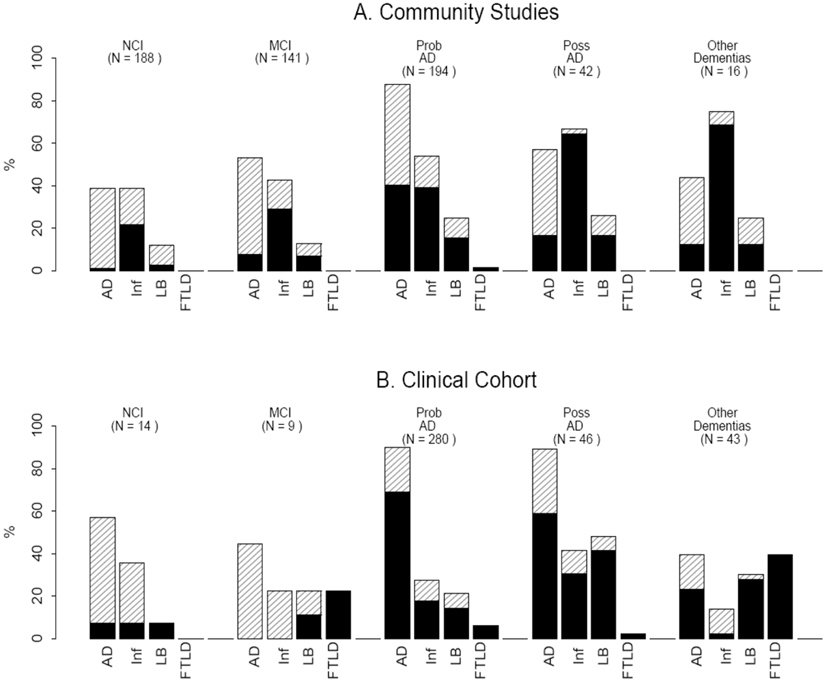
The 392 persons in the clinic cohort were younger (mean age = 77.5) and more likely to have dementia compared to community-based persons (p < 0.001). Few persons in the clinic cohort had no cognitive impairment or MCI (Table 1, Fig. 1). The clinic cohort had a higher proportion of persons with a pathologic diagnosis of AD and the amount of pathology was more likely to be high. The clinic subjects also had more Lewy bodies, particularly neocortical Lewy bodies, but a lesser proportion with cerebral infarcts (all p′s < 0.001). Atypical neuropathologies [Frontotemporal lobar degeneration (FTLD), Progressive supranuclear palsy (PSP), Corticobasal degeneration (CBD), Multisystem atrophy (MSA), and Creutzfeld Jakob disease (CJD)] were more common in the clinic cohort (Table 1, Fig. 1).
Fig. 1.
Distribution of neuropathology in persons from community studies (A) compared to clinical cohort (B) with and without dementia. Clinical diagnoses: NCI (no cognitive impairment); MCI (mild cognitive impairment); prob AD (probable Alzheimer’s disease), poss AD (possible Alzheimer’s disease); other dementias (includes non-AD dementias, see text). Pathologic diagnoses: AD (Alzheimer’s disease; black bar = High likelihood NIA-Reagan; diagonal lines = intermediate likelihood NIA-Reagan); Inf (Cerebral infarcts; black bar = macroscopic infarcts; diagonal lines = microscopic infarcts only); LB (Lewy bodies; black bar = neocortical Lewy bodies; diagonal lines = nigral or limbic Lewy bodies only); FTLD (frontotemporal lobar degeneration or other atypical pathologies). See text for further information regarding clinical and neuropathologic diagnoses.
No cognitive impairment in the community versus clinic
Almost 200 persons had no cognitive impairment in the two community studies (Table 2, Fig. 1) compared to 14 in the clinic cohort. Compared to subjects in the community with no cognitive impairment, those in the clinic were similar in age and proportion of significant neuropathologies, with over half in both the community and clinic having significant neuropathology (Table 2, Fig. 1). Though numbers were small, a pathologic diagnosis of AD was more common in the clinic; there were comparable proportions of Lewy body disease pathology and infarcts in the clinic compared to community cohort.
Table 2.
Demographics [mean (SD)] and distribution [number (%)] of pathology in persons with no cognitive impairment in community compared to clinic cohorts
| Community cohorts |
Clinical cohort |
|
|---|---|---|
| Number | 188 | 14 |
| Age at death | 83.8 (SD = 6.4) | 81.6 (SD = 10.7) |
| Education | 17.0 (SD = 3.9) | 14.7 (SD = 3.0) |
| MMSE | 28.3 (SD = 1.6) | 27.8 (SD = 1.6) |
| Pathologic diagnosis of AD (NIA-Reagan) |
73 (38.8%) | 8 (57.1%) |
| High | 2 (1.1%) | 1 (7.1%) |
| Intermediate | 71 (37.8%) | 7 (50.0%) |
| Infarct (any) | 73 (38.8%) | 5 (35.7%) |
| Macroscopic | 41 (21.8%) | 1 (7.1%) |
| Microscopic | 41 (21.8%) | 5 (35.7%) |
| Lewy bodies (any) | 23 (12.2%) | 1 (7.1%) |
| Neocortical | 5 (2.7%) | 1 (7.1%) |
| One pathology (AD, infarcts, or Lewy bodies) |
87 (46.3%) | 6 (42.9%) |
| Mixed pathology | 16 (8.5%) | 2 (14.3%) |
| FTLD or other atypical pathology |
0 | 0 |
Mild cognitive impairment in the community versus clinic
Eighty-seven persons in Religious Orders Study and 54 in the Memory and Aging Project had a final diagnosis of MCI, compared to only nine persons with MCI in the clinic sample. Similar to the community sample, about half of those in the clinic with MCI had pathologic diagnosis of AD, specifically intermediate likelihood by NIA-Reagan criteria. Lewy bodies were only seen in 12.8% of the community subjects compared to 22.2% of the clinic subjects. In contrast, community subjects were much more likely to have infarcts (Table 3, Fig.1).
Table 3.
Demographics [mean (SD)] and distribution [number (%)] with mild cognitive impairment in community compared to clinic cohorts
| Community cohorts |
Clinical cohort |
|
|---|---|---|
| Number | 141 | 9 |
| Age at death | 87.0 (SD = 6.5) | 78.9 (SD = 10.7) |
| Education | 16.7 (SD = 3.8) | 16.1 (SD = 2.8) |
| MMSE | 26.0 (SD = 3.8) | 26.8 (SD = 1.2) |
| Pathologic diagnosis of AD (NIA-Reagan) |
75 (53.2%) | 4 (44.4%) |
| High | 11 (7.8%) | 0 |
| Intermediate | 64 (45.4%) | 4 (44.4%) |
| Any infarct | 60 (42.6%) | 2 (22.2%) |
| Macroscopic | 41 (29.1%) | 0 |
| Microscopic | 32 (22.7%) | 2 (22.2%) |
| Lewy bodies (any) | 18 (12.8%) | 2 (22.2%) |
| Neocortical | 8 (5.7%) | 1 (11.1%) |
| One pathology (AD, infarcts, or Lewy bodies) |
75 (53.2%) | 3 (33.3%) |
| Mixed pathology | 27 (19.1%) | 1 (11.1%) |
| FTLD or other atypical pathology |
0 | 2 (22.2%) |
Probable AD in the community versus clinic
In both the community and clinic, about 90% of persons diagnosed with probable AD were confirmed to have AD by pathology (Table 4, Fig. 1). In the clinic, however, a greater proportion of persons with AD pathology had high likelihood by NIA-Reagan criteria (p < 0.001). The proportions of non-AD pathologies were different in community compared to clinic subjects. Macroscopic infarcts were much more common in the community (p < 0.001), as were mixed pathologies (p < 0.001). Lewy bodies were similarly represented in the community compared to the clinic cohort of persons with a clinical diagnosis of probable AD. The clinic cohort was more likely to have an atypical pathologic diagnosis in lieu of a pathologic diagnosis of AD, including FTLD (n = 12), PSP (n = 2), CBD (n = 1), or CJD (n = 2). These diagnoses were uncommon in the community cohort, with only one case of FTLD, one case of PSP, and one case of MSA.
Table 4.
Demographics [mean (SD)] and distribution [number (%)] with clinically probable AD in community compared to clinic cohorts
| Community cohorts |
Clinical cohort |
|
|---|---|---|
| Number | 194 | 280 |
| Age at death | 89.8 (SD = 5.5) | 79.2 (SD = 10.1) |
| Education | 16.8 (SD = 3.6) | 12.8 (SD = 3.2) |
| MMSE | 14.1 (SD = 8.5) | 6.0 (SD = 8.0) |
| Pathologic diagnosis of AD (NIA-Reagan) |
170 (87.6%) | 252 (90%) |
| High | 78 (40.2%) | 193 (68.9%) |
| Intermediate | 92 (47.4%) | 59 (21.1%) |
| Infarcts (any) | 105 (54.1%) | 77 (27.5%) |
| Macroscopic | 76 (39.2%) | 50 (17.9%) |
| Microscopic | 60 (30.9%) | 49 (17.5%) |
| Lewy bodies (any) | 48 (24.7%) | 60 (21.4%) |
| Neocortical | 30 (15.5%) | 40 (14.3%) |
| One pathology (AD, infarcts, or Lewy bodies) |
96 (49.5%) | 185 (66.1%) |
| Mixed pathology | 86 (44.3%) | 77 (27.5%) |
| FTLD or other atypical pathology |
3 (1.5%) | 17 (6.1%) |
Possible AD in the community versus clinic
Fewer persons in the community cohort had a clinical diagnosis of possible AD (AD mixed with another disorder) compared to the clinic cohort. Those in the community were older than the clinic subjects. Neuropathologically, the proportion of persons with clinically possible AD in the clinic that were confirmed to have AD was greater compared to the community cohorts (p < 0.001, Table 5, Fig. 1). There were also more infarcts in the community cohort. Conversely, persons with possible AD in the clinic cohort were more likely to have Lewy bodies, mixed pathologies, oratypical pathologies compared to the community subjects (Table 5, Fig. 1).
Table 5.
Demographics [mean (SD)] and distribution [number (%)] with clinically possible AD in community compared to clinic cohorts
| Community cohorts |
Clinical cohort |
|
|---|---|---|
| Number | 42 | 46 |
| Age at death | 87.2 (SD = 6.6) | 81.3 (SD = 6.3) |
| Education | 16.9 (SD = 3.1) | 14.3 (SD = 3.5) |
| MMSE | 13.4 (SD = 8.4) | 5.8 (SD = 7.5) |
| Pathologic diagnosis of AD (NIA-Reagan) |
24 (57.1%) | 41 (89.1%) |
| High | 7 (16.7%) | 27 (58.7%) |
| Intermediate | 17 (40.5%) | 14 (30.4%) |
| Infarcts (any) | 28 (66.7%) | 19 (41.3%) |
| Macroscopic | 27 (64.3%) | 14 (30.4%) |
| Microscopic | 16 (38.1%) | 10 (21.7%) |
| Lewy bodies (any) | 11 (26.2%) | 22 (47.8%) |
| Neocortical | 7 (16.7%) | 19 (41.3%) |
| One pathology (AD, infarcts, or Lewy bodies) |
21 (50%) | 13 (28.3%) |
| Mixed pathology | 17 (40.5%) | 29 (63%) |
| FTLD or other atypical pathology |
0 | 1 (2.2%) |
Other dementias in the community versus clinic
The clinic cohort had a greater proportion of persons clinically diagnosed with other dementias (p < 0.001). The clinic cohort with other dementias were younger and neuropathologically more likely to have atypical forms of dementia (almost 40%), whereas the community cohort diagnosed with other dementias were much more likely to have infarcts (almost 75%). There were a similar proportion in both the community and clinic “other” dementia groups with a pathologic diagnosis of AD and Lewy body disease (Table 6, Fig. 1).
Table 6.
Demographics [mean (SD)] and distribution [number (%)] in persons with other dementias in community compared to clinic cohorts
| Community cohorts |
Clinical cohort |
|
|---|---|---|
| Number | 16 | 43 |
| Age at death | 83.8 (SD = 7.7) | 70.0 (SD = 11.7) |
| Education | 17.4 (SD = 3.9) | 13.7 (SD = 2.6) |
| MMSE | 14.8 (SD = 8.6) | 7.4 (SD = 9.1) |
| Pathologic diagnosis of AD (NIA-Reagan) |
7 (43.8%) | 17 (39.5%) |
| High | 2 (12.5%) | 10 (23.2%) |
| Intermediate | 5 (31.2%) | 7 (16.3%) |
| Infarcts (any) | 12 (75.0%) | 6 (14.0%) |
| Macroscopic | 11 (68.8%) | 1 (2.3%) |
| Microscopic | 7 (43.8%) | 5 (11.6%) |
| Lewy bodies (any) | 4 (25.0%) | 13 (30.2%) |
| Neocortical | 2 (12.5%) | 12 (27.9%) |
| One pathology (AD, infarcts, or Lewy bodies) |
8 (50.0%) | 15 (34.9%) |
| Mixed pathology | 5 (31.2%) | 7 (16.3%) |
| FTLD or other atypical pathology |
0 | 17 (39.5%) |
DISCUSSION
Clinical-pathologic studies of aging and dementia from specialty clinics rather than population or community cohorts may be vulnerable to selection bias. We investigated the effect of sample selection on age-related brain pathology in older persons with and without dementia from two longitudinal community-based cohorts compared to one specialty clinic-based cohort. We found important differences between the cohorts. Community subjects with probable AD were more likely than clinic subjects to have intermediate compared to high likelihood pathologic AD, and more likely to have cerebral infarcts and mixed pathologies. Community MCI subjects showed a similar proportion with pathologic AD but a higher frequency of infarcts and mixed pathologies compared to clinic subjects. Community subjects with other dementias were much less likely to have evidence of frontotemporal lobar degeneration or other atypical pathologies.
Selection or referral bias has been previously reported in subjects with AD [1–4]. Studies have indicated significant sociodemographic [1,3,4], clinical [3,4], and genetic [2] differences between subjects referred to specialty clinics compared to other registry and population cohorts. One study [1] reported differences in neuropathology, most notably an increase in dementia due to cerebrovascular disease in the community cohort. We extend these findings by merging two community cohorts and comparing them to a clinic cohort that employed similar clinical and neuropathologic methods. In addition, we compared neuropathologic data in five diagnostic groups: no cognitive impairment, MCI, probable AD, possible AD, and other dementias.
AD pathology was less severe in the community sample with dementia compared to the clinic cohort. One obvious explanation is that the older persons with dementia in the community had less severe cognitive impairment compared to demented persons in a specialty clinic. Indeed, we had previously reported less severe cognitive impairment in older persons with dementia in the community [17]. There are several explanations for the differences in severity of cognitive impairment and AD pathology in the clinic versus the community. First, community subjects are recruited after the age of 65 without dementia and develop dementia during the study, whereas clinic patients are typically recruited at baseline with a history of dementia and impairment worsens during the course of the study. Second, doctors may be more apt to refer younger patients with a more rapid progression as evidenced by the younger age for the clinic patients. Third, behavioral issues, mobility disabilities, and incontinence may be related to an increased likelihood of referral as well as more severe cognitive impairment and more widely distributed AD pathology. In addition, clinic subjects may have fewer co-morbidities and greater financial, social, and personal resources, resulting in longer survival after diagnosis, less mixed pathologies, and more severe pathology at death. Finally age itself may be a factor. Older age has been associated with less AD pathology and a lesser association with dementia [35].
Mixed pathologies and infarcts were more common in the community sample with probable AD compared to the clinic. Mixed rather than single pathologies were also seen more commonly in the community of MCI subjects compared to the clinic, though the numbers were very small. Interestingly, stroke was not an exclusionary criterion for enrollment in the clinical core. There are other possible explanations for discordant mixed pathology and cerebrovascular disease between clinic and community subjects. Individuals with vascular cognitive impairment may be more likely to visit a stroke and or general neurology clinic rather than be referred to a Memory disorders or AD clinic. In addition, persons who seek medical attention such as those in the clinic may have healthier lifestyles which may make them less prone toward vascular events. Finally, subjects with cognitive impairment in the community were older, allowing additional pathologies such as infarcts to accumulate. Indeed, the younger age of clinic-based compared to community-based subjects is a potential threat to the generalizability of a broad range of studies on dementia [36].
Similar biases are likely responsible for the increase in Lewy body disease and atypical pathologies (e.g., frontotemporal lobar degeneration) in the clinic cohort, including age, associated disabilities, and behavioral issues. Lewy bodies have been previously reported to be associated with advancing age [37]. In addition, the well known association of neocortical Lewy body disease with such problems as hallucinations, REM sleep behavior disorder, and falls are likely to prompt the solicitation of medical advice or referral. The significant behavioral issues associated with atypical pathologies, such as frontotemporal lobar degenerations, are likely to have similar consequences.
The neuropathologic disparities between the community and clinic cohorts with and without dementia may not be surprising; yet, the real-world consequences have broad implications for many types of research. For instance, because infarcts and mixed pathologies are more common in persons with probable AD in the community, trials in specialty clinics are likely to underestimate the potential benefit at preventing or treating vascular disease in older persons with preclinical or clinical AD. Moreover, selection bias will have the potential to exaggerate expected effect sizes for treatments aimed specifically at AD pathology in the community. Because the burden of dementia will dramatically increase in the older age groups over the next decades, it is important to target prevention and treatment studies toward persons that best reflect the population of older persons.
Whereas, the clinic under-represents older persons with infarcts and mixed pathologies, it likely over-represents persons with atypical pathologies. This also has real-world consequences. For instance, when dementia subtypes are extrapolated from autopsy studies derived from specialty clinics to the community, the prevalence of cortical Lewy body disease and frontotemporal lobar degeneration may be overestimated. Though the clinical-pathologic spectrum of both Lewy body disease and frontotemporal lobar degeneration continues to be explored and the prevalence, particularly of frontotemporal lobar degeneration, is currently uncertain, one should be cautious in extrapolating prevalence data from specialty clinics. These data may influence the allocation of resources and alter the direction of research studies.
There are limitations to this study. We had very few persons without dementia in the clinic sample to compare to the community sample. The community subjects were drawn from two different cohorts that are likely to have important differences. There are also advantages. The community subjects from the two cohorts were found to be very similar in both clinical and pathologic features allowing us to pool this data. All three studies had high autopsy rates, lessening the chance for bias when comparing the community to the clinic. Finally, all three groups had their neuropathology evaluated at the same center, lessening the potential for inter-rater differences in the interpretation of pathology.
In summary, sample selection may have a profound impact on the frequency, distribution, and types of neuropathology found in older persons with and without dementia. Persons from the community, compared to the clinic, tend to show less severe AD pathology, more cerebral infarcts, less neocortical Lewy body disease, and less atypical pathologies (e.g., frontotemporal lobar degeneration). These neuropathologic differences vary by diagnostic group. One should be aware of possible selection bias when using or reporting neuropathologic data derived from specialty clinics of older persons with and without dementia.
ACKNOWLEDGMENTS
The authors thank the patients and families of the Rush Alzheimer’s Disease Center and Memory Clinic, the nuns, priests, and brothers from across the country participating in the Religious Orders Study, the older persons from across northeastern Illinois participating in the Rush Memory and Aging Project. We thank the staff of the Rush Alzheimer’s Disease Center and Rush Institute for Health Aging.
Supported by the National Institute on Aging (R01 AG15819, R01 AG17917, P30 AG10161, K08 AG00849)
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=115).
REFERENCES
- 1.Massoud F, Devi G, Stern Y, Lawton A, Goldman JE, Liu Y, Chin SS, Mayeux R. A clinicopathological comparison of community-based and clinic-based cohorts of patients with dementia. Arch Neurol. 1999;56:1368–1373. doi: 10.1001/archneur.56.11.1368. [DOI] [PubMed] [Google Scholar]
- 2.Tsuang D, Kukull W, Sheppard L, Barnhart RL, Peskind EE, Edland SD, Schellenberg G, Raskind M, Larson EB. Impact of sample selection on APOE e4 allele frequency: A comparison of two Alzheimer’s disease samples. J Am Geriatrics Soc. 1996;44:704–707. doi: 10.1111/j.1532-5415.1996.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 3.Kokmen E, Ozsarfati Y, Beard M, O’Brien PC, Rocca WA. Impact of referral bias on Clinical and Epidemiological Studies of Alzheimer’s disease. J Clin Epidemiol. 1996;49:79–83. doi: 10.1016/0895-4356(95)00031-3. [DOI] [PubMed] [Google Scholar]
- 4.Barnhardt RL, van Belle G, Edland SD, Larson E. Referral bias in Alzheimer’s disease. J Clin Epidemiol. 1997;50:365–366. doi: 10.1016/s0895-4356(96)00384-8. [DOI] [PubMed] [Google Scholar]
- 5.Lim A, Tsuang D, Kukull W, Nochlin D, Leverenz J, Mc-Cormick W, Bowen J, Teri L, Thompson J, Peskind ER, Raskind M, Larson EB. Clinico-neuropathological correlation of Alzheimer’s disease in a community-based case series. J Am Geriatr Soc. 1999;47:564–569. doi: 10.1111/j.1532-5415.1999.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 6.White L, Small BJ, Petrovitch H, Ross GW, Masaki K, Abbott RD, Hardman J, Davis D, Nelson J, Markesbery W. Recent clinical-pathologic research on the causes of dementia in later life: update from the Honolulu-Asia Aging Study. J Geriatr Psychiatry Neurol. 2005;18:224–227. doi: 10.1177/0891988705281872. [DOI] [PubMed] [Google Scholar]
- 7.Neuropathology Group of the Medical Research Council Cognitive Function and Aging Study (MRC CFAS) Pathologic correlates of late onset dementia in a multicentre, community based population in England and Wales. Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 8.Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 9.Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66:1136–1146. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 11.Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, Jicha GA, Ivnik RJ, Smith GE, Tangalos EG, Braak H, Kokmen E. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 12.Galvin JE, Powlishta KK, Wilkins K, McKeel DW, Jr, Xiong C, Grant E, Storandt M, Morris JC. Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Arch Neurol. 2005;62:758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- 13.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 14.Saito Y, Murayama S. Neuropathology of mild cognitive impairment. Neuropathology. 2007;27:578–584. doi: 10.1111/j.1440-1789.2007.00806.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 16.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: Study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 17.Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident AD. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 19.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. The natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 20.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. JINS. 2005;11:400–407. [PubMed] [Google Scholar]
- 21.Wilson RS, Gilley DW, Bennett DA, Hebert LE, Beckett LA, Evans DA. Person-specific paths of cognitive decline in Alzheimer’s disease and their relation to age. Psychol Aging. 2000;15:18–28. doi: 10.1037//0882-7974.15.1.18. [DOI] [PubMed] [Google Scholar]
- 22.Hui JS, Wilson RS, Bennett DA, Bienias JL, Gilley DW, Evans DA. Rate of cognitive decline and mortality in Alzheimer’s disease. Neurology. 2003;61:1356–1361. doi: 10.1212/01.wnl.0000094327.68399.59. [DOI] [PubMed] [Google Scholar]
- 23.Barnes LL, Wilson RS, Li Y, Gilley DW, Bennett DA, Evans DA. Change in cognitive function in Alzheimer’s disease in African American and white persons. Neuroepidemiology. 2005;25:16–22. doi: 10.1159/000089231. [DOI] [PubMed] [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A. Vascular dementia: Diagnostic criteria for research studies - Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 26.Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, Watts R. Core Assessment Program for Intracerebral Transplantations (CAPIT) Movement Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 27.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The consortium to establish a registry for Alzheimer’s disease (CERAD). I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1169. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 28.Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, Small GW, Miller B, Stevens JC. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 29.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer’s disease pathology. Neurology. 2004;62:1148–1152. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 30.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 31.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 32.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18(4 Suppl):S1–S2. [PubMed] [Google Scholar]
- 33.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 34.SAS Institute Inc. SAS OnlineDoc® 9.1.3. Cary, NC: SAS Institute Inc; 2002–2005. [Google Scholar]
- 35.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 36.Schoenmaker N, Van Gool WA. The age gap between patients in clinical studies and in the general population: a pitfall for dementia research. Lancet Neurol. 2004;3:627–630. doi: 10.1016/S1474-4422(04)00884-1. [DOI] [PubMed] [Google Scholar]
- 37.Wakisaka Y, Furuta A, Tankzaki Y, Kiyohara Y, Iida M, Iwaki T. Age-associated prevalence and risk factors of Lewy body pathology in a general population: the Hisayama study. Acta Neuropathol. 2003;106:374–382. doi: 10.1007/s00401-003-0750-x. [DOI] [PubMed] [Google Scholar]