Abstract
Methionine adenosyltransferase (MAT) is an essential enzyme required for S-adenosylmethionine biosynthesis. Hepatic MAT activity falls during chronic liver injury, and mice lacking Mat1a develop spontaneous hepatocellular carcinoma by 18 months. We have previously demonstrated that CD133+CD45− oval cells isolated from 16-month-old Mat1a−/− mice represent a liver cancer stem cell population. The transforming growth factor β (TGF-β) pathway constitutes a central signaling network in proliferation, apoptosis, and tumorigenesis. In this study, we tested the response of tumorigenic liver stem cells to TGF-β. CD133+CD45− oval cells were isolated from premalignant 16-month-old Mat1a−/− mice by flow cytometry and expanded as five clone lines derived from a single cell. All clone lines demonstrated expression of both hepatocyte and cholangiocyte markers and maintained a small population (0.5% to 2%) of CD133+ cells in vitro, and three of five clone lines produced tumors. Although TGF-β1 inhibited cell growth equally in CD133− and CD133+ cells from each clone line, the CD133+ population demonstrated significant resistance to TGF-β–induced apoptosis compared with CD133+ cells. Furthermore, CD133+ cells demonstrated a substantial increase in mitogen-activated protein kinase (MAPK) pathway activation, as demonstrated by phosphorylated extra-cellular signal-regulated kinase levels before and after TGF-β stimulation. MAPK inhibition using mitogen-activated protein kinase kinase 1 (MEK1) inhibitor PD98059 led to a significant increase in TGF-β–induced apoptosis in CD133+ cells. Conversely, a constitutively active form of MEK1 blocked the apoptotic effects of TGF-β in CD133− cells.
Conclusion
CD133+ liver cancer stem cells exhibit relative resistance to TGF-β–induced apoptosis. One mechanism of resistance to TGF-β–induced apoptosis in CD133+ cancer stem cells is an activated mitogen-activated protein kinase/extracellular signal-regulated kinase pathway.
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality worldwide.1 The prognosis of HCC depends on the cancer stages at the time of diagnosis. Although surgical therapies have led to an improvement in the 5-year survival of select patients, most patients with HCC gain no significant benefit from traditional chemotherapy.2
Recently, a number of studies have demonstrated that solid tumors such as colon,3 pancreatic,4 brain,5 and prostate cancers6 are initiated from cancer stem cells (CSCs). CSCs are resistant to injury and may account for the relative failure of traditional chemotherapy.7 Several studies have linked CD133 expression to liver CSCs, demonstrating that CD133+ cells from established HCC cell lines displayed significant tumorigenic capacity.8,9 In patients with HCC, a hepatoblast phenotype correlates with a significantly worse prognosis.10
In a previous study, we defined a CD133+ CSC population isolated from methionine adenosyltransferase 1a (Mat1a)-deficient mice during premalignant liver injury.11 Methionine adenosyltransferase (MAT) is an essential enzyme responsible for the synthesis of S-adenosylmethionine, the principal methyl donor required for glutathione biosynthesis.12 The relationship between hepatic MAT activity and chronic liver disease in human patients is well established.12–14 Mat1a knockout mice demonstrated hepatosteatosis, oxidative liver injury, and spontaneous development of HCC by 18 months.14 What was unknown from our initial research was a specific mechanism of survival of the CD133+ cell population.
Resistance to transforming growth factor β (TGF-β) has been postulated to be an early event in HCC development. 15,16 TGF-β is the prototype of a large family of structurally related growth and differentiation factors that initiates its signals from a receptor complex,17 and intermediary activated Smads translocate into the nucleus, where they induce or suppress transcription of defined genes.18 In hepatocytes, TGF-β acts as a principle growth inhibitor,19 mediated by inducing expression of the CDK inhibitors p21 and p15, and down-regulating c-myc, cyclin D, and cyclin E.20 In addition, TGF-β induces apoptosis in several established human liver cell lines, including HepG2 hepatoma and HepG3 HCC cells.21 However, the precise role of TGF-β in HCC progression remains complex and depends on the stage of the tumor.15
In order to understand the role of TGF-β in the homeostasis of CD133+ liver CSCs, we tested both the cell growth inhibitory and apoptotic effects of TGF-β on CD133+ CSCs with Mat1a deficiency. Although there is no significant difference in the cell growth inhibition in CD133+ and CD133− cells in response to TGF-β, CD133+ cells did exhibit relative resistance to the apoptotic effects of TGF-β as compared with CD133− cells. Our results indicate that one mechanism for the resistance to TGF-β–induced apoptosis in CD133+ CSCs is an activated mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (Erk) pathway.
Materials and Methods
Reagents
See Supplementary Fig. 1 for detailed list of all reagents.
Cell Culture
Mat1a−/− cells expanded from a single CD133+CD45− cell were cultured in 1:1 Dulbecco’s modified Eagle’s medium/F12 (Sigma) containing 10% fetal bovine serum as described.11,22 CD45 depletion was conducted using Miltenyi CD45 microbead antibodies (Miltenyi Biotec Inc, Auburn, CA) per the manufacturer’s protocol, followed by CD133+CD45− cell flow cytometry (FACS) isolation for single cell expansion. Unless otherwise specified, 2 × 104 cells/cm2 were plated.
Animal Care
Mice were fed a standard diet (Harlan Teklad irradiated mouse diet 7912, Madison, WI) ad libitum and housed in a temperature-controlled animal facility with a 12-hour light/dark cycle. All procedures were in compliance with our institution’s guidelines for the use of laboratory animals and were approved by the Institutional Animal Care and Use Committee.
Tumor Formation Assay
Cells were counted with trypan blue exclusion, as described,11,22 and were resuspended in phosphate-buffered saline (PBS) for transplantation at a concentration of 2 × 106 live cells/200 µL (1:1 Matrigel/PBS). Six-week-old female nude mice (Jackson Laboratory, Bar Harbor, ME) or syngeneic wild-type mice were used for tumor formation analysis using subcutaneous inoculation, and tumors were isolated after 15 weeks.
Real-Time Polymerase Chain Reaction
Total RNA was extracted from 2 × 104 cells/cm2 in six-well plates using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. RNA was quantified using an ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE) and complementary DNA constructed as described.22 Real-time polymerase chain reaction (PCR) experiments were conducted using an ABI-Prism 7700 Thermal Cycler and TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA). The housekeeping gene β-Actin was used for all ΔΔCt calculations. Relative expression was calculated for the genes p15, p21, cyclin D1, and c-myc and was assessed using primer/probe sets (Applied Biosystems). Standard RT-PCR was conducted using the primers listed in Supplementary Fig. 1. Primers were selected in separate exons, with PCR conditions as described.11
Anchorage-Independent Growth Assays
After 0.8% bottom soft agar gel was plated in six-well culture plates, 20,000 cells were prepared in 0.3% agar gel in each well and loaded to the top of the bottom agar when it was completely solidified. The plates were placed in a 37°C humidified incubator with 5% CO2. One hundred microliters of fresh medium was added to the top of agar every other day. After 21 days, cells were stained with 0.005% crystal violet.
Apoptosis Assays
For DNA laddering assays, after 1 hour in serum-free medium, cells were treated with 5 ng/mL of TGF-β1 for 24 hours and collected for DNA isolation using a Suicide Track DNA Ladder Isolation Kit (Calbiochem, La Jolla, CA). DNA fragments were separated using 1.5% agarose gel and visualized using ethidium bromide staining. For caspase-3 assays, cells were treated with serum-free medium for 1 hour, then with 5 ng/mL of TGF-β1 for the indicated duration. Cells were trypsinized, fixed, permeabilized, and stained with activated caspase-3–phycoerythrin antibody (BD Biosciences, San Diego, CA). For annexin V/propridium iodine (PI) staining, cells were pretreated with TGF-β as described above, trypsinized, and stained using the Annexin V/PI Apoptosis Kit (BioVision, Mountain View, CA) according to the manufacturer’s protocol. FACS analysis was conducted on a BD FACS-Calibur. Analysis was conducted using FlowJo (Tree Star, Ashland, OR). For apoptosis assays, 2 × 104 cells/cm2 cells were plated on 60-mm dishes.
Western Blot Analysis
For Western blot analysis, cell lysates were harvested by the addition of lysis buffer (40 mM Tris [pH 7.4], 150 mM NaCl, 10 mM ethylene diamine tetraacetic acid, 10% glycerol, 1% Triton X-100, 10 mM glycerophosphate, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride) supplemented with protease inhibitor cocktail tablets (Roche, Indianapolis, IN). Forty micrograms of protein lysates were separated on a Nu-PAGE 4% to 12% Bis-Tris Gel (Invitrogen, Carlsbad, CA) and transferred to a polyvinylidene difluoride membrane (Invitrogen), as described.23 Signals were detected using the enhanced chemiluminescence solutions (Thermo Fisher Scientific, Rockford, IL). Densitometry was analyzed using ImageJ 1.40 software (National Institutes of Health) and normalized with pan-Erk1/2 expression levels.
CD133+ Cell Isolation
CD133+ cell isolation was performed using Miltenyi MACS systems according to the manufacturer’s protocol as described.24 Cells were trypsinized and suspended in 500 µL of 1 × PBS/2 mM ethylene diamine tetraacetic acid/0.5% bovine serum albumin buffer and incubated with magnetic microbeads conjugated with anti–Prominin-1 antibody (catalog #130-092-333) prior to separation using Miltenyi LS column.
Tritiated Thymidine Incorporation Assay
2 × 104 cells/cm2 were plated in triplicate in six-well plates. Cells were pulsed with 1 µCi/mL tritiated thymidine for 2 hours, then washed with 1 × PBS, and precipitated with 10% trichloroacetic acid for 10 minutes, and solubilized with 0.2N sodium hydroxide/salmon DNA buffer before quantitation with a scintillation counter.
Cell Viability Assay
Cell viability was performed using the XTT [2,3-bis(2-methoxy-4-nitro-5- sulfophenyl)-2H-tetrazolium-5-carboxanilide] kit (Trevigen, catalog #4891-025-K) according to the manufacturer’s protocol. 1 × 104 cells/well were plated in 96-well plates. Twenty-four hours after either β-galactosidase (β-Gal) or a constitutively active form of mitogen-activated protein kinase kinase 1 (CA-MEK1) adenoviral infection, cells were treated in serum-free medium for 1 hour, then were incubated in the presence or absence of 5 ng/mL of TGF-β for an additional 12 hours prior to analysis.
Complementary DNA Microarray
Complementary DNA from CD133+ and CD133− cells were hybridized to Illumina Mouse ref. 8 gene chip (Illumina, San Diego, CA) according to the manufacturer’s standard protocol. Housekeeping genes were used as standards to generate expression levels, and data analysis was conducted using a 1.4-fold or greater change in expression, with P < 0.01 considered significant.
Adenovirus Infection
All recombinant adenoviruses were expanded, purified, and titrated using BD Clontech’s Adeno-X Rapid Titer Kit per manufacturer’s protocol (Mountain View, CA) in a HEK293 monolayer of cells. Using either CA-MEK1 or β-Gal adenoviral constructs above, cells were infected using the indicated virus multiplicity of infection (MOI) as described.25
Statistical Analyses
The paired 2-tailed Student t test was used when comparing 2 groups. A p value less than 0.05 was considered significant.
Results
Mat1a−/− Clone Lines Maintain Bipotent Capacity of Liver Oval Cells
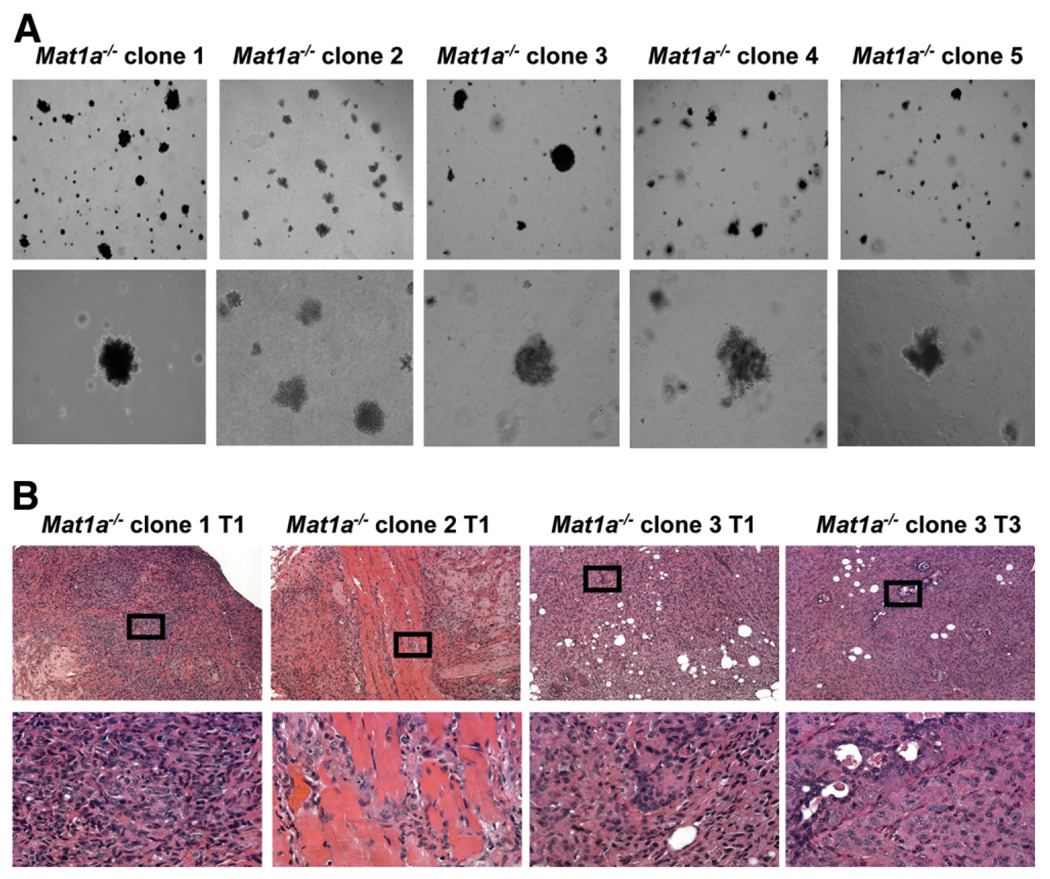
Five clone lines were expanded from single C133+CD45− non-parenchymal cells isolated from 16-month-old Mat1a−/− mice, as depicted in Fig. 1A.11 As shown in Fig. 1B, all five clone lines express both hepatocyte and cholangiocyte markers, such as Albumin and Ck-19. In addition, each clone line expresses a number of oval cell associated genes: Hnf3β, Hnf1α, and αFP.22,26 Western blot confirms αFP and CD133 protein expression in all clone lines (Fig. 1C). There was no difference in gene expression between early (passages 3–6) and later (passages 30–35) cells (data not shown). Within each clone line, the number of CD133+ cells remained relatively stable at 0.5% to 2% across multiple passages.
Fig. 1.
Single-cell isolation for clonal expansion. (A) Schematic representation of single-cell FACS isolation of CD133+CD45− cells from 16-month-old Mat1a−/− mice. On day 1, single cells were plated into individual wells of a 96-well plate. After 21 days, 10/96 wells showed colonies with growth over 50% of the well bottom. Five of these colonies were selected for expansion (CD133+ clone lines 1–5). (B) RT-PCR. In each clone line 1–5, coexpression of Albumin and CK-19 indicated bipotential stem cells, with strong expression of oval cell–associated transcription factors hepatocyte nuclear factor 1α and 3β (HNF1α and HNF3β) and ABCG2 transmembrane pump. Strong expression of growth factor receptors c-Met and epithelial growth factor receptor (EGFr) were also detected by RT-PCR. (C) Western blot. High levels of prominin-1 (CD133) and αFP protein were detected in each clone line (+ control = positive control for Western blot).
Anchorage-Independent Growth and Xenograft Tumor Formation from Mat1a−/− CD133+ Clone Lines
Our previous study demonstrated that bulk culture of CD133+ cells isolated from Mat1a−/− mice produced tumors in 40% of immune-deficient mice.11 As shown in Fig. 2A, all five clone lines grew in an anchorage-independent manner. In order to assess the tumor-forming ability of CD133+ cell-derived clone lines in vivo, a tumor model with immune-deficient mice was used. Two million cells isolated from each clone line were subcutaneously inoculated into immune-deficient mice (n = 4 per cell line, five lines total, passage 5). Of the five lines expanded from single CD133+ cell (clone lines 1–5), three lines formed tumors in nude mice at passage 5 (line 1 2/4, line 2 ¼, line 3 4/4, line 4 0/4, and line 5 0/4). There was no tumor formation in the control mice injected with Matrigel and PBS carrier. Tumor histology revealed hepatoma-like cells with mixed epithelial cell morphology and columnar/cuboidal cells, and the average tumor size was 200 ± 80 mg (Fig. 2B). Two million cells from tumorigenic line 3 were also transplanted into syngeneic wild-type mice. The small tumors in 25% of transplantations demonstrated hepatoma-like cells on hematoxylin-eosin staining (2/8, tumor size 50 and 100 mg) (Supplementary Fig. 2A–B). Subsequent analyses focus on tumorigenic lines: CSC clone lines 1, 2, and 3.
Fig. 2.
CD133+ Cancer stem cells. (A) All five CSC clone lines demonstrated anchorage-independent growth in soft agar at 21 days (4× and 20× objective), and (B) three of the five lines formed subcutaneous tumors in nude mice (2 × 106 cells injected, passage 5). Hematoxylin-eosin staining demonstrates tumor histology with mixed hepatoma-like populations of liver epithelial tumors (4× and 40× objectives).
Cell Growth Inhibition in Response to TGF-β
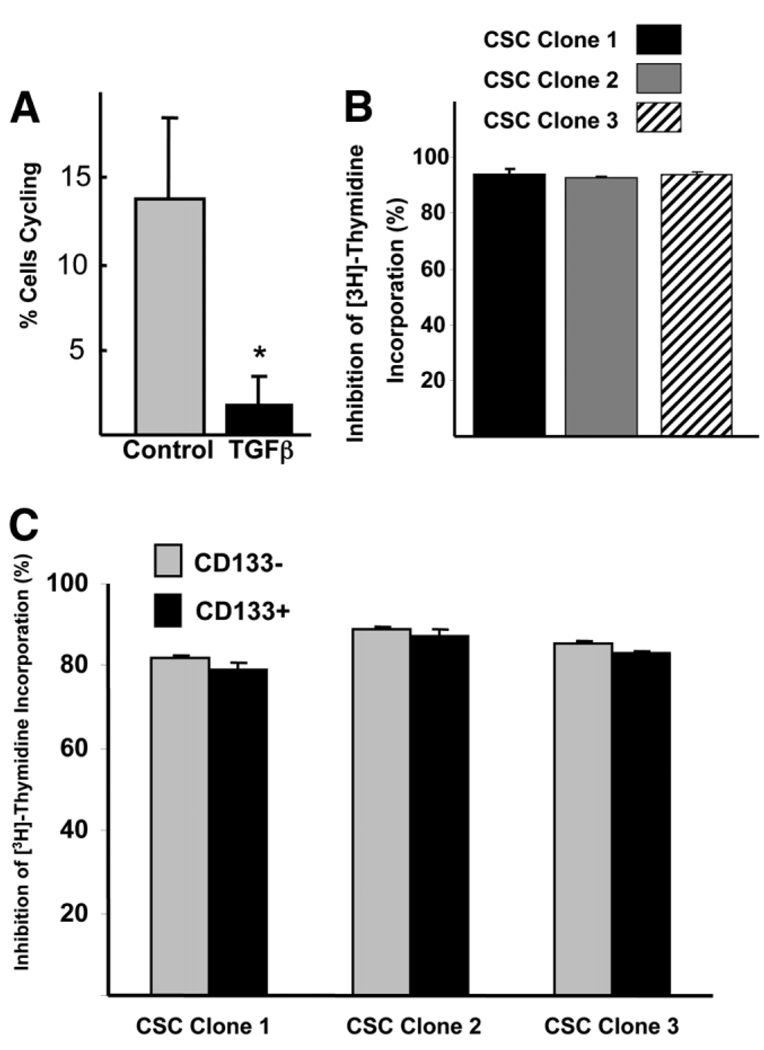
Given the growth inhibition effects of TGF-β, we tested the proliferation response of CSC clone lines after TGF-β stimulation. In serum-free conditions, 14 ± 5% of CSC clone line 1–3 cells enter S phase of cell cycle (1 hour bromodeoxyuridine pulse). After 5 ng/mL of TGF-β1 stimulation (24 hours), the number of cells entering S phase was significantly decreased by nearly 90% compared with serum-free controls, indicating that Mat1a−/− CSCs are sensitive to growth inhibition by TGF-β (Fig. 3A). This level of inhibition was observed in all three CSC clone lines (Fig. 3B). When the CSC clone lines were separated based on CD133 expression, CD133− and CD133+ cells were equally sensitive to growth inhibition by TGF-β1, showing 85% inhibition of [3H]-thymidine incorporation (Fig. 3C). For this and future analyses, isolation of CD133+ and CD133− cells was conducted using cells from the same culture plate.
Fig. 3.
CSC clone lines demonstrate growth inhibition in response to TGF-β stimulation. (A) Compared with serum-free baseline conditions, 24-hour TGF-β stimulation results in a significant decrease in cell cycling using bromodeoxyuridine 1-hour pulse (n = 3 lines, each in triplicate, + standard deviation). *P < 0.05. (B) TGF-β stimulation results in similar decrease in cell cycling in all three CSC cell lines. (C) No difference in cell cycle inhibition in response to TGF-β stimulation between C133+ and CD133− cells isolated from each CSC clone line using [3H]-thymidine incorporation. (B, C) Twenty-four-hour TGF-β stimulation, 2-hour [3H]-thymidine pulse, percent inhibition after TGF-β stimulation compared with serum-free controls, each line in triplicate, + standard deviation (P > 0.05 for each line CD133+ versus CD133−).
No Difference in TGF-β Signal Proteins
In order to examine if TGF-β signal pathway elements were differentially expressed in CD133+ and CD133− cells, we used standard immunoblot assays to measure the protein levels of TGF-β receptor, Smad2/3, and Smad4, as well as the inhibitory Smad6/7 proteins. There was no substantial difference in the protein expression of either TGF-β receptor or Smad proteins between CD133+ and CD133− cells with and without TGF-β stimulation (Supplementary Fig. 3). We could not detect Smad6/7 proteins in either CD133− or CD133+ cells.
Analysis of Expression of TGF-β Inducible Genes Related to Cell Cycle Regulation
To further characterize any difference in the responsiveness after TGF-β–induced G1 phase cell cycle arrest, we used TaqMan real-time RT-PCR to examine TGF-β–regulated genes. p15INK4b(p15) and p21WAF1/CIP1(p21), the potent inhibitors of cyclin-dependent kinases, function as cell cycle inhibitors by blocking cyclin D and cyclin E. As shown in Fig. 4A, in both CD133− and CD133+ cells, the expression of p21 was up-regulated, whereas cyclin D1 and c-myc were down-regulated 4 hours after TGF-β stimulation. The expression of c-myc and cyclin D1 remained at a suppressed level 12 hours after TGF-β treatment (Fig. 4B). There was no significant difference between CD133+ and CD133− cells in the fold changes of p15, p21, c-myc, and cyclin D1 messenger RNA (mRNA) levels after TGF-β stimulation.
Fig. 4.
Real-time PCR analysis of CD133+ and CD133− cells in response to TGF-β stimulation. Real-time PCR analysis of TGF-β down-stream targets, p15 and p21, and cell cycle regulators, c-myc and cyclin D1, (A) 4 hours and (B) 12 hours after TGF-β stimulation (5 ng/mL). No significant difference between CD133+ and CD133− cells was detected (2 × 104 cells/cm2 plated, data presented as fold change in expression, n = 3 lines each in triplicate + standard error).
CD133+ Cells Demonstrate Resistance to TGF-β–Induced Apoptosis
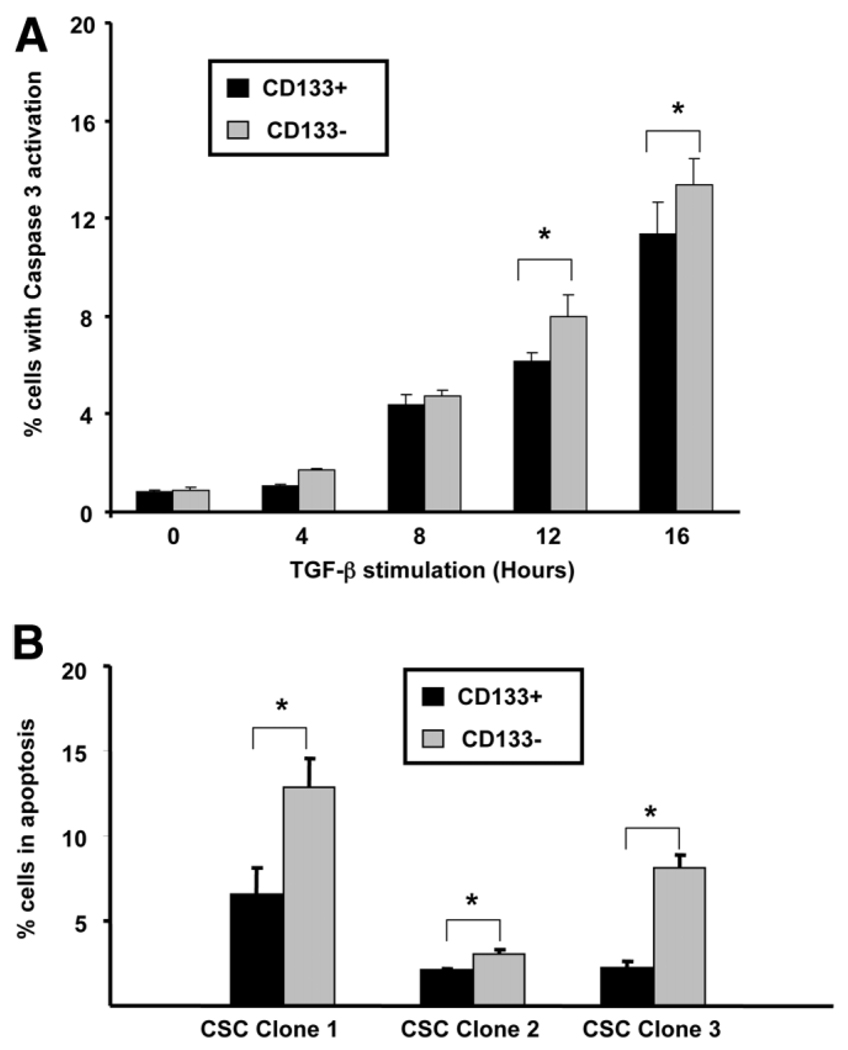
TGF-β can function by inhibition of cell cycle and induction of apoptosis in murine primary hepatocytes and hepatocytic cell lines,21 as well as several HCC cell lines.27,28 Apoptosis was determined using DNA laddering, activated caspase-3 labeling, and annexin V/PI staining. When CSC clone lines were exposed to TGF-β1 for 24 hours, DNA laddering was detectable in both the detached and the attached fractions but not in control serum-free cells (Supplementary Fig. 4). Using activated caspase-3 FACS analysis, the number of apoptotic cells increased in both CD133+ and CD133− cell fractions with increased time of TGF-β stimulation (Fig. 5A). For all future experiments, we chose a 12-hour time point of TGF-β incubation. When we tested CD133+ and CD133− cells, obtained from the same culture plate of the CSC clone lines, the CD133+ cells (lines 1–3) demonstrated a significant resistance to TGF-β–induced apoptosis compared with CD133− cells, displaying a 1.5-to 3-fold reduction in the number of apoptotic cells stained with annexin V/PI on FACS analysis (Fig. 5B).
Fig. 5.
CD133+ cells demonstrate resistance to TGF-β–induced apoptosis. (A) Timing of TGF-β–induced apoptosis in CD133+ and CD133− cells with activated caspase-3 levels increasing from 4 to 16 hours using intracellular active caspase-3 FACS analysis (2 × 104 cells/cm2 plated from CSC clone line 1, each time point in triplicate + standard deviation). *P < 0.05. (B) CD133+ cells demonstrate less apoptosis compared with CD133− cells from the same culture plates from each of the CSC clone lines 1–3. Apoptosis determined using annexin V/PI staining and FACS analysis (2 × 104 cells/cm2 plated, each line in triplicate, 50,000 events counted/replicate + standard error). *P < 0.05.
MAPK/Erk Was Constitutively Activated in Mat1a−/− Clone Lines
In the mRNA microarray analysis, the Ras/MAPK/Erk signal pathway components (RAB5C RAS oncogene, MEK1, and p14) are all up-regulated in CD133+ cells compared with CD133− cells (Supplementary Fig. 5A). Among these genes, MEK1 lies upstream of MAP/Erk, and MEK1 stimulates the enzymatic activity of MAPKs. To test the hypothesis that the Ras/MAPK/Erk pathway may execute an antiapoptotic role in Mat1a−/− CD133+ CSCs, we isolated CD133− and CD133+ cells from CSC clone lines to determine the activated Erk levels. As shown in Fig. 6A,B, Erk was constitutively phosphorylated in both CD133− and CD133+ cells, with an overall 1.8-fold increase in phosphorylated Erk1/2 (p-Erk1/2) level in CD133+ cells compared with CD133− cells when signals were normalized with pan-Erk1/2. In addition, as shown in Fig. 6C, TGF-β suppressed p-Erk1/2 after a short period of TGF-β exposure in both populations.
Fig. 6.
Elevated p-Erk in CD133+ cells. (A) Western blot analysis demonstrates elevated p-Erk in CD133+ cells compared with CD133− cells in each of the CSC clone lines 1–3. pan-Erk was used as a loading control. (B) Densitometry analysis of all three CSC lines revealed a significant increase in p-Erk levels in CD133+ cells (2 × 104 cells/cm2 plated, n = 3 lines + standard error). *P < 0.05. (C) Western blot analysis demonstrated that TGF-β stimulation results in a decrease in p-Erk levels, with a smaller decrease in CD133+ cells.
Specific MEK1 Chemical Inhibitor Increased Sensitivity to TGF-β–Induced Apoptosis in CD133+ Cells
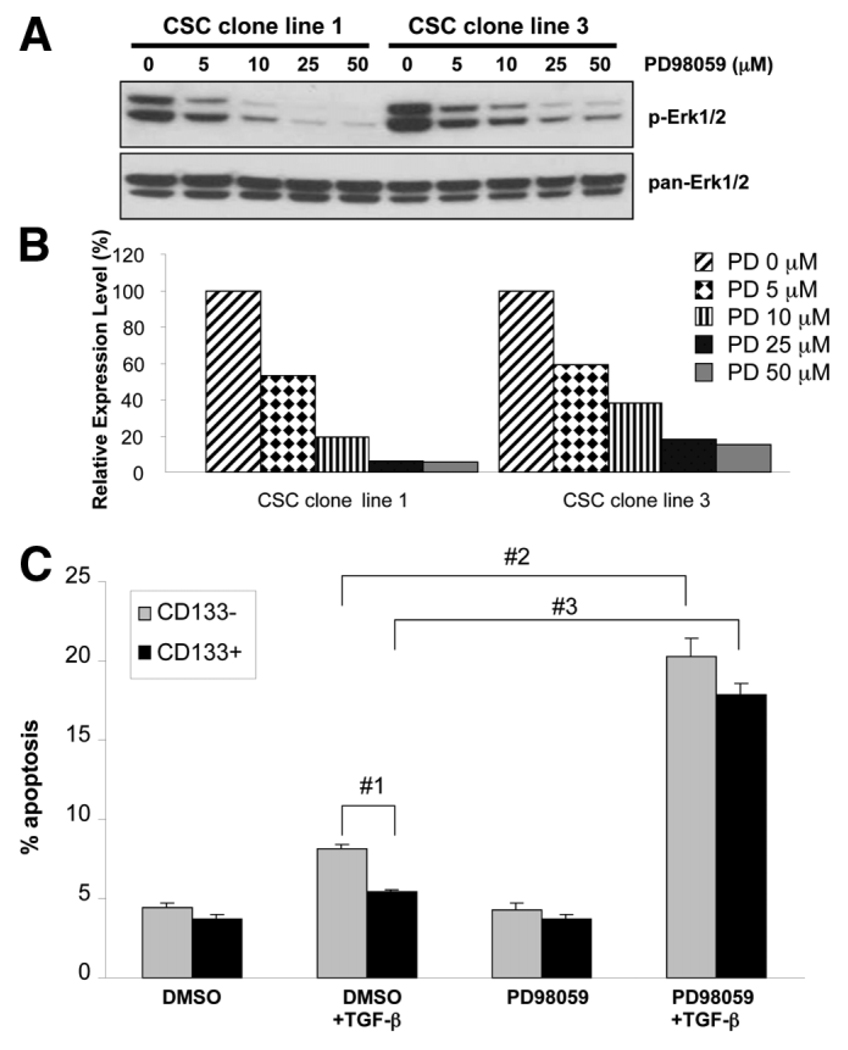
In order to verify whether blockade of the MAPK pathway is capable of reversing the resistance of Mat1a−/− CD133+ CSCs to TGF-β–induced apoptosis, we used PD98059, an inhibitor that blocks MEK1, the upstream kinase of Erk1/2. As shown in Fig. 7A, p-Erk1/2 levels were reduced in a dose-dependent manner by PD98059. At 25 µM of PD98059, p-Erk1/2 was inhibited 80% to 90% in CSC clone lines 1 and 3 (Fig. 7B). CD133+ and CD133− cells from the CSC clone lines were treated with 25 µM of PD98059 for 1 hour, cultured in serum-free medium for 1 hour, and stimulated with 5 ng/mL of TGF-β1 for 12 hours. As shown in Fig. 7C, 5.4 ± 0.2% of CD133+ cells underwent apoptosis upon TGF-β stimulation after DMSO pre-treatment. After pretreatment with PD98059, TGF-β stimulation significantly increased the number of CD133+ cells undergoing apoptosis to 17.8 ± 0.4% (P < 0.05 versus DMSO group), demonstrating that the survival advantage of CD133+ cells is reversed with MEK1 inhibition. A similar increase in apoptosis was also observed in CD133− cells after PD98059/TGF-β treatment. Pretreatment with either DMSO or PD98059 without TGF-β stimulation did not result in a significant change in the number of apoptotic cells.
Fig. 7.
Mek1 inhibition reverses survival advantage of CD133+ cells by decreasing p-Erk levels. The MEK1 inhibitor PD98059 was used to block Erk activation. (A) Erk phosphorylation decreased with increasing dose of PD98059. pan-Erk was used as a loading control. (B) Graph of densitometry comparing p-Erk with pan-Erk in CSC cell lines 1 and 3. (C) PD98059 reverses the survival advantages of CD133+ cells. TGF-β stimulation with PD98059 pretreatment resulted in a significant increase in apoptosis in both CD133+ and CD133− cells. Apoptosis measured using annexin V/PI staining and FACS analysis (2 × 104 cells/cm2 plated, n = 3, 50,000 events/replicate + standard error). #1,#2,#3P < 0.05.
CA-MEK1 Protects CD133− Cells from TGF-β–Induced Apoptosis
In order to determine if superactivated MAPK signals are capable of antagonizing the apoptosis induced by TGF-β in CD133− cells, we employed an adenoviral construct that expresses CA-MEK1. CA-MEK1 contains S218E/S222E mutations and is activated without ligand binding.29 To determine appropriate adenovirus concentrations, Mat1a−/− CSC clone lines were infected with adenovirus-expressing β-Galactosidase (β-Gal) with an MOI of 0, 5, 10, 25, 50, and 100. Twenty-four hours after adenoviral infection, over 95% of cells were positively stained with X-Gal at MOI of 100 adenovirus and 80% of cells contain positive staining at MOI 50 (Supplementary Fig 6). When we infected CSC clone lines with CA-MEK1 adenovirus, Erk1/2 was phosphorylated in a dose-dependent manner (Fig. 8A,B).
Fig. 8.
Forced increase in p-Erk provides a survival advantage in CD133− cells. Adenovirus vectors with β-Gal or CA-MEK1 were used at different MOI. (A) Western blot of p-Erk and pan-Erk demonstrated a marked increase in p-Erk levels in cells infected with CA-MEK1 adenovirus compared with β-Gal adenovirus, with increased levels at higher MOI. (B) Graph of densitometry analysis of p-Erk/pan-Erk ratio demonstrates a marked increase in the p-Erk/pan-Erk ratio with increasing MOI of CA-MEK1 adenovirus. (C) CA-MEK1 confers a survival advantage in CD133− cells after TGF-β stimulation (2 × 104 cells/cm2 plated annexin V/PI FACS analysis, n = 3, 50,000 events/replicate + standard error). #1,#2,#3P < 0.05.
To confirm that CA-MEK1 is capable of antagonizing TGF-β–induced apoptosis in CD133− cells from CSC clone lines, we used adenoviral infection with MOI 50. Both CD133− and CD133+ cells were infected with either β-Gal or CA-MEK1 adenovirus for 24 hours. As shown in Fig. 8C, the number of apoptotic cells was significantly increased in CD133− cells infected with β-Gal adenovirus 12 hours after TGF-β stimulation compared with CD133− cells infected with CA-MEK1 adenovirus (β-Gal 15.3 ± 1.6% versus CA-MEK1 2.2 ± 0.2% [P< 0.001]). CD133+ cells infected with either β-Gal or CA-MEK1 demonstrated a relative resistance to TGF-β-induced apoptosis compared with CD133− cells in either the β-Gal or CA-MEK1 group. To further test the CD133− cell survival after CA-MEK1 adenoviral infection, we tested the cell viability using the XTT assay. CD133− cell viability was significantly reduced in cells infected with β-Gal adenovirus after TGF-β treatment, and CD133− cell viability remained at pretreatment levels in the CA-MEK1 adenovirus-infected cells (Supplementary Fig. 7). These results further indicated that superactivated MAPK pathway signaling in CD133+ cancer stem cells provides a protective role against TGF-β–induced apoptosis.
Induction of CD133 Expression in Cells with Superactivated Erk
Our final series of experiments tested the potential relationship between CD133 expression and the superactivated MAPK pathway. The CA-MEK1 adenoviral construct was able to induce a five-fold increase in CD133 expression compared with the β-Gal adenovirus, as measured by FACS CD133 cell surface staining (β-Gal 0.5 ± 0.3% versus CA-MEK1 2.3 ± 0.3% [P < 0.05]) (Supplementary Fig. 8). Inhibition of MEK-1 with PD98059 had no significant effect on CD133 expression (data not shown).
Discussion
Our previous study demonstrated that CD133+ cells represent a liver CSC population in Mat1a−/− mice.11 Given this work, our primary goal was to determine a mechanism of CD133+ CSC survival during chronic injury.
Liver stem cells proliferate during chronic liver injury.22 The majority of HCC develops on this background of chronic injury, such as during chronic hepatitis B or C infection.1,30 During chronic injury due to viral infection, TGF-β is produced by non-parenchymal cells and acts as a negative regulator of hepatocyte proliferation.31 Under this circumstance, liver stem cells with an ability to antagonize the cell growth inhibitory or apoptotic effects of TGF-β are potentially able to repopulate the damaged liver. Although deregulated TGF-β has been studied in HCC progression,16 the exact role of TGF-β in the homeostasis of liver progenitor cells remains largely unknown.
In some studies, hepatic progenitor cells display resistance to proapoptotic and antiproliferative effects of endogenous TGF-β compared with the well-differentiated mature hepatocytes.32 In fetal hepatocytes, Sanchez et al.33 observed that 50% of the cells survive despite increasing concentration of TGF-β. These surviving fetal liver cells were less differentiated with respect to liver-specific transcription factor activity, were still able to undergo growth arrest in response to TGF-β, and appeared completely unresponsive to TGF-β–induced apoptosis.
In terms of progression from chronic injury to HCC, several studies have indicated that a significant subset of HCC originates from liver CSCs. In studies of established HCC cell lines such as Huh7, only cells expressing CD133 are capable of expanding and forming tumors in vivo.34 Given that CD133 is a marker of oval cells22 as well as liver CSCs, we postulate that these tumorigenic CD133+ CSCs, isolated from Mat1a−/− mice, are derived from liver stem cells.11
In our current study, we used Mat1a−/− mice to demonstrate that tumorigenic CD133+ liver progenitor cells have acquired a survival advantage against TGF-β–induced apoptosis. Compared with CD133− cells, we did not see a significant difference in the cell growth inhibition by TGF-β in CD133+ cells. In addition, when comparing CD133+ to CD133− cells, we also did not observe a significant change in mRNA levels for the cell cycle proteins p15, p21, cyclin D1, and c-myc.
Furthermore, in both CD133− and CD133+ cells, the inhibitory proteins Smad6/7 are not detectable; and there was a very low level of Smad6/7 mRNA expression. In one study, rat oval cells were less sensitive to TGF-β–induced cell growth inhibition due to the up-regulated Smad6.19 This study suggests that inhibitory Smad6 plays a critical role in the regulation of cell proliferation in oval cells. In our study, the very low levels of Smad6/7 mRNA and undetectable protein in Mat1a−/− CSC clone lines may explain why both CD133− and CD133+ cells are equally sensitive to TGF-β growth arrest. Furthermore, it has been reported that TGF-β–mediated apoptosis is not dependent on the Smad pathway,35 indicating that the cell growth inhibitory and apoptotic effects of TGF-β are mediated by distinct signaling pathways.
In this study, up-regulated MAP kinase signaling was associated with C133+ cell survival against TGF-β–induced apoptosis. Up-regulated MAPK signaling has been well documented in HCC,36 indicating that Erk activation is important for liver cancer cell proliferation and survival. In chronic viral hepatitis, hepatitis C virus core protein and hepatitis B × gene protein can activate the Ras/MAPK/Erk pathway and play critical roles in the initiation and development of HCC.37,38 Alterations in the MAPK pathway with elevated Erk levels have been described in Mat1a deletion mice, which develop HCC spontaneously by 18 months.39 Moreover, the specific inhibitors of MEK1/2, PD98059, and U0126 and Erk1/2 antisense oligonucleotide can inhibit HCC cellular proliferation in a dose-dependent manner.40 However, the dysregulation of Ras/MAPK/Erk signals in the initiation and maintenance of liver CSCs remains largely unknown. Interestingly, a recent report indicates that mitogen-activated protein kinase 2, a member of the MAPK/Erk pathway, was up-regulated in prostate progenitor cells expressing CD133.41
We previously demonstrated increased k-Ras expression within specific populations of tumorigenic stem cells isolated from Mat1a-deleted mice.11 We now demonstrate that activated MAPK signaling appears to confer a relative resistance to TGF-β–induced apoptosis in CD133+ cells compared with CD133− cells. For the first time, our results demonstrate that increased MAPK signaling results in an overactivated Erk1/2 specifically in CD133+ CSCs. Our working hypothesis is that CD133+ CSCs play a critical role in the tumorigenesis through an acquired survival advantage: resistance to TGF-β–mediated apoptosis. Furthermore, our observations suggest that aberrant MAPK/Erk pathway in liver cancer stem cells may play a pivotal role in the initiation and development of HCC.
The molecular mechanism of TGF-β and MAP kinase signals in the homeostasis of liver stem cells still needs to be elucidated. Our future work will focus on the mechanism underlying the transcriptional/translational regulation of CD133 in liver stem cells and to define molecular therapeutic targets of liver CSCs.
Supplementary Material
Acknowledgment
We thank Dr. Jeffery D. Molkentin (Department of Pediatrics, Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, OH) for providing adenoviruses encoding constitutively active MEK1 and Dr. Jane McAllister (Department of Cellular & Molecular Physiology, the Penn State College of Medicine, Hershey, Pennsylvania) for providing adenoviral β-Gal construct. We would like to acknowledge Drs. Vrana and Freeman of the Functional Genomics Core at the Penn State College of Medicine. Important Penn State Functional Genomics Core Facility instrument purchases were made possible through Tobacco Settlement Funds and through the Penn State Cancer Institute contract with the Department of the Navy.
Supported by an AGA/AstraZeneca Fellow/Faculty Transition Award (to C. B. R.). This study was made possible by Grant D1BTH06321-01 from the Office for the Advancement of Telehealth, Health Resources and Services Administration, Department of Health and Human Services (to C. B. R.); National Institutes of Health Grants 1K08DK080928-01 (to C. B. R.), DK51719 (to S. C. L.), and AT1576 (to S. C. L. and J. M. M.); and Plan Nacional of I+D SAF 2005-00855, HEPADIP-EULSHM-CT-205, and ETORTEK-2005 (to J. M.).
Abbreviations
- CA-MEK1
constitutively active form of MEK1
- CSC
cancer stem cell
- Erk
extracellular signal-regulated kinase
- FACS
flow cytometry; β-Gal, β-galactosidase
- HCC
hepatocellular carcinoma
- MAPK
mitogen-activated protein kinase
- MAT
methionine adenosyltransferase
- MEK1
mitogen-activated protein kinase kinase 1
- MOI
multiplicity of infection
- mRNA
messenger RNA
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- p-Erk
phosphorylated extracellular signal-regulated kinase
- PI
propridium iodine
- RT-PCR
reverse-transcription polymerase chain reaction
- TGF-β
transforming growth factor β
- XTT
2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide.
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–S78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Fuster J, Bruix J. Prognosis of hepatocellular carcinoma. Hepatogastroenterology. 2002;49:7–11. [PubMed] [Google Scholar]
- 3.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 4.Gou S, Liu T, Wang C, Yin T, Li K, Yang M, et al. Establishment of clonal colony-forming assay for propagation of pancreatic cancer cells with stem cell properties. Pancreas. 2007;34:429–435. doi: 10.1097/MPA.0b013e318033f9f4. [DOI] [PubMed] [Google Scholar]
- 5.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 6.Miki J, Furusato B, Li H, Gu Y, Takahashi H, Egawa S, et al. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 2007;67:3153–3161. doi: 10.1158/0008-5472.CAN-06-4429. [DOI] [PubMed] [Google Scholar]
- 7.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 8.Sell S, Dunsford HA. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989;134:1347–1363. [PMC free article] [PubMed] [Google Scholar]
- 9.Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. HEPATOLOGY. 2006;44:240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 10.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 11.Rountree CB, Senadheera S, Mato JM, Crooks GM, Lu SC. Expansion of liver cancer stem cells during aging in methionine adenosyltransferase 1A-deficient mice. HEPATOLOGY. 2008;47:1288–1297. doi: 10.1002/hep.22141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mato JM, Lu SC. Role of S-adenosyl-L-methionine in liver health and injury. HEPATOLOGY. 2007;45:1306–1312. doi: 10.1002/hep.21650. [DOI] [PubMed] [Google Scholar]
- 13.Avila MA, Berasain C, Torres L, Martin-Duce A, Corrales FJ, Yang H, et al. Reduced mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J Hepatol. 2000;33:907–914. doi: 10.1016/s0168-8278(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 14.Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, et al. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci U S A. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulouarn C, Factor VM, Thorgeirsson SS. Transforming growth factor-beta gene expression signature in mouse hepatocytes predicts clinical out-come in human cancer. HEPATOLOGY. 2008;47:2059–2067. doi: 10.1002/hep.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitisin K, Ganesan N, Tang Y, Jogunoori W, Volpe EA, Kim SS, et al. Disruption of transforming growth factor-beta signaling through beta-spectrin ELF leads to hepatocellular cancer through cyclin D1 activation. Oncogene. 2007;26:7103–7110. doi: 10.1038/sj.onc.1210513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massague J, Andres J, Attisano L, Cheifetz S, Lopez-Casillas F, Ohtsuki M, et al. TGF-beta receptors. Mol Reprod Dev. 1992;32:99–104. doi: 10.1002/mrd.1080320204. [DOI] [PubMed] [Google Scholar]
- 18.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen LN, Furuya MH, Wolfraim LA, Nguyen AP, Holdren MS, Campbell JS, et al. Transforming growth factor-beta differentially regulates oval cell and hepatocyte proliferation. HEPATOLOGY. 2007;45:31–41. doi: 10.1002/hep.21466. [DOI] [PubMed] [Google Scholar]
- 20.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 21.Gressner AM, Lahme B, Mannherz HG, Polzar B. TGF-beta-mediated hepatocellular apoptosis by rat and human hepatoma cells and primary rat hepatocytes. J Hepatol. 1997;26:1079–1092. doi: 10.1016/s0168-8278(97)80117-1. [DOI] [PubMed] [Google Scholar]
- 22.Rountree CB, Barsky L, Ge S, Zhu J, Senadheera S, Crooks GM. A CD133-expressing murine liver oval cell population with bilineage potential. Stem Cells. 2007;25:2419–2429. doi: 10.1634/stemcells.2007-0176. [DOI] [PubMed] [Google Scholar]
- 23.Ilangovan U, Ding W, Zhong Y, Wilson CL, Groppe JC, Trbovich JT, et al. Structure and dynamics of the homodimeric dynein light chain km23. J Mol Biol. 2005;352:338–354. doi: 10.1016/j.jmb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Rountree CB, Senadheera S, Mato JM, Crooks GM, Lu SC. Expansion of liver cancer stem cells during aging in methionine adenosyltransferase 1A-deficient mice. HEPATOLOGY. 2008;47:1288–1297. doi: 10.1002/hep.22141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson-Degrave VL, Wickenheisser JK, Hendricks KL, Asano T, Fujishiro M, Legro RS, et al. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol. 2005;19:379–390. doi: 10.1210/me.2004-0178. [DOI] [PubMed] [Google Scholar]
- 26.Nagy P, Bisgaard HC, Thorgeirsson SS. Expression of hepatic transcription factors during liver development and oval cell differentiation. J Cell Biol. 1994;126:223–233. doi: 10.1083/jcb.126.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inagaki M, Moustakas A, Lin HY, Lodish HF, Carr BI. Growth inhibition by transforming growth factor beta (TGF-beta) type I is restored in TGF-beta-resistant hepatoma cells after expression of TGF-beta receptor type II cDNA. Proc Natl Acad Sci U S A. 1993;90:5359–5363. doi: 10.1073/pnas.90.11.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Ozaki I, Mizuta T, Yoshimura T, Matsuhashi S, Eguchi Y, et al. Transforming growth factor-beta 1-induced apoptosis is blocked by beta 1-integrin-mediated mitogen-activated protein kinase activation in human hepatoma cells. Cancer Sci. 2004;95:878–886. doi: 10.1111/j.1349-7006.2004.tb02197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 30.Chan HL, Sung JJ. Hepatocellular carcinoma and hepatitis B virus. Semin Liver Dis. 2006;26:153–161. doi: 10.1055/s-2006-939753. [DOI] [PubMed] [Google Scholar]
- 31.Grasl-Kraupp B, Rossmanith W, Ruttkay-Nedecky B, Mullauer L, Kammerer B, Bursch W, et al. Levels of transforming growth factor beta and transforming growth factor beta receptors in rat liver during growth, regression by apoptosis and neoplasia. HEPATOLOGY. 1998;28:717–726. doi: 10.1002/hep.510280318. [DOI] [PubMed] [Google Scholar]
- 32.Clark JB, Rice L, Sadiq T, Brittain E, Song L, Wang J, et al. Hepatic progenitor cell resistance to TGF-beta1’s proliferative and apoptotic effects. Biochem Biophys Res Commun. 2005;329:337–344. doi: 10.1016/j.bbrc.2005.01.129. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez A, Alvarez AM, Lopez Pedrosa JM, Roncero C, Benito M, Fabregat I. Apoptotic response to TGF-beta in fetal hepatocytes depends upon their state of differentiation. Exp Cell Res. 1999;252:281–291. doi: 10.1006/excr.1999.4624. [DOI] [PubMed] [Google Scholar]
- 34.Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 35.McGinnis KM, Gnegy ME, Park YH, Mukerjee N, Wang KK. Procaspase-3 and poly(ADP)ribose polymerase (PARP) are calpain substrates. Biochem Biophys Res Commun. 1999;263:94–99. doi: 10.1006/bbrc.1999.1315. [DOI] [PubMed] [Google Scholar]
- 36.Huynh H, Nguyen TT, Chow KH, Tan PH, Soo KC, Tran E. Over-expression of the mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: its role in tumor progression and apoptosis. BMC Gastroenterol. 2003;3:19. doi: 10.1186/1471-230X-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi J, Aoki H, Kajino K, Moriyama M, Arakawa Y, Hino O. Hepatitis C virus core protein activates the MAPK/ERK cascade synergistically with tumor promoter TPA, but not with epidermal growth factor or transforming growth factor alpha. HEPATOLOGY. 2000;32:958–961. doi: 10.1053/jhep.2000.19343. [DOI] [PubMed] [Google Scholar]
- 38.Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Chantar ML, Corrales FJ, Martinez-Cruz LA, Garcia-Trevijano ER, Huang ZZ, Chen L, et al. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J. 2002;16:1292–1294. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- 40.Wiesenauer CA, Yip-Schneider MT, Wang Y, Schmidt CM. Multiple anticancer effects of blocking MEK-ERK signaling in hepatocellular carcinoma. J Am Coll Surg. 2004;198:410–421. doi: 10.1016/j.jamcollsurg.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Shepherd CJ, Rizzo S, Ledaki I, Davies M, Brewer D, Attard G, et al. Expression profiling of CD133+ and CD133− epithelial cells from human prostate. Prostate. 2008;68:1007–1024. doi: 10.1002/pros.20765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.