Abstract
Dopaminergic neurotransmission has been implicated in associative learning processes related to drugs of abuse. However, it is not clear whether blockade of activation of dopamine receptors alters conditioned incentive properties of nicotine-associated cues. Using a response-reinstatement procedure, this study examined the effects of antagonists selective for the D1 and the D2 subtypes of dopamine receptors on cue-induced reinstatement of nicotine-seeking behavior. Male Sprague-Dawley rats were trained in 30 daily 1 h sessions to intravenously self-administer nicotine (0.03 mg/kg/infusion) on a FR5 schedule and associate a conditioned stimulus (cue) with each nicotine delivery. After extinction of responding by withholding nicotine (saline substitution) and its cue, the reinstatement tests were conducted following subcutaneous administration of a D1 antagonist SCH23390 (0, 5, 10, 30 μg/kg) or a D2 antagonist eticlopride (0, 5, 10, 30 μg/kg) in different groups of animals. Both SCH23390 and eticlopride significantly attenuated the magnitude of cue-elicited reinstatement of nicotine-seeking responding. These results indicate that activation of dopaminergic D1 and D2 receptors may play a role in mediating the conditioned motivational effects of nicotine-associated cues as measured in the response-reinstatement procedure. These findings suggest that manipulation of dopaminergic neurotransmission at the D1 and/or D2 receptors may prove to be a potential target for the development of pharmacotherapy for prevention of environmental nicotine cue-triggered smoking relapse.
Keywords: Antagonists, conditioned stimuli, dopamine receptors, eticlopride, nicotine-seeking, reinstatement, SCH23390, self-administration, rat
Introduction
Cigarette smoking and nicotine dependence is a chronic relapsing disorder. The high rates of relapse in abstinent smokers present a formidable challenge for long-term success of smoking cessation treatments. One important factor critically contributing to the resumption of drug use in abstinent addicts is exposure to environmental stimuli previously associated with drug intake (Carter and Tiffany 1999; Childress et al. 1993; Drummond et al. 1995; Niaura et al. 1989; O’Brien et al. 1998; Stewart et al. 1984). Indeed, presentation of cigarette cues such as the visual stimuli that were previously associated with nicotine self-administration via cigarette smoking has been shown to reliably elicit strong craving for smoking along with physiological responses (Carter and Tiffany 1999; LaRowe et al. 2007; Miranda et al. 2008; Niaura et al. 1988; Sayette et al. 2001). Recently, using an operant response-reinstatement procedure in laboratory animals it has been demonstrated that cues conditioned to intravenous nicotine self-administration effectively reinstate nicotine-seeking behavior after extinction (Cohen et al. 2005; LeSage et al. 2004; Liu et al. 2006, 2007, 2008; Paterson et al. 2005). These animal studies not only provide experimental support for the significance of environmental cues in triggering relapse to smoking in abstinent smokers but also validate the procedure as a reliable animal model for elucidation of the mechanisms of nicotine-seeking behavior and for medication development.
Corticomesolimbic dopaminergic neurotransmission has been implicated in mediation of the reinforcing actions of nicotine (Balfour et al. 1998; Clarke 1990; Di Chiara 2000; Picciotto and Corrigall 2002; Watkins et al. 2000). For instance, acute nicotine administration increases in vivo dopamine release in the nucleus accumbens, a terminal field of the dopamine circuitry (Balfour et al. 1998; Brazell et al. 1990; Damsma et al. 1989; Di Chiara and Imperato 1988; Ferrari et al. 2002; Maisonneuve et al. 1997; Marshall et al. 1997; Mifsud et al. 1989; Nisell et al. 1994). Neuroadapative changes in the corticomesolimbic dopamine system have been observed after chronic nicotine exposure via either active self-administration or passive administration by experimenters (Benwell et al. 1995; Carboni et al. 2000; Rahman et al. 2004). In nicotine self-administration studies, 6-hydroxydopamine-induced lesion of the nucleus accumbens (Corrigall et al. 1992) and blockade of activation of the D1 and D2 receptors (Corrigall and Coen 1991) significantly reduced nicotine intake.
Recently, increasing human studies using functional magnetic resonance imaging and positron emission tomography indicate that activation of the corticomesolimbic dopamine circuitry may also be implicated in mediation of the conditioned responses to smoking/nicotine cues. In both clinical and preclinical settings, exposure to environmental stimuli previously associated with cigarette smoking and nicotine intake has been found to activate neurotransmission in the components of corticomesolimbic dopamine circuitry, such as anterior cingulate cortex, amygdale, nucleus accumbens (Brody et al. 2007; David et al. 2005; Franklin et al. 2007; McBride et al. 2006; McClernon et al. 2007b; Schiltz et al. 2005; Schroeder et al. 2001; Smolka et al. 2006). Genetic analysis studies have revealed significant association of smoking cue reactivity including cigarette craving with genetic polymorphism in the components of dopaminergic neurotransmission pathways (Erblich et al. 2005; Franklin et al. 2009; Hutchison et al. 2002; McClernon et al. 2007a; Munafo and Johnstone 2008).
In animal studies, however, little attention has been paid to the issue of whether dopaminergic neurotransmission plays a role in mediation of the conditioned incentive properties of nicotine-associated cues. To address this issue, the present study, by using a response reinstatement procedure in rats, examined whether pharmacological blockade of dopaminergic neurotransmission alters the behavioral motivation by a nicotine-associated cue. Specifically, this study examined the effects of the dopamine D1 antagonist SCH23390 and the D2 antagonist eticlopride on cue-induced reinstatement of extinguished nicotine-seeking behavior.
Methods
Subjects
Male Sprague-Dawley rats (Charles River), weighing 225–250 g at arrival, were used. Animals were individually housed in a humidity- and temperature-controlled (21–22 °C) vivarium on a reversed light/dark cycle (lights on 19:00 h; off 07:00 h) with unlimited access to water. After the first week of acclimation to the vivarium, rats were placed on a food-restriction (20 g chow/day) regimen throughout the experiments. Training and experimental sessions were conducted during the dark phase at the same time each day (09:00 – 15:00 h). All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996).
Apparatus
Self-administration training, extinction, and reinstatement test sessions were conducted in operant conditioning chambers located inside sound-attenuating, ventilated cubicles (Med Associates, St. Albans, VT). The chambers were equipped with two retractable response levers on one side panel and with a 28-V white light above each lever as well as a red chamber light on the top of the chambers. Between the two levers was a food pellet trough. Intravenous nicotine injections were delivered by a drug delivery system with a syringe pump (Med Associates, model PHM100-10 rpm). All experimental events and data collection were automatically controlled by an interfaced computer.
Food Training
To facilitate nicotine self-administration training, rats were first trained to press a lever for food reward. These food training sessions began on the second day of food-restriction regimen. Introduction of levers and illumination of the red chamber light signaled the start of the sessions. The right lever was assigned as the active lever, each response on which was rewarded with delivery of a 45 mg food pellet. Sessions lasted 1 hour with a maximum delivery of 45 food pellets. Within 3–5 sessions the reinforcement schedule was increased to a fixed-ratio (FR) 5. Food training finished after rats earned all the 45 food pellets on the FR5 schedule. During these food training sessions, no stimulus other than constant illumination of the red chamber light was presented.
Surgery
After food training, the rats were anesthetized with an isoflurane-oxygen mixure (1–3% isoflurane) and implanted with jugular catheters. Catheters were constructed from a 15 cm piece of Silastic tubing (0.31 mm ID and 0.63 mm OD, Dow Corning) attached to a 22 gauge stainless steel guide cannula. The latter was bent and molded onto a durable polyester mesh (Plastic One) with dental cement and became the catheter base. Through an incision on the rat back, the base was anchored underneath the skin at the level of scapula and the catheter passed subcutaneously to the ventral lower neck region and inserted into the right jugular vein (≈3.5 cm). Animals were allowed at least 7 days to recover from surgery. During the recovery period, the catheters were flushed daily with 0.1 ml of sterile saline containing heparin (30 U/ml) and Timentin (66.7 mg/ml) to maintain catheter patency and prevent infection. Thereafter, the catheters were flushed with the heparinized saline prior to and after the experimental sessions throughout the studies. If there was no session on the weekend, the catheter was flushed once/day.
Nicotine self-administration/conditioning
After recovery from surgery, rats were trained to self-administer nicotine intravenously (0.03 mg/kg/infusion, free base) and associate a cue with nicotine delivery. In the training sessions, animals were placed in the operant conditioning chambers and connected to a drug delivery system. The daily 1-h sessions were initiated by introduction of the two levers and illumination of the red chamber light. Once the FR requirement on the active lever was met, an infusion of nicotine was dispensed in a volume of 0.1 ml/kg in approximately 1 s. Each nicotine infusion was paired with a presentation of the cue consisting of a 5 s tone (83 dB) and turn-on of the active lever light for 20 s. The latter signaled a 20 s timeout period during which time responses were recorded (included in the total number of responses) but not reinforced. Responses on the inactive lever had no consequence. A FR1 schedule was used for days 1–5, a FR2 for days 6–8 and a FR5 for the remainder of the experiments. All rats received 30 daily self-administration/conditioning sessions to equalize their operant experience and nicotine intake.
Extinction
After completion of the self-administration/conditioning phase, rats were subjected to daily extinction sessions, in which the nicotine-maintained lever responding was extinguished by withholding nicotine and its associated cue. Thus, responses on the active lever resulted in the delivery of saline rather than nicotine and the cue was not presented. The FR5 schedule and the 20 s timeout period were still in effect for saline infusions. The criterion for extinction was 3 consecutive sessions in which the number of responses/session was ≤20% of that averaged across the last 3 self-administration/conditioning sessions.
Reinstatement test
One day after the final extinction session, reinstatement tests were conducted. As happened in the self-administration/conditioning and extinction phases, the test session started with introduction of the two levers and illumination of the red chamber light. During the sessions, responses on the active lever resulted in presentation of the cue and saline infusion on the FR5 schedule with the 20 s timeout period. As such, there was still no availability of nicotine. Responses on the inactive lever had no consequence. The test sessions lasted 1 hour.
Effect of SCH23390 on cue-induced reinstatement of nicotine-seeking
Rats were designated into four groups (n=8–10) in a pseudorandom order so that each group had similar profiles of lever responding and nicotine infusions. Thirty min before the reinstatement test session, SCH23390 (0, 5, 10, 30 μg/kg in a volume of 1 ml/kg, dissolved in sterile saline) was subcutaneously administered to different groups of rats. The test session was performed as described above.
Effect of eticlopride on cue-induced reinstatement of nicotine-seeking
Rats were divided into four groups (n=8–9) with a similar rate of lever responding and nicotine infusions in each group. Eticlopride (0, 5, 10, 30 μg/kg in a volume of 1 ml/kg, dissolved in sterile saline) was subcutaneously injected to different groups of rats 30 min before the reinstatement test session, which was performed as described above.
Effect of SCH23390 and eticlopride on food self-administration
Due to the fact that both SCH23390 (highest dose) and eticlopride (all three doses) effectively attenuated lever responding in the reinstatement tests (see results), the effects of these two agents at their respective effective doses on food self-administration were also examined in the respective drug/dose groups of rats. After completion of the drug/reinstatement tests, the rats from highest SCH23390 dose group and three eticlopride dose groups were re-trained to self-administer food pellets. Specifically, responses on the redesignated active lever (inactive lever during nicotine self-administration) were rewarded on a FR10 schedule with 20 s timeout period while responding on the inactive lever was not rewarded. Stable responses for food pellets were established within 7 daily 30 min sessions. Then, in the 8th and 10th sessions (no drug treatment for the 9th session) the effects of drug pretreatment were examined by using a within-subject and counterbalanced design. Individual groups received the same drug/dose treatment respectively as in the reinstatement tests. The saline vehicle control was tested within each individual drug/dose group. That is, half rats of each group received drug injection (SCH23390: 30 μg/kg; eticlopride: 5, 10, 30 μg/kg respectively) and the other half received a saline vehicle in the 8th session. The reversed treatment order happened in the 10th session.
Data Analyses
Data are presented as the mean (±SEM) number of lever responses and earned nicotine infusions. One-way ANOVA was used to analyze data obtained from the self-administration/conditioning phase averaged across the final three sessions and drug/reinstatement tests, followed by the Fisher’s PLSD post hoc tests to verify differences among individual means. Because saline vehicle control was performed within each individual drug/dose group, the data obtained from food self-administration tests were analyzed by using a paired t-test for each drug/dose group.
Results
Nicotine self-administration/conditioning and extinction
Rats readily acquired stable level of nicotine intake in the 30 daily 1-h self-administration/conditioning training sessions. Averaged across all subjects over the final 3 sessions, animals emitted mean±SEM responses of 89.5±6.2 on the active lever and 5.4±1.2 on the inactive lever. Correspondingly, rats earned 15.2±2.2 nicotine infusions in the daily 1-h sessions. In the first extinction session, animals emitted a mean±SEM number of responses of 71.6±9.5 on the active lever and 10.1± 3.3 on the inactive lever. During subsequent extinction sessions, lever responses gradually decreased. To equalize the number of the extinction sessions and synchronize the reinstatement tests with drug pretreatment, three rats were excluded during the extinction phase because they failed to meet extinction criterion within 10 sessions. Since rats were first divided into the two antagonist testing conditions and then further subdivided into 4 groups for different doses under each drug condition in a pseudorandom (counterbalanced) manner, there was no difference between the two drug conditions and among different dose groups under each individual drug condition in lever responses and nicotine infusions earned during the self-administration/conditioning, or in lever responses during the extinction phases as well as body weights immediately before the reinstatement test session, details of which are shown in Table 1.
Table 1.
Profiles of mean (± S.E.M.) lever responses and corresponding nicotine infusions averaged across the final three sessions of the self-administration/conditioning training phase, responses on the active lever averaged across the last three extinction sessions, and body weights measured before the reinstatement test session.
| Group (drug/dose) | Nicotine SA responses |
Nicotine Infusions | Extinction (active lever) | B. W (g) | |
|---|---|---|---|---|---|
| Active lever | Inactive lever | ||||
| SCH23390 (μg/kg) | |||||
| 0 | 88.9±10.2 | 7.6±2.3 | 15.3±2.6 | 18.3±2.9 | 398±20 |
| 5 | 95.6±18.2 | 11.3±5.6 | 18.1±4.1 | 19.0±3.2 | 411±28 |
| 10 | 79.8±15.2 | 8.6±2.9 | 14.9±3.1 | 16.6±2.4 | 378±25 |
| 30 | 82.1±9.9 | 6.3±3.5 | 15.1±1.8 | 18.6±2.9 | 376±18 |
| Eticlopride (μg/kg) | |||||
| 0 | 76.9±18.3 | 8.3±2.9 | 14.3±3.9 | 17.0±2.9 | 402±23 |
| 5 | 85.3±12.3 | 6.9±3.2 | 15.5±2.8 | 18.7±2.3 | 378±16 |
| 10 | 91.3±20.1 | 14.3±5.2 | 17.2±4.3 | 18.5±3.6 | 386±20 |
| 30 | 89.0±16.5 | 8.2±3.4 | 16.8±3.6 | 17.4±3.0 | 391±21 |
Effect of SCH23390 on cue-induced reinstatement of nicotine-seeking
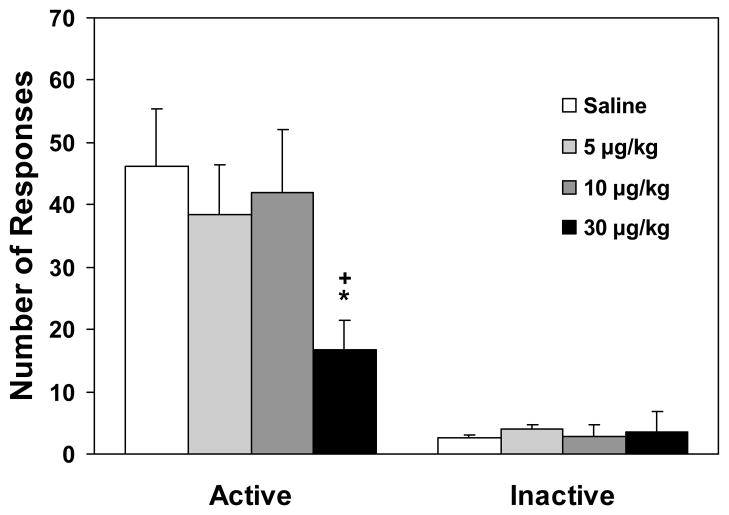
One-way ANOVA on the number of responses on the active lever yielded a near-significant dose effect [F(3,33)=2.64, p=0.06] and subsequent Fisher’s PLSD post hoc tests showed a significant (p<0.05) difference between the highest dose (30 μg/kg) versus saline control and the 10 μg/kg group (Figure 1). However, similar analysis revealed no significant change in the number of responses on the inactive lever. These data indicate that SCH23390 at 30 μg/kg reduced the magnitude of cue-induced reinstatement of nicotine-seeking behavior.
Figure 1.
Effect of SCH23390 on lever-pressing responses in the reinstatement tests (n=8–10). Data are expressed as mean±SEM. * p < 0.05 different from saline control; + p < 0.05 different from 10 μg/kg group.
Effect of eticlopride on cue-induced reinstatement of nicotine-seeking
Pretreatment with eticlopride dose-dependently decreased the magnitude of the cue-induced reinstatement of nicotine-seeking responses. One-way ANOVA on the number of responses on the active lever yielded a significant dose effect [F(3,30)=12.26, p<0.001]. Further Fisher’s PLSD post hoc tests verified significant difference among the individual group means, details of which are depicted in Figure 2. Similar analysis on the number of the inactive lever responses did not show a significant dose effect [F(3,30)=1.72, NS].
Figure 2.
Effect of eticlopride on lever-pressing responses in the reinstatement tests (n=8–9). Data are expressed as mean±SEM. * p < 0.05, *** p < 0.001 different from saline control; + p < 0.05, ++ p < 0.01, different from 5 μg/kg group.
Effect of SCH23390 and eticlopride on food self-administration
After completion of the drug/nicotine-seeking tests, rats were successfully re-trained to lever-press for food reward in 7 daily sessions. There was no observable difference among groups (for subsequent drug/dose treatment tests) in the number of both responses and food pellets earned. The detailed numbers of lever responses and food pellets earned in the last pretest session are shown in Table 2.
Table 2.
Mean (± S.E.M.) lever responses and food pellets earned in the last session of food self-administration training before drug treatment tests.
| Group (drug/dose) | Food SA responses |
Food Pellets | |
|---|---|---|---|
| Active lever | Inactive lever | ||
| SCH23390 (μg/kg) | |||
| 30 | 294±36 | 7±3 | 28±4 |
| Eticlopride (μg/kg) | |||
| 5 | 283±32 | 6±2 | 28±3 |
| 10 | 268±19 | 5±1 | 25±2 |
| 30 | 289±27 | 7±2 | 27±3 |
Figure 3 shows the total number of responses on the active lever rats made for food reinforcement after pretreatment with SCH23390 or eticlopride. There was no difference of responses between SCH23390 (30 μg/kg) and saline control condition (p>0.5). Further analysis on the number of responses pooled in 15 min bins of the session revealed no significant change after SCH23390 pretreatment. Similarly, no significant change in the number of active lever responses was observed after pretreatment with eticlopride at the two lower doses (5 and 10 μg/kg). However, there was a significant (p<0.05) difference in both the total and the first two 15 min bins in the number of active lever responses between the highest dose (30 μg/kg) of eticlopride and saline control condition, indicating a significant suppression of food self-administering behavior.
Figure 3.
Effect of SCH23390 (top) and eticlopride (below) on active lever responses for food self-administration. Data are expressed as mean±SEM. * p < 0.05 different from saline control.
In all groups, responses on the inactive lever remained quite low in the food self-administration sessions as shown in Table 2 and no change was observed after pretreatment with these drug/doses (data not shown).
Discussion
This study examined the effects of pharmacological blockade of dopaminergic neurotransmission at the D1 and D2 receptors on conditioned reinstatement of nicotine-seeking behavior after extinction. The finding that both the D1 antagonist SCH23390 and the D2 antagonist eticlopride effectively attenuated the magnitude of nicotine-seeking responding elicited by presentation of a previous nicotine-associated cue indicates a role of dopaminergic neurotransmission via the D1 and D2 receptors in mediation of the conditioned incentive properties of nicotine cues. Therefore, it is suggested that manipulations targeting these receptors may have clinical potential for prevention of drug cue-triggered relapse to tobacco smoking in abstinent smokers.
The first set of data showed that the dopamine D1 antagonist SCH23390 at its highest dose (30 μg/kg) effectively attenuated lever responding for the presentation of the nicotine cue. The lack of effect of this agent on the food self-administering behavior ruled out any nonspecific suppression of operant behavior. This result indicates that SCH23390 selectively decreased the cue-elicited reinstatement of nicotine-seeking responding. It is consistent with a previous report showing that SCH23390 suppressed nicotine cue-maintained lever responding (Cohen et al. 2005). In that study, after training for self-administration of 0.03 mg/kg/infusion nicotine with presentation of a tone/light cue (i.e., 1 s tone + 20 s lever light) lever responding was maintained by presentation of the cue without nicotine injection rather than extinguished by withholding both nicotine and its cue as happened in the present study. SCH23390 dose-dependently suppressed the cue-maintained responding. Although obtained from procedures with some differences, the present results together with the data of Cohen et al. (2005) demonstrate that blockade of dopamine D1 receptor activation decreases the conditioned incentive value of nicotine-associated cues in the animal models of nicotine-seeking. In addition, Bevins et al (2001) reported that SCH23390 blocked a contextual nicotine cue-induced increase in locomotor activity in a passive association procedure, i.e., a particular context was associated with experimenter-administered nicotine. Taken together, these findings indicate that activation of dopamine D1 receptors may play a role in mediation of the conditioned incentive properties of nicotine-associated environmental cues.
The dopamine D2 antagonist eticlopride strongly suppressed the active lever responding during the reinstatement test session. The effect was observed across all the three doses used. However, it should be noted that eticlopride at its highest dose (30 μg/kg) also reduced food self-administration behavior in the same set of rats that had completed nicotine tests and been re-trained for food pellet reward. Thus, it is likely that the highest dose of eticlopride produced nonspecific suppression on operant behavior. This effect is similar to the documentation in the literature showing that D2 antagonists are in general prone to produce a suppressant effect on locomotor activity, the cataleptogenic properties of D2 blockade (Amalric et al. 1993; Ferrari and Giuliani 1995; Skjoldager and Fowler 1988). An alternative explanation for eticlopride suppression on food responding is that the D2 receptor blockade may attenuate rats’ motivation to acquire food reward. For instance, it was previously reported that eticlopride at 30 μg/kg significantly reduced the breaking point in a progressive ratio test for food self-administration (Blokland et al. 2005). As to the two lower doses (5 and 10 μg/kg) of eticlopride, since they did not alter responding in the food self-administration tests, it could be concluded that etclopride in this dose range specifically attenuated cue-induced reinstatement of nicotine-seeking behavior and this conclusion suggests a selective decrease in conditioned incentive value of the nicotine cue by blockade of dopaminergic neurotransmission at the D2 receptors. This is the first study to demonstrate D2 antagonist attenuation of cue-reinstated nicotine-seeking behavior, so this finding extends the involvement of activation of the D2 receptors in mediation of the incentive value of cues that are conditioned to other drugs of abuse such as alcohol (Liu and Weiss 2002) to the motivational effects of nicotine-associated cues. Further, from a clinical perspective, the present results suggest that the D2 antagonists may prove to be good candidates for smoking cessation treatment, although careful selection of effective doses seems to be critical owing to the fact that these agents are prone to produce extrapyramidal side effects at higher doses.
The implication of dopaminergic neurotransmission via the D1 and D2 receptors in conditioned motivation by nicotine cues is in line with ample evidence demonstrating the important roles of activation of the D1 and D2 receptors in the mediation of conditioned reinforcing properties of the cues that are associated with other drugs of abuse as well as natural rewards such as food and water (Di Chiara and Bassareo 2007; Sutton and Beninger 1999; Wise 2006). Microdialysis and voltametric studies in rodents have demonstrated an increase in dopamine release in the mesolimbic circuitries in response to the presentation of cues that were previously conditioned to food and drugs such as cocaine and alcohol (Bassareo and Di Chiara 1999; Katner and Weiss 1999; McCullough and Salamone 1992; Roitman et al. 2004; Weiss et al. 2000). However, the precise mechanisms underlying a broad spectrum of dopamine antagonism to interfere with the conditioned incentive salience of environmental cues are not quite clear. It is possible that blockade of dopaminergic neurotransmission might reduce motivation to obtain the conditioned reinforcers (cues) and/or dampen perception of the cues. These issues remain to be investigated.
The results of the present study are complementary to reports using discriminative stimulus tests. For instance, both the D1 antagonist SCH23390 and the D2 antagonists haloperidol and spiperone have been found to decrease the discriminative stimulus effects of nicotine (Corrigall and Coen 1994a; b). A D1 agonist SKF82958 could substitute for nicotine in a discriminative stimulus test although the D2/D3 agonists did not (Gasior et al. 1999). However, in a conditioned place preference (CPP) test (Spina et al. 2006), neither the D1 nor the D2 antagonists in nucleus accumbens altered the expression of nicotine-induced CPP although the D1 (but not the D2) antagonist in the shell of the nucleus accumbens impaired the acquisition of the CPP, indicating interference with the primary reinforcement of nicotine. A recent human study using positron emission tomography did not find increased dopamine release in the ventral striatal region when tobacco-dependent smokers smoked a denicotinized cigarette (Brody et al. 2009), suggesting no significant change in dopamine activity in response to exposure to nicotine cues. These conflicting data ask for more investigation on whether and how nicotine cue exposure changes dopaminergic neurotransmission.
In conclusion, this study demonstrates that blockade of activation of the D1 and D2 receptors attenuates the motivational effects of a nicotine-associated cue in the response-reinstatement procedure. This finding suggests that manipulation of dopaminergic neurotransmission at the D1 and/or D2 receptors may prove to be a potential target for the development of pharmacotherapy for prevention of smoking relapse triggered by exposure to environmental nicotine-associated cues. Therefore, these data lend support for the continued clinical effort to test the effectiveness of antipsychotics for prevention of smoking relapse. For instance, haloperidol has been found to reduce smoking of denicotinized cigarettes, which indicates a decreased reaction to nicotine cue exposure (Brauer et al. 2001; but see Mahler and de Wit 2005), and a D2 receptor antagonist olanzapine to attenuate cue-induced craving for cigarette smoking (Hutchison et al. 2004; Rohsenow et al. 2008).
Acknowledgments
This work was supported by NIH grant DA017288 (X. Liu) from the National Institute on Drug Abuse.
References
- Amalric M, Berhow M, Polis I, Koob GF. Selective effects of low-dose D2 dopamine receptor antagonism in a reaction-time task in rats. Neuropsychopharmacology. 1993;8:195–200. doi: 10.1038/npp.1993.21. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Benwell ME, Birrell CE, Kelly RJ, Al-Aloul M. Sensitization of the mesoaccumbens dopamine response to nicotine. Pharmacol Biochem Behav. 1998;59:1021–1030. doi: 10.1016/s0091-3057(97)00537-6. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Birrell CE. Desensitization of the nicotine-induced mesolimbic dopamine responses during constant infusion with nicotine. Br J Pharmacol. 1995;114:454–460. doi: 10.1111/j.1476-5381.1995.tb13248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: dopaminergic and GABAergic influences on conditioned expression. Pharmacol Biochem Behav. 2001;68:135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Blokland A, Sik A, Lieben C. Evaluation of DOI, 8-OH-DPAT, eticlopride and amphetamine on impulsive responding in a reaction time task in rats. Behav Pharmacol. 2005;16:93–100. doi: 10.1097/00008877-200503000-00004. [DOI] [PubMed] [Google Scholar]
- Brauer LH, Cramblett MJ, Paxton DA, Rose JE. Haloperidol reduces smoking of both nicotine-containing and denicotinized cigarettes. Psychopharmacology (Berl) 2001;159:31–37. doi: 10.1007/s002130100894. [DOI] [PubMed] [Google Scholar]
- Brazell MP, Mitchell SN, Joseph MH, Gray JA. Acute administration of nicotine increases the in vivo extracellular levels of dopamine, 3,4-dihydroxyphenylacetic acid and ascorbic acid preferentially in the nucleus accumbens of the rat: comparison with caudate-putamen. Neuropharmacology. 1990;29:1177–1185. doi: 10.1016/0028-3908(90)90042-p. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL, Costello MR, Farahi J, Saxena S, Monterosso J, London ED. Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology. 2009;34:282–289. doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Bortone L, Giua C, Di Chiara G. Dissociation of physical abstinence signs from changes in extracellular dopamine in the nucleus accumbens and in the prefrontal cortex of nicotine dependent rats. Drug Alcohol Depend. 2000;58:93–102. doi: 10.1016/s0376-8716(99)00064-2. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Clarke PB. Mesolimbic dopamine activation--the key to nicotine reinforcement? Ciba Found Symp. 1990;152:153–162. doi: 10.1002/9780470513965.ch9. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacology. 2005;30:145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology (Berl) 1991;104:171–176. doi: 10.1007/BF02244174. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Dopamine mechanisms play at best a small role in the nicotine discriminative stimulus. Pharmacol Biochem Behav. 1994a;48:817–820. doi: 10.1016/0091-3057(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine self-administration and locomotor activity are not modified by the 5-HT3 antagonists ICS 205–930 and MDL 72222. Pharmacol Biochem Behav. 1994b;49:67–71. doi: 10.1016/0091-3057(94)90457-x. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Damsma G, Day J, Fibiger HC. Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. Eur J Pharmacol. 1989;168:363–368. doi: 10.1016/0014-2999(89)90798-x. [DOI] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine dopamine concentrations in the mesolimbic system of of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DC, Tiffany ST, Glautier S, Remington B. Addictive behavior: cue exposure theory and practice. Wiley, Wiley; 1995. [Google Scholar]
- Erblich J, Lerman C, Self DW, Diaz GA, Bovbjerg DH. Effects of dopamine D2 receptor (DRD2) and transporter (SLC6A3) polymorphisms on smoking cue-induced cigarette craving among African-American smokers. Mol Psychiatry. 2005;10:407–414. doi: 10.1038/sj.mp.4001588. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Giuliani D. Behavioural assessment in rats of the antipsychotic potential of the potent dopamine D2 receptor antagonist, (−)eticlopride. Pharmacol Res. 1995;31:261–267. doi: 10.1016/1043-6618(95)80030-1. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Le Novere N, Picciotto MR, Changeux JP, Zoli M. Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur J Neurosci. 2002;15:1810–1818. doi: 10.1046/j.1460-9568.2001.02009.x. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y, Jens W, Cruz J, Kampman K, Ehrman R, Berrettini W, Detre JA, O’Brien CP, Childress AR. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34:717–728. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Shoaib M, Yasar S, Jaszyna M, Goldberg SR. Acquisition of nicotine discrimination and discriminative stimulus effects of nicotine in rats chronically exposed to caffeine. J Pharmacol Exp Ther. 1999;288:1053–1073. [PubMed] [Google Scholar]
- Hutchison KE, LaChance H, Niaura R, Bryan A, Smolen A. The DRD4 VNTR polymorphism influences reactivity to smoking cues. J Abnorm Psychol. 2002;111:134–143. doi: 10.1037//0021-843x.111.1.134. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Rutter MC, Niaura R, Swift RM, Pickworth WB, Sobik L. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology (Berl) 2004;175:407–413. doi: 10.1007/s00213-004-1837-3. [DOI] [PubMed] [Google Scholar]
- Katner SN, Weiss F. Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res. 1999;23:1751–1760. [PubMed] [Google Scholar]
- LaRowe SD, Saladin ME, Carpenter MJ, Upadhyaya HP. Reactivity to nicotine cues over repeated cue reactivity sessions. Addict Behav. 2007;32:2888–2899. doi: 10.1016/j.addbeh.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Dufek M, Keyler DE, Pentel PR. Reinstatement of nicotine self-administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol Biochem Behav. 2004;79:507–513. doi: 10.1016/j.pbb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent versus nondependent rats. J Pharmacol Exp Ther. 2002;300:882–889. doi: 10.1124/jpet.300.3.882. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Yee SK, Nobuta H, Poland RE, Pechnick RN. Reinstatement of nicotine-seeking behavior by drug-associated stimuli after extinction in rats. Psychopharmacology (Berl) 2006;184:417–425. doi: 10.1007/s00213-005-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Yee SK, Nobuta H, Sved AF, Pechnick RN, Poland RE. Mecamylamine attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Neuropsychopharmacology. 2007;32:710–718. doi: 10.1038/sj.npp.1301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology (Berl) 2008;196:365–375. doi: 10.1007/s00213-007-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Effects of haloperidol on reactions to smoking cues in humans. Behav Pharmacol. 2005;16:123–126. doi: 10.1097/00008877-200503000-00008. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Mann GL, Deibel CR, Glick SD. Ibogaine and the dopaminergic response to nicotine. Psychopharmacology (Berl) 1997;129:249–256. doi: 10.1007/s002130050187. [DOI] [PubMed] [Google Scholar]
- Marshall DL, Redfern PH, Wonnacott S. Presynaptic nicotinic modulation of dopamine release in the three ascending pathways studied by in vivo microdialysis: comparison of naive and chronic nicotine-treated rats. J Neurochem. 1997;68:1511–1519. doi: 10.1046/j.1471-4159.1997.68041511.x. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an FMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hutchison KE, Rose JE, Kozink RV. DRD4 VNTR polymorphism is associated with transient fMRI-BOLD responses to smoking cues. Psychopharmacology (Berl) 2007a;194:433–441. doi: 10.1007/s00213-007-0860-6. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual Differences in Nicotine Dependence, Withdrawal Symptoms, and Sex Predict Transient fMRI-BOLD Responses to Smoking Cues. Neuropsychopharmacology. 2007b;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Salamone JD. Involvement of nucleus accumbens dopamine in the motor activity induced by periodic food presentation: a microdialysis and behavioral study. Brain Res. 1992;592:29–36. doi: 10.1016/0006-8993(92)91654-w. [DOI] [PubMed] [Google Scholar]
- Mifsud JC, Hernandez L, Hoebel BG. Nicotine infused into the nucleus accumbens increases synaptic dopamine as measured by in vivo microdialysis. Brain Res. 1989;478:365–367. doi: 10.1016/0006-8993(89)91518-7. [DOI] [PubMed] [Google Scholar]
- Miranda R, Jr, Rohsenow DJ, Monti PM, Tidey J, Ray L. Effects of repeated days of smoking cue exposure on urge to smoke and physiological reactivity. Addict Behav. 2008;33:347–353. doi: 10.1016/j.addbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Johnstone EC. Smoking status moderates the association of the dopamine D4 receptor (DRD4) gene VNTR polymorphism with selective processing of smoking-related cues. Addict Biol. 2008;13:435–439. doi: 10.1111/j.1369-1600.2008.00098.x. [DOI] [PubMed] [Google Scholar]
- Niaura R, Abrams D, Demuth B, Pinto R, Monti P. Responses to smoking-related stimuli and early relapse to smoking. Addict Behav. 1989;14:419–428. doi: 10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. Repeated administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine-seeking in rats. Neuropsychopharmacology. 2005;30:119–128. doi: 10.1038/sj.npp.1300524. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22:3338–3341. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Engleman EA, Corrigall WA. Neuroadaptive changes in the mesoaccumbens dopamine system after chronic nicotine self-administration: a microdialysis study. Neuroscience. 2004;129:415–424. doi: 10.1016/j.neuroscience.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Tidey JW, Miranda R, McGeary JE, Swift RM, Hutchison KE, Sirota AD, Monti PM. Olanzapine reduces urge to smoke and nicotine withdrawal symptoms in community smokers. Exp Clin Psychopharmacol. 2008;16:215–222. doi: 10.1037/1064-1297.16.3.215. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96:1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz CA, Kelley AE, Landry CF. Contextual cues associated with nicotine administration increase arc mRNA expression in corticolimbic areas of the rat brain. Eur J Neurosci. 2005;21:1703–1711. doi: 10.1111/j.1460-9568.2005.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105:535–545. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Skjoldager P, Fowler SC. Effects of pimozide, across doses and within sessions, on discriminated lever release performance in rats. Psychopharmacology (Berl) 1988;96:21–28. doi: 10.1007/BF02431528. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Spina L, Fenu S, Longoni R, Rivas E, Di Chiara G. Nicotine-conditioned single-trial place preference: selective role of nucleus accumbens shell dopamine D1 receptors in acquisition. Psychopharmacology (Berl) 2006;184:447–455. doi: 10.1007/s00213-005-0211-4. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Sutton MA, Beninger RJ. Psychopharmacology of conditioned reward: evidence for a rewarding signal at D1-like dopamine receptors. Psychopharmacology (Berl) 1999;144:95–110. doi: 10.1007/s002130050982. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]