SUMMARY
Background
Microsatellite instability (MSI) occurs in chronically inflamed colorectal tissue and may evolve to colitis-associated cancer. In vitro data suggest that mesalazine (5-ASA) improves MSI.
Aim
To analyse the changes in MSI in 156 distal colonic biopsies of 39 patients with ulcerative colitis that had been treated within a randomized, double-blind trial comparing 5-ASA with E. coli Nissle (EcN).
Methods
Two biopsies had been collected before and after 1 year of treatment. MSI testing was performed using a panel of eight markers, including 3 dinucleotide and 5 mononucleotide repeats.
Results
No MSI was observed with any of the mono-repeats, and among dinucleotide repeats, only D5S346 (maps to APC) and D17S250 (p53) were consistently informative. Overall, 31/156 (20%) biopsies displayed MSI. After 1 year, 3/11 patients displayed MSI improvement [change to microsatellite stability (MSS); 1 on 5-ASA, 2 on EcN] at D5S346 and 4/11 showed MSI worsening (change from MSS to MSI; all 5-ASA). For D17S250, the corresponding data were for 3/9 patients (2 on 5-ASA, 1 on EcN) and 2/9 (both on 5-ASA), respectively.
Conclusions
In the set of biopsies taken from patients treated with 1.5 g 5-ASA for 1 year, there was no improvement in the prevalence of MSI in the distal colon.
INTRODUCTION
Colorectal cancer (CRC) is a serious complication in patients with ulcerative colitis (UC) or Crohn’s colitis. Early age at diagnosis, the extent and severity of inflammatory disease, the presence of primary sclerosing cholangitis and a family history of cancer have been established as independent risk factors for the development of colitis-associated cancer (CAC).1 The phenotype of CAC is slightly different from that of sporadic CRC;2 patients are generally affected earlier, location is frequently in the right colon and tumours develop (sometimes multifocally) from nonpolypous flat mucosa.
Currently, we recognized at least three mutational mechanisms that destabilize the genome and may drive colorectal carcinogenesis: chromosomal instability (CIN), microsatellite instability (MSI) and the CpG-methylator phenotype (CIMP).3, 4 In each instance, the genomic or epigenetic instability leads to the accumulation of mutations or epigenetic alterations of DNA. This concept – the so-called mutator phenotype – had been hypothesized for a long period of time5, 6 before it was recognized in MSI CRCs. MSI is the fingerprint of replication errors that had passed through the postreplication DNA check by the mismatch repair (MMR) system without a proper response.7 In MSI, frame-shift mutations (i.e. deletion or addition of DNA base pairs) within repetitive DNA sequences (i.e. microsatellites) accumulate. Most microsatellites are located in intronic, noncoding DNA sequences, so that they usually do not alter gene function. However, if microsatellites are located within the coding region of genes, these mutations shift the reading frame, causing changes in the amino acid sequence (missense mutations) and/or protein truncation (nonsense mutation), and typically a loss of protein function. Certain tumour suppressor genes (such as TGFBR2, IGF2R and ACVR2) carry mononucleotide repeats within their coding regions and are a common target of mutational events in MSI-positive tumours.8-10 MSI-positive tumours are located more frequently in the right colon and may develop from flat mucosa, similar to CAC. Interestingly, MSI is frequently found in UC-associated dysplasia or CAC,11 and also in up to 50% of nondysplastic chronically inflamed mucosal biopsies.11-14 Studies have failed to identify germline mutations in the MMR genes of patients with CAC,12, 15 but have identified hMLH1 promoter methylation (by the CIMP pathway) as a possible cause of the MSI.16 The largest fraction of UC-associated dysplastic lesions or cancers, however, demonstrates a specific subtype of MSI, called MSI-low.11-14 In this setting, principally dinucleotide repeats are affected.7
We have previously demonstrated that in vitro oxidative stress may temporarily relax the MMR system, and thereby allow insertion/deletion mutations to occur at a (CA)13 repeat.17, 18 The appropriate G2/M checkpoint arrest after such replication errors depends on the presence of functional hMSH2, a mechanism that seems to be inactivated in CAC.19 We also showed that mesalazine (5-ASA), which is commonly used for treatment of UC and considered to prevent from CAC,1, 20 may interfere with this mutational mechanism by an as yet unknown mechanism that may involve activation of a replication checkpoint.21-23 Thus, there is experimental evidence suggesting that 5-ASA may interfere with colitis-associated carcinogenesis by improving replication fidelity. In this study, we hypothesize that 5-ASA might reduce the progression of MSI in patients with UC, and studied the presence of MSI in biopsy material from a randomized, controlled clinical trial of patients before and after treatment with 5-ASA or E. coli Nissle (EcN).24
MATERIALS AND METHODS
Biopsy material from a previously conducted randomized, double-blind, controlled trial was made available by the sponsor (Ardeypharm GmbH, Herdecke, Germany).24 The clinical trial compared the maintenance of remission in UC using eudragit-L-coated 5-ASA (Salofalk, 1.5 g/day; Dr. Falk Pharma GmbH, Freiburg, Germany) vs. a probiotic drug, EcN (Mutaflor, 200 mg/day; Ardeypharm GmbH). Details of this trial have been published previously elsewhere.24 In a subgroup of patients, two biopsies from the rectum and sigmoid colon were taken before and at the completion of the one-year trial (i.e. four biopsies per patient). From the 327 patients who participated in the trial, biopsies were sampled from 171 patients. The biopsies were examined by a single pathologist using a four-point scale, and did not show any dysplasia.25
The investigators of this study were blinded regarding the allocation of patients to the two groups (5-ASA vs. EcN). Biopsies were coded and the file was kept with the sponsor until the database had been closed. From the total of 171 patients in whom biopsies had been taken, cases were selected using three clinical criteria:
disease location beyond proctitis only,
disease duration more than 1 year and
no 5-ASA treatment within 2 months prior to the trial.
The primary endpoint of the study was the number of individuals with a change from MSI before treatment to microsatellite stability (MSS; i.e. non-MSI) at any locus (i.e. improvement). This was compared with the number of individuals with unchanged MSS or MSI before and after treatment (i.e. unchanged), or from MSS to MSI or loss of heterozygosity (LOH) upon completion of treatment (i.e. worsening). The study was approved by the institutional review board of the Medical University of Vienna.
Microsatellite instability analysis
Microsatellite analyses on all 156 tissue biopsies were performed using a panel of eight microsatellite markers that consisted of five standard markers (D2S123, D5S346, D17S250, BAT25 and BAT26) and three additional mononucleotide repeat markers (NR21, NR24 and NR27) as described previously elsewhere.7, 26 The 3 dinucleotide repeat polymorphic markers were utilized for both MSI and LOH analyses. Unlike dinucleotide repeat markers, the 5 mononucleotide markers are quasi-monomorphic in nature, do not require simultaneous amplification of matching normal DNA and can be amplified in a single multiplex PCR-amplification reaction.
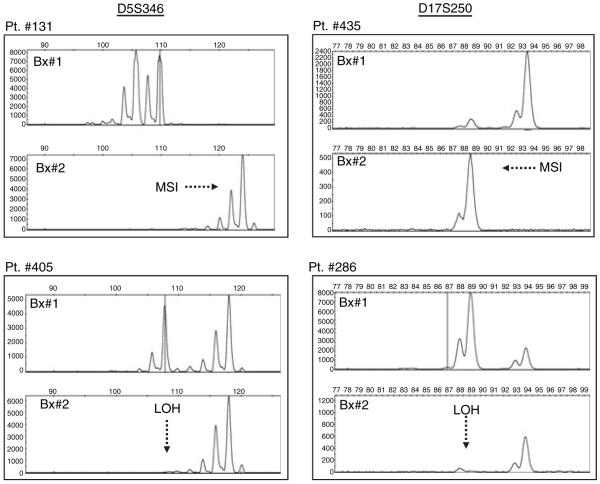
As indicated in Table 1, the antisense primer in each pair was labelled with a fluorescent dye, FAM for BAT-26, NR-21 and D17S250; HEX for BAT-25, NR-27 and D5S346; and NED for NR-24. Each PCR reaction consisted of a 25 μL reaction mixture containing 100 ng of genomic DNA, 1× HotStarTaq master mix (Qiagen, Hilden, Germany) and 1 μm of each primer. The PCR reactions were carried out in a PTC 200 DNA Engine System (MJ Research Inc., Watertown, MA, USA). PCR conditions included denaturing at 94 °C for 15 min, followed by 40 cycles of denaturing at 94 °C, annealing at 55 °C and extension at 72 °C for 30 s. The extension time of the final cycle was 10 min. In every PCR, we used a positive template control (with the same amount of DNA from the microsatellite unstable colon cancer cell line HCT116), and a template blank (with no DNA, but containing all other reagents, to control contamination). MSI was detected with the aid of an ABI PRISM 3100 Avant Genetic Analyzer, and the electropherograms were analysed with GeneMapper software (version 3.5; Applied Biosystems, Foster City, CA, USA). A patient was considered to be MSI positive if there was altered electrophoretic mobility of the microsatellite repeat sequence in pre- and post-treatment biopsies, or LOH positive if allelic loss was observed at any of the marker (Figure 1).
Table 1.
Microsatellite markers and PCR conditions for PCR analysis
| Marker name |
Gene | Microsatellite repeat |
Primer sequences* | Annealing Temp (°C) |
Product size |
|---|---|---|---|---|---|
| D5S346 | APC | CA(9) | 5′-ACTCACTCTAGTGATAAATCGGG-3′ 5′-AGCAGATAAGACAGTATTACTAGTT-3′ |
55 | 122 bp |
| D17S250 | p53 | CA(12) | 5′-AAGAGCTGAGACTCCATCTC-3′ 5′-ACAGCTCCTTTAATGGCAGG-3′ |
52 | 150 bp |
| D2S123 | hMSH2 | CA(13) | 5′-TCAACATTGCTGGAAGTTCT-3′ 5′-GACTTTCCACCTATGGGACT-3′ |
50 | 196bp |
| BAT-26 | hMSH2 | A(26) | 5′-CTGCGGTAATCAAGTTTTTAG-3′ 5′-AACCATTCAACATTTTTAACCC-3′ |
55 | 183 bp |
| BAT-25 | c-kit | A(25) | 5′-TACCAGGTGGCAAAGGGCA-3′ 5′-TCTGCATTTTAACTATGGCTC-3′ |
55 | 153 bp |
| NR-24 | Zinc finger-2 | A(24) | 5′-GCTGAATTTTACCTCCTGAC-3′ 5′-ATTGTGCCATTGCATTCCAA-3′ |
55 | 131 bp |
| NR-21 | SLC7A8 | A(21) | 5′-GAGTCGCTGGCACAGTTCTA-3′ 5′-CTGGTCACTCGCGTTTACAA-3′ |
55 | 109 bp |
| NR-27 | Inhibitor of apoptosis protein-1 |
A(27) | 5′-AACCATGCTTGCAAACCACT-3′ 5′-CGATAATACTAGCAATGACC-3′ |
55 | 87 bp |
In each case, the antisense primer was labelled with a fluorescent dye: FAM for BAT-26, NR-21 and D17S250; HEX for BAT-25, NR-27 and D5S346; and NED for NR-24.
Figure 1.
A representative figure illustrating microsatellite instability (MSI) and loss of heterozygosity (LOH) at D5S346 and D17S250 markers pre- and post-5-ASA and/or EcN treatments. On the left, the electropherogram depicts the presence of MSI at D5S346 marker in patient #131, as indicated by an increased allele length after 1-year of 5-ASA treatment. The bottom left panel shows LOH at D5S346 marker in patient #405. The two electropherograms on the right show similar data for the D17S250 marker, with patient #435 showing MSI with 5-ASA, while patient #286 shows evidence for LOH with EcN treatment.
Statistics
Baseline characteristics in the study groups were compared using the Mann–Whitney–Wilcoxon’s rank sum test for parametric variables, or using the chi-square test for categorical variables by SPSS for Windows Version 15.0 (SPSS: Chicago, IL, USA). For all tests, P-values below 0.05 were considered statistically significant.
RESULTS
Clinical characteristics of selected biopsies
A total of 156 paraffin-embedded biopsy tissue sections were obtained from 39 patients who had been treated in a randomized, double-blind, 1-year, UC maintenance trial comparing 5-ASA (n = 25) with EcN (n = 14). The selection of cases and analysis of biopsies was performed blinded to the treatment. Any imbalance between the two groups happened only by chance. The cases were selected from a total of 171 patients in whom biopsies had been taken according to predefined criteria (disease location beyond proctitis, disease duration over 1 year and no 5-ASA therapy within 2 months prior to the study) to increase the likelihood of identifying MSI in this set of patients in remission, and independent of the treatment allocation. Most patients had proctosigmoiditis only, with a median disease duration of 8 years (range 2–33; Table 2). All biopsies were histologically scored and did not display dysplasia. The histological grading showed mostly disease in remission and did not differ between the two groups.
Table 2.
Clinical characteristics of study population
| 5-ASA (n = 25) |
EcN (n = 14) |
P | |
|---|---|---|---|
| Age (median-range) | 45 (19–67) | 38.5 (31–67) | P = 0.608 |
| Female, n (%) | 14 (56) | 5 (36) | P = 0.230 |
| Location, n (%) | |||
| Proctitis | 0 (0) | 0 (0) | P = 0.950 |
| Proctosigmoiditis | 15 (60) | 8 (57) | |
| Left-sided colitis | 4 (16) | 2 (14) | |
| Extensive colitis | 6 (24) | 4 (29) | |
| Duration (median-range) | 7 (2–33) | 10 (2–20) | P = 0.608 |
| Histological grade, n (%) | |||
| Remission | 20 (80) | 8 (57) | P = 0.177 |
| Mild | 4 (16) | 3 (21) | |
| Moderate | 1 (4) | 3 (21) | |
MSI and LOH in biopsies of patients with ulcerative colitis
A panel of 8 microsatellite markers was selected that included the 5 NCI workshop recommended markers (D2S123, D5S346, D17S250, BAT25 and BAT26) and 3 mono-repeat markers (NR21, NR24 and NR27). Some of these markers are tightly linked with key genes, including APC, p53 and MSH2, which play an important role in colorectal carcinogenesis. As described in the methods, the 5 mononucleotide repeat markers utilize a single PCR reaction and obviate the need for amplification of matched normal DNA from the tumour. We first amplified all 156 biopsies for these mononucleotide repeat markers, and failed to detect genetic alterations at any of these markers.
We next amplified the three dinucleotide repeats (D2S123, D5S346 and D17S250), and among these, only D5S346 and D17S250 were consistently informative in most of the biopsies. Twenty-five percent (39/156) of all biopsies were not informative at any of these two markers, with more non-informative cases occurring at the D17S250 marker (77/156; 49%) than at the D5S346 (54/156; 35%) marker (Table 3). Taking all markers into consideration, 20% (31/156) of the biopsies demonstrated MSI, 10% (16/156) showed allelic losses (LOH) and 45% (70/156) were MSS. D5S346 showed 14% MSI, 5% LOH and 46% MSS (the rest giving only one band after PCR, being non-informative). Similar frequencies of genetic alterations were present at the D17S250 marker with 8% MSI, 5% LOH and 38% MSS. The median duration of disease in patients who displayed MSI was longer than that in patients without (12.8 vs. 5.0 years; P = 0.016).
Table 3.
Frequency of genetic alterations in all biopsies (n = 156)
| Subset | Any marker % (no. of biopsies) |
D5S346 % (no. of biopsies) |
D17S250 % (no. of biopsies) |
|---|---|---|---|
| Non-informative | 25 (39) | 35 (54) | 49 (77) |
| MSS | 45 (70) | 46 (71) | 38 (59) |
| MSI | 20 (31) | 14 (22) | 8 (12) |
| LOH | 10 (16) | 6 (9) | 5 (8) |
Changes in MSI upon treatment with 5-ASA or EcN
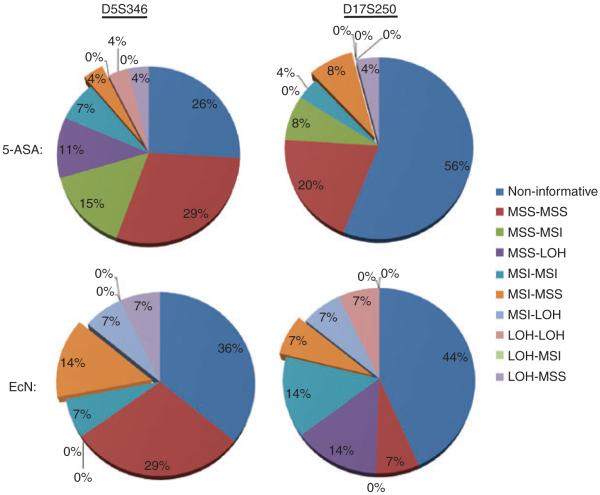
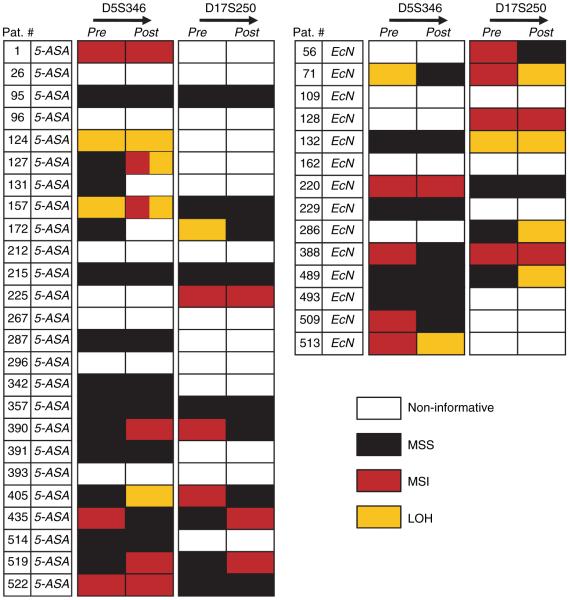
When data were analysed in the context of 5-ASA and EcN treatments, 69% (27/39) of the patients were informative at the D5S346 marker, and 49% (19/39) were informative at the D17S250 marker (Table 4; Figure 2). Among these, 16/27 subjects did not show any genetic changes in pre- and post-treatment biopsies after 5-ASA or EcN treatments at the D5S346 marker, and 10/19 patients did not at D17S250. At D5S346, 3/11 patients (27%) changed from MSI to MSS (considered MSI improvement, 1 on 5-ASA, 2 on EcN) and 4/11 (36%) changed from MSS to MSI (considered MSI worsening, all on 5-ASA). Two of the 5-ASA-treated patients (#131 and 172) showed overlapping events, with one of the two biopsies each from these individuals changed from MSS to MSI and MSS to LOH, respectively (Figure 3).
Table 4.
Changes at D5S346 and D17S250 in 5-ASA- (n = 25; 100 biopsies) and EcN- (n = 14; 56 biopsies) treated patients
| Subset | 5-ASA % (n) |
D5S346 |
D17S250 |
Any Group % (n) |
|||
|---|---|---|---|---|---|---|---|
| EcN % (n) | Any Group % (n) | 5-ASA % (n) | EcN % (n) | ||||
| Non-informative | 28 (7) | 36 (5) | 33 (12) | 56 (14) | 43 (6) | 51 (20) | |
| Improvement | MSI-MSS | 4 (1) | 14 (2) | 8 (3) | 8 (2) | 7 (1) | 8 (3) |
| LOH-MSS | 4 (1) | 7 (1) | 5 (2) | 4 (1) | 0 (0) | 3 (1) | |
| Worsening | MSS-MSI | 16 (4)* | 0 (0) | 10 (4)* | 8 (2) | 0 (0) | 5 (2) |
| MSS-LOH | 12 (3)* | 0 (0) | 8 (3)* | 0 (0) | 14 (2) | 5 (2) | |
| No change | MSS-MSS | 32 (8) | 29 (4) | 33 (12) | 20 (5) | 7 (1) | 15 (6) |
| MSI-MSI | 8 (2) | 7 (1) | 8 (3) | 4 (1) | 14 (2) | 8 (3) | |
| LOH-LOH | 4 (1) | 0 (0) | 3 (1) | 0 (0) | 7 (1) | 3 (1) | |
| Indifferent | MSI-LOH | 0 (0) | 7 (1) | 3 (1) | 0 (0) | 7 (1) | 3 (1) |
| LOH-MSI | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
Two patients showed overlapping MSI and LOH events; therefore, the percent change and total number of patients (n) reflect a deviation from expected values.
Figure 2.
The pie charts summarize detailed frequencies of genetic alterations in various subsets of biopsies, with 5-ASA and EcN treatment at D5S346 and D17S250 markers. Although majority of biopsies demonstrated alterations at both D5 and D17 markers after 1 year of 5-ASA or EcN treatment (indicated by specific percent change), a smaller subset of biopsies stayed unaltered (shown as 0%) during this time period.
Figure 3.
The figure illustrates individual results from the 39 patients (25 with 5-ASA and 14 with EcN). The results indicate cumulative changes in each of the two biopsies obtained pre- and post-treatment for both D5S346 and D17S250 markers. In most cases, both biopsies showed similar data. However, in patients #131 and #172, each of the two biopsies changed from MSS to MSI, and from MSI to LOH, after 1 year of 5-ASA treatment.
At the D17S250 marker, the corresponding data for MSI improvement were 3/9 patients (33%; 2 on 5-ASA, 1 on EcN) and for MSI worsening 2/9 (22%; both on 5-ASA). Taken together, there was no difference in overall improvement or worsening of MSI between the 5-ASA and EcN treatment groups at the two dinucleotide repeat markers among the informative subjects.
DISCUSSION
In UC, CRC development occurs more commonly, and at an earlier age. Previous studies have highlighted various biological effects of 5-ASA with regard to the prevention of CAC. In consideration of 5-ASA’s in vitro activity in reducing mutations at a (CA)13 repeat,22 we studied changes in microsatellite mutations in rectal and distal sigmoid colon biopsies from patients with UC who had been treated with either 5-ASA or EcN.24 From the two markers that amplified best, no consistent trend towards a protective effect for 5-ASA or EcN was found. As expected, mononucleotide markers were not mutated at all.12 The presence of MSI was associated with longer disease duration.
In a recent, elegant study, clonality of mutations and local expansion of founder mutations within colonic regions (e.g. rectum or sigmoid colon) were demonstrated for colitis-associated neoplasia.27 Our hypothesis is consistent with this conclusion and our data fit this model, as we detected stable mutational patterns (i.e. either MSI or LOH) through the one-year period in six biopsy pairs before and after treatment. A change from MSI to MSS is therefore very provocative, but may simply be caused by endoscopic sampling difference (biopsies after 1 year were taken from different parts of the rectal or sigmoid mucosa), and does not necessarily demonstrate dynamic changes in cellular populations. As changes occurred in both directions (worsening and improvement) with both compounds (5-ASA and EcN), sampling variation seems the most likely explanation for our mixed effects.
Besides the possibility that 5-ASA has no antimutational effects in vivo, these somewhat disappointing results may have resulted from methodological shortcomings. First, the DNA quality of the samples may have degraded over time (due to extended formaldehyde exposure, as the samples were shipped prior to paraffin embedding), which may render PCR amplification unreliable. Thus, despite extensive laboratory efforts, only two markers could be analysed reliably. Second, biopsies had been taken 1 year apart by different endoscopists. Thus it is likely that the biopsy locations were different, and sampling variation is quite likely. We did not attempt to analyse rectal and sigmoid biopsy pairs, which would have been preferable. Third, all patients were in clinical remission at study entry, and the number of mutations at baseline was low. Thus, despite our effort to select for high risk patients within the cohorts in the randomized controlled trial, the selected group may have displayed a low rate of MSI (21% total) due to the absence of mucosal inflammatory activity. In fact, previous work has suggested a higher frequency (50%) of MSI in nondysplastic colitis tissue.12 This possibility is further supported by the distal disease location (60% had no more than proctosigmoiditis), and absence of severe histological disease activity (80% were in remission). It turned out that from this sample (with few mutations at baseline), a sensitive assessment of the protective effects of 5-ASA is unlikely. Last, the type of 5-ASA preparation (eudragit-L-coated) and the dose (0.5g TID) may have caused insufficient mucosal concentrations in the distal colon.28 In fact, this preparation has been shown to release the drug in the ileocaecal region.29 More delayed-release preparations such as MMX-mesalazine and once-daily administration of a higher dose (above 2 gram;30) may have led to different results. The combination of these shortcomings diminishes the strength of our study.
The selection of samples for these laboratory analyses was performed in a blinded fashion. This is the reason for the unbalanced representation of clinical features between the two treatment groups. As we did not directly compare the two groups, but rather compared patients’ pre- and post-5-ASA treatment, these differences did not affect our results. The small sample size in the EcN group (n = 14), however, precludes any conclusion of the effects of EcN on mucosal mutations.31
Besides the improvement in replication fidelity, other therapeutic mechanisms of 5-ASA in tumour prevention have been envisioned.1 5-ASA is potentially an effective oxygen scavenger32 and treatment with the drug has been reported to increase apoptosis in vitro.33 In contrast to this study, the effect of 5-ASA on the induction of apoptosis has been observed in mucosal samples.34 In this study, 5-ASA enemas were taken for 14 days, providing a sufficient time and luminal concentration of the compound. A similar study with high intra-luminal 5-ASA concentrations is needed before ruling out an in vivo effect on replication fidelity.
ACKNOWLEDGEMENTS
Declaration of personal interests: WK and MS received lecturing and consulting honoraria from Dr Falk Pharma, Shire Pharmaceuticals, Merckle-Recordatti and Ardeypharm. CG has research collaborations with Giuliani SpA and Shire Pharmaceuticals and received research support, lecturing or consulting honoraria from Ferring and Dr Falk Pharma. Declaration of funding interests: This study was funded in part by the International Organization of Inflammatory Bowel Diseases (CG), the Austrian Science Fund (FWF 18270) and R01 CA072851 (CRB). Biopsy material was provided by Ardeypharm GmbH, Herdecke, Germany.
REFERENCES
- 1.Rubin DT, Cruz-Correa MR, Gasche C, et al. Colorectal cancer prevention in inflammatory bowel disease and the role of 5-aminosalicylic acid: a clinical review and update. Inflamm Bowel Dis. 2008;14:265–74. doi: 10.1002/ibd.20297. [DOI] [PubMed] [Google Scholar]
- 2.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 3.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–7. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 4.Ahuja N, Li Q, Mohan AL, Baylin SB, Issa JP. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res. 1998;58:5489–94. [PubMed] [Google Scholar]
- 5.Loeb LA. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991;51:3075–9. [PubMed] [Google Scholar]
- 6.Loeb LA, Loeb KR, Anderson JP. Multiple mutations and cancer. Proc Natl Acad Sci US A. 2003;100:776–81. doi: 10.1073/pnas.0334858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57. [PubMed] [Google Scholar]
- 8.Jung B, Doctolero RT, Tajima A, et al. Loss of activin receptor type 2 protein expression in microsatellite unstable colon cancers. Gastroenterology. 2004;126:654–9. doi: 10.1053/j.gastro.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz S, Wang J, Myeroff L, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–8. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 10.Jass JR, Iino H, Ruszkiewicz A, et al. Neoplastic progression occurs through mutator pathways in hyperplastic polyposis of the colorectum. Gut. 2000;47:43–9. doi: 10.1136/gut.47.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki H, Harpaz N, Tarmin L, et al. Microsatellite instability in ulcerative colitis-associated colorectal dysplasias and cancers. Cancer Res. 1994;54:4841–4. [PubMed] [Google Scholar]
- 12.Brentnall TA, Crispin DA, Bronner MP, et al. Microsatellite instability in nonneoplastic mucosa from patients with chronic ulcerative colitis. Cancer Res. 1996;56:1237–40. [PubMed] [Google Scholar]
- 13.Cravo ML, Albuquerque CM, Salazar de SL, et al. Microsatellite instability in non-neoplastic mucosa of patients with ulcerative colitis: effect of folate supplementation. Am J Gastroenterol. 1998;93:2060–4. doi: 10.1111/j.1572-0241.1998.00592.x. [DOI] [PubMed] [Google Scholar]
- 14.Park WS, Pham T, Wang C, et al. Loss of heterozygosity and microsatellite instability in non-neoplastic mucosa from patients with chronic ulcerative colitis. Int J Mol Med. 1998;2:221–4. [PubMed] [Google Scholar]
- 15.Noffsinger AE, Belli JM, Fogt F, Fischer J, Goldman H, Fenoglio-Preiser CM. A germline hMSH2 alteration is unrelated to colonic microsatellite instability in patients with ulcerative colitis. Hum Pathol. 1999;30:8–12. doi: 10.1016/s0046-8177(99)90293-9. [DOI] [PubMed] [Google Scholar]
- 16.Fleisher AS, Esteller M, Harpaz N, et al. Microsatellite instability in inflammatory bowel disease-associated neoplastic lesions is associated with hypermethylation and diminished expression of the DNA mismatch repair gene, hMLH1. Cancer Res. 2000;60:4864–8. [PubMed] [Google Scholar]
- 17.Gasche C, Chang CL, Rhees J, Goel A, Boland CR. Oxidative stress increases frameshift mutations in human colorectal cancer cells. Cancer Res. 2001;61:7444–8. [PubMed] [Google Scholar]
- 18.Chang CL, Marra G, Chauhan DP, et al. Oxidative stress inactivates the human DNA mismatch repair system. Am J Physiol Cell Physiol. 2002;283:C148–54. doi: 10.1152/ajpcell.00422.2001. [DOI] [PubMed] [Google Scholar]
- 19.Campregher C, Luciani MG, Gasche C. Activated neutrophils induce an hMSH2-dependent G2/M checkpoint arrest and replication errors at a (CA)13-repeat in colon epithelial cells. Gut. 2008;57:780–7. doi: 10.1136/gut.2007.141556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100:1345–53. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]
- 21.Gasche C, Chang CL, Natarajan L, et al. Identification of frame-shift intermediate mutant cells. Proc Natl Acad Sci U S A. 2003;100:1914–9. doi: 10.1073/pnas.0437965100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasche C, Goel A, Natarajan L, Boland CR. Mesalazine improves replication fidelity in cultured colorectal cells. Cancer Res. 2005;65:3993–7. doi: 10.1158/0008-5472.CAN-04-3824. [DOI] [PubMed] [Google Scholar]
- 23.Luciani MG, Campregher C, Fortune JM, Kunkel TA, Gasche C. 5-ASA affects cell cycle progression in colorectal cells by reversibly activating a replication checkpoint. Gastroenterology. 2007;132:221–35. doi: 10.1053/j.gastro.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruis W, Fric P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–23. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991;32:174–8. doi: 10.1136/gut.32.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung WT, Li MS, Goel A, Boland CR. JC virus T-antigen expression in sporadic adenomatous polyps of the colon. Cancer. 2008;112:1028–36. doi: 10.1002/cncr.23266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leedham SJ, Graham TA, Oukrif D, et al. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology. 2009;136:542–50. doi: 10.1053/j.gastro.2008.10.086. [DOI] [PubMed] [Google Scholar]
- 28.Klotz U, Maier KE, Fischer C, Bauer KH. A new slow-release form of 5-aminosalicylic acid for the oral treatment of inflammatory bowel disease. Biopharmaceutic and clinical pharmacokinetic characteristics. Arzneimittelforschung. 1985;35:636–9. [PubMed] [Google Scholar]
- 29.Hardy JG, Healey JN, Reynolds JR. Evaluation of an enteric-coated delayed-release 5-aminosalicylic acid tablet in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 1987;1:273–80. doi: 10.1111/j.1365-2036.1987.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 30.Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000;14:145–53. doi: 10.1046/j.1365-2036.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 31.Sonnenborn U, Janosch D, Dubbert S, Stroff T, Schulze L, Mueller H. Antimutagenic activity of the probiotic E. Coli strain Nissile 1917. J Crohn’s and Colitis. 2009;3:S136. Abstract. [Google Scholar]
- 32.Simmonds NJ, Millar AD, Blake DR, Rampton DS. Antioxidant effects of aminosalicylates and potential new drugs for inflammatory bowel disease: assessment in cell-free systems and inflamed human colorectal biopsies. Aliment Pharmacol Ther. 1999;13:363–72. doi: 10.1046/j.1365-2036.1999.00484.x. [DOI] [PubMed] [Google Scholar]
- 33.Reinacher-Schick A, Schoeneck A, Graeven U, Schwarte-Waldhoff I, Schmiegel W. Mesalazine causes a mitotic arrest and induces caspase-dependent apoptosis in colon carcinoma cells. Carcinogenesis. 2003;24:443–51. doi: 10.1093/carcin/24.3.443. [DOI] [PubMed] [Google Scholar]
- 34.Bus PJ, Nagtegaal ID, Verspaget HW, et al. Mesalazine-induced apoptosis of colorectal cancer: on the verge of a new chemopreventive era? Aliment Pharmacol Ther. 1999;13:1397–402. doi: 10.1046/j.1365-2036.1999.00652.x. [DOI] [PubMed] [Google Scholar]