Abstract
The E3 ubiquitin ligases Cdc20-anaphase-promoting complex (Cdc20-APC) and Cdh1-APC play key roles in cell cycle transitions in proliferating cells. Remarkably, these ubiquitin ligases are also expressed in postmitotic neurons, raising interest in non-mitotic functions of the APC. Cdh1-APC has been implicated in diverse functions in the nervous system, from the control of axon growth and patterning to synapse development to neuron survival. However, until recently the question of whether Cdc20-APC harbors functions in neurons remained unanswered. New evidence from Kim et al. (2009) has uncovered a novel role for Cdc20-APC in in dendrite growth and elaboration in post-mitotic neurons. Interestingly, the histone deacetylase HDAC6 augments Cdc20-APC activity at the centrosome by promoting Cdc20 polyubiquitination. In turn, Cdc20-APC triggers the degradation of the centrosomally localized protein Id1 and thereby promotes dendrite growth and elaboration. These findings have advanced our understanding of APC biology in neuronal connectivity in the brain.
The E3 ubiquitin ligase anaphase-promoting complex (APC) plays an essential role in the control of the cell cycle in proliferating cells.1-4 The APC associates with one of two coactivators, Cdc20 and Cdh1, which have unique substrate specificities, and thereby orchestrates critical mitotic transitions and promotes G1 maintenance.5, 6 The evolving concept that cell cycle proteins have critical functions in post-mitotic neurons has sparked interest in the APC in neurobiology.7-10 Cdh1-APC restrains axon growth in mammalian brain neurons via ubiquitination and degradation of the transcriptional regulators SnoN and Id2.11-15 Cdh1-APC has also been implicated in the control of synapse development and function in flies and nematodes.16-18 Cdh1 heterozygous mice have defects in long-phase LTP and harbor behavioral deficits.19, 20 Cdh1-APC has been reported to control additional neuronal responses, including cell survival and the glyoclytic state in neurons.21, 22 Despite these emerging diverse functions of Cdh1-APC in post-mitotic neurons, a role for the Cdh1 homolog, Cdc20, remained unexplored until recently.
Kim et al. (2009) addressed the question of whether Cdc20-APC has additional non-mitotic functions during neuronal development.23 Remarkably, Cdc20 was found to be expressed during the period of dendrite development in primary granule neurons based on immunoblotting and in situ hybridization analyses. To probe the function of endogenous Cdc20 in post-mitotic neurons, the authors utilized an RNAi approach. Cdc20 knockdown dramatically reduced dendrite length and arborization in primary neurons as well as in granule neurons in the rat cerebellum in vitro, indicating that endogenous Cdc20 is required for dendrite growth and elaboration in the mammalian brain (Figure 1A).
Figure 1. Centrosomal Cdc20-APC controls dendrite morphogenesis in post-mitotic neurons.
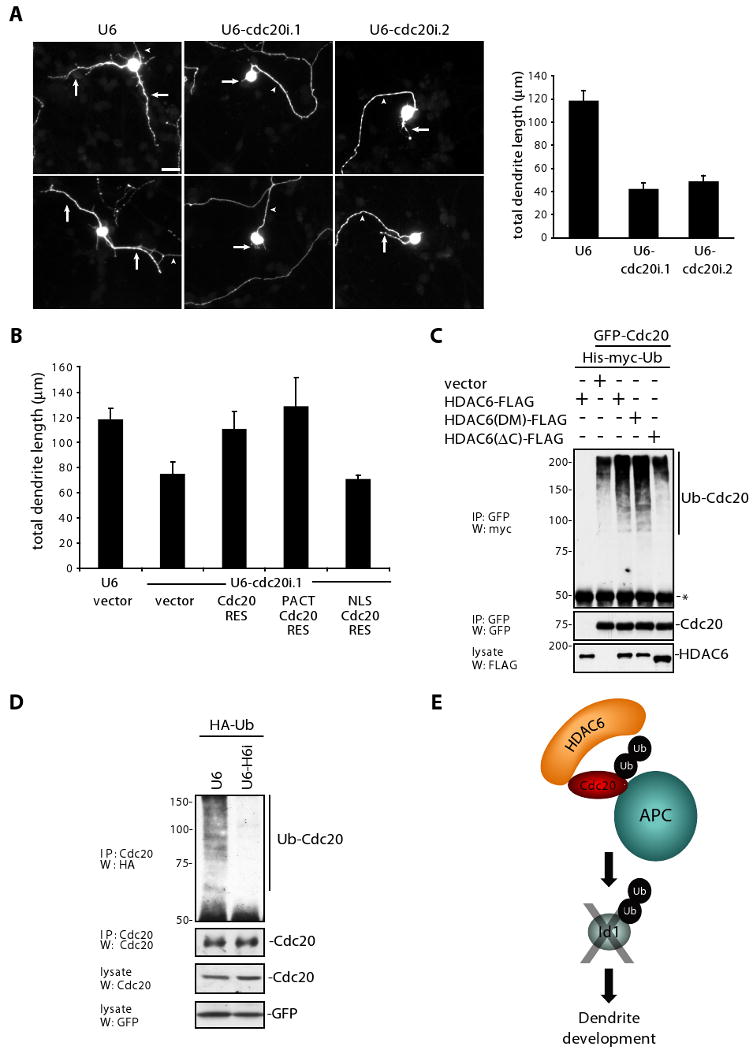
(A) Left: Granule neurons transfected with Cdc20 RNAi or control U6 plasmid along with a GFP expression plasmid were analyzed by immunocytochemistry with the GFP antibody four days after transfection. Representative neurons are shown. Arrows indicate dendrites and arrowheads denote axons. Scale bar represents 10 μm. Right: Total dendrite length from granule neurons transfected with Cdc20 RNAi or control U6 plasmid was measured. Cdc20 knockdown significantly reduced total dendrite length in transfected neurons compared to control U6-transfection (ANOVA; p < 0.0001).
(B) Granule neurons were transfected with the Cdc20 RNAi or control U6 plasmid along with an expression plasmid encoding NLS-Cdc20-RES, PACT-Cdc20-RES, PACT, PACT-Cdh1, or control vector and a GFP expression plasmid. Granule neurons were analyzed as in (A). Centrosomally localized Cdc20 (PACT-Cdc20-RES), but not nuclearly localized Cdc20 (NLS-Cdc20-RES) significantly increased total dendrite length compared to the control vector in the background of Cdc20 RNAi (ANOVA, p < 0.0001).
(C) 293T cells were transfected with the GFP-Cdc20 expression plasmid or control vector along with a His-myc-ubiquitin expression plasmid and the indicated FLAG-HDAC6 expression plasmids. Lysates were sonicated and immunoprecipitated with GFP antibody. Immunoprecipitates and lysates were immunoblotted with the antibodies as indicated. Asterisk indicates IgG heavy chain.
(D) Granule neurons were transfected with the HDAC6 RNAi or control U6 plasmid and expression plasmids encoding HA-ubiquitin and GFP to determine transfection efficiency. Lysates were sonicated and immunoprecipitated with the Cdc20 antibody and immunoprecipitates and lysates were immunoblotted with the antibodies as indicated.
(E) The centrosomal HDAC6/Cdc20-APC/Id1 ubiquitin signaling pathway controls dendrite morphogenesis in post-mitotic neurons.
Reprinted with permission from Cell.
To determine how Cdc20-APC controls dendrite morphogenesis, the authors first explored the subcellular localization of endogenous Cdc20. Immunocytochemical and biochemical analyses revealed that Cdc20 is concentrated at the centrosome in post-mitotic neurons. The importance of Cdc20's subcellular localization in its function was then assessed by localizing an RNAi-resistant Cdc20 rescue protein to the nucleus or centrosome, the latter using the centrosomal targeting domain of pericentrin/AKAP450 (PACT domain). Centrosomal Cdc20, but not nuclear Cdc20, reversed the Cdc20 RNAi-induced phenotype (Figure 1B). In complementary structure-function analyses, the authors identified amino acids 111-129 within the N-terminal domain of Cdc20 as the putative centrosomal localization signal (CLS) and demonstrated a requirement for CLS in Cdc20-dependent dendrite growth and elaboration in neurons.
The centrosomal localization of Cdc20 immediately suggested that other centrosomally localized proteins may regulate Cdc20-APC activity in post-mitotic neurons. Among the candidate regulators of Cdc20-APC, HDAC6 was intriguing due to its localization at the basal body in non-neuronal cell types, role in ciliary morphogenesis, and C-terminal zinc-finger ubiquitin binding (ZnF-UBP) domain. HDAC6 and Cdc20 formed a robust complex endogenously in neurons, and HDAC6 knockdown reduced centrosomal Cdc20-APC activity in a reporter assay, suggesting that HDAC6 positively regulates Cdc20-APC. In agreement with the model that HDAC6 promotes Cdc20-APC activity in neurons, HDAC6 knockdown phenocopied the effect of Cdc20 RNAi, substantially reducing dendrite growth and elaboration.
Initially, Kim et al. explored the possibility that the HDAC6 deacetylase domain regulates the acetylation status of Cdc20, but HDAC6 did not appear to alter the global acetylation status of Cdc20. The authors next turned their attention to the C-terminal ZnF-UBP domain of HDAC6. The ZnF-UBP may stabilize polyubiquitinated proteins,24 and recent studies suggest that Cdc20 autoubiquitination stimulates Cdc20-APC activity in proliferating cells.25, 26 To test if HDAC6 promotes Cdc20 polyubiqutination and subsequent activity, the authors expressed mutants of HDAC6 in cells. HDAC6 robustly augmented Cdc20 polyubiquitination, and the ZnF-UBP domain was required for this effect (Figure 1C). Accordingly, HDAC6 knockdown substantially reduced endogenous Cdc20 polyubiquitination in neurons (Figure 1D). In further structure-function analyses, Kim and colleagues demonstrated that the ZnF-UBP domain but not the deacetylase activity, is required for HDAC6-induced dendrite growth. To demonstrate that Cdc20 polyubiquitination in vitro is critical for dendrite morphogenesis, the authors induced knockdown of USP44, a Cdc20-specific deubiquitinase. USP44 knockdown increased Cdc20 polyubiquitination and centrosomal Cdc20-APC activity in neurons, and correspondingly promoted the elaboration of dendrites. Thus, HDAC6 enhances the polyubiquitination status of endogenous Cdc20, which increases the activity of centrosomal Cdc20-APC and drives dendrite development.
A major question that remained to be addressed was how centrosomal Cdc20-APC regulates dendrite development. Kim and colleagues focused on centrosomal proteins that contain a conserved destruction box (D-box), which serves as a recognition motif in substrates of Cdc20-APC. The helix-loop-helix protein Id1 fulfilled both these criteria. Levels of Id1 substantially increased with MG132 treatment, suggesting that Id1 is regulated by the proteasome in neurons. Cdc20-APC catalyzed Id1 ubiquitination in vitro. Cdc20 knockdown in neurons increased endogenous Id1 levels, suggesting that Id1 is a physiologically relevant substrate of neuronal Cdc20-APC. To test if Cdc20-APC regulates Id1 to control dendrite development, Kim et al. induced knockdown of Id1 in neurons. Id1 knockdown stimulated dendrite growth and elaboration in primary neurons and in granule neurons in the cerebellum in vitro. Epistasis analyses revealed that Id1 operates downstream of Cdc20 to orchestrate dendrite growth. These data support the conclusion that centrosomal Cdc20-APC promotes the ubiquitination of Id1 to control dendrite development in the mammalian brain (Figure 1E).
The role of the centrosomal Cdc20-APC ubiquitin ligase pathway in dendrite morphogenesis stands in contrast to that of the Cdh1-APC signaling pathway, which operates in the nucleus to regulate axon growth.13, 15 Thus the APC may act as a critical regulator of diverse aspects of neuronal morphology by specifically recruiting Cdc20 and Cdh1 in unique subcellular locales.
The identification of Cdc20-APC as the first ubiquitin ligase to control dendrite development in the mammalian brain raises fundamental questions regarding the physiological mechanisms that control dendrite development and suggest new mechanisms that may contribute to the pathogenesis of neurological diseases. Previous studies have implicated transcription factors as an important cell-intrinsic mechanism for controlling dendrite development and maturation.27-29 Therefore, important issues for future investigation include how centrosomal Id1 and its regulation by Cdc20-APC ultimately influence dendrite growth and branching and whether these cellular mechanisms operate independently of or in corroboration with other pathways regulating dendrite morphogenesis. Importantly, the identification of the HDAC6/Cdc20-APC/Id1 pathway suggests that the centrosome is a local platform for cellular signaling. Because the centrosome has been implicated in neuronal migration and axon specification and growth30 as well as in neurodevelopmental disorders such as lissencephaly and schizophrenia,31, 32 components of the centrosomal Cdc20-APC signaling pathway may have additional roles in these centrosomally-mediated physiologic and pathophysiologic processes.
The identification of Cdc20-APC as a novel mediator of dendrite development reinforces the concept that cell cycle proteins may have functions in post-mitotic neurons and suggests the intriguing hypothesis that regulators of Cdc20-APC in proliferating cells may be involved in the control of dendrite morphogenesis. These valuable insights from the cell cycle field may also illuminate as-of-yet uncharacterized functions of the APC in neuronal development and function. An additional ramification of investigations of cell cycle proteins in neurons is that novel mechanisms elucidated in post-mitotic neurons may be relevant to important biological processes in proliferating cells. HDAC6 controls primary cilium morphogenesis in epithelial cells, suggesting the idea that Cdc20-APC may operate downstream of HDAC6 to control the primary cilium as well. More speculatively, identification of the HDAC6/Cdc20-APC/Id1 signaling pathway raises the possibility that HDAC6 may also regulate Cdc20-APC and mitosis in proliferating cells.
Recently, Cdc20-APC has also been implicated in pre-synaptic differentiation in addition to its functions in dendrite morphogenesis.33 Together, these findings raise the interesting prospect that the major cell cycle regulator Cdc20-APC may have diverse roles in neurobiology. It remains to be seen how many new tricks an old dog can learn.
References
- 1.Sullivan M, Morgan DO. Finishing mitosis, one step at a time. Nat Rev Mol Cell Biol. 2007;8:894–903. doi: 10.1038/nrm2276. [DOI] [PubMed] [Google Scholar]
- 2.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–56. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 3.Wolf DA, Jackson PK. Cell cycle: oiling the gears of anaphase. Curr Biol. 1998;8:R636–9. doi: 10.1016/s0960-9822(07)00410-1. [DOI] [PubMed] [Google Scholar]
- 4.Baker DJ, Dawlaty MM, Galardy P, van Deursen JM. Mitotic regulation of the anaphase-promoting complex. Cell Mol Life Sci. 2007;64:589–600. doi: 10.1007/s00018-007-6443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Peters JM. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell. 2002;9:931–43. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- 7.Becker EB, Bonni A. Beyond proliferation--cell cycle control of neuronal survival and differentiation in the developing mammalian brain. Semin Cell Dev Biol. 2005;16:439–48. doi: 10.1016/j.semcdb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Herrup K, Yang Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci. 2007;8:368–78. doi: 10.1038/nrn2124. [DOI] [PubMed] [Google Scholar]
- 9.Liu DX, Greene LA. Neuronal apoptosis at the G1/S cell cycle checkpoint. Cell Tissue Res. 2001;305:217–28. doi: 10.1007/s004410100396. [DOI] [PubMed] [Google Scholar]
- 10.Kim AH, Bonni A. Thinking within the D box: initial identification of Cdh1-APC substrates in the nervous system. Mol Cell Neurosci. 2007;34:281–7. doi: 10.1016/j.mcn.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Huynh MA, Stegmuller J, Litterman N, Bonni A. Regulation of Cdh1-APC function in axon growth by Cdh1 phosphorylation. J Neurosci. 2009;29:4322–7. doi: 10.1523/JNEUROSCI.5329-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikeuchi Y, Stegmuller J, Netherton S, Huynh MA, Masu M, Frank D, Bonni S, Bonni A. A SnoN-Ccd1 pathway promotes axonal morphogenesis in the mammalian brain. J Neurosci. 2009;29:4312–21. doi: 10.1523/JNEUROSCI.0126-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konishi Y, Stegmuller J, Matsuda T, Bonni S, Bonni A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science. 2004;303:1026–30. doi: 10.1126/science.1093712. [DOI] [PubMed] [Google Scholar]
- 14.Stegmuller J, Huynh MA, Yuan Z, Konishi Y, Bonni A. TGFbeta-Smad2 signaling regulates the Cdh1-APC/SnoN pathway of axonal morphogenesis. J Neurosci. 2008;28:1961–9. doi: 10.1523/JNEUROSCI.3061-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stegmuller J, Konishi Y, Huynh MA, Yuan Z, Dibacco S, Bonni A. Cell-intrinsic regulation of axonal morphogenesis by the Cdh1-APC target SnoN. Neuron. 2006;50:389–400. doi: 10.1016/j.neuron.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans Neuron. 2002;35:107–20. doi: 10.1016/s0896-6273(02)00749-3. [DOI] [PubMed] [Google Scholar]
- 17.Juo P, Kaplan JM. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr Biol. 2004;14:2057–62. doi: 10.1016/j.cub.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 18.van Roessel P, Elliott DA, Robinson IM, Prokop A, Brand AH. Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell. 2004;119:707–18. doi: 10.1016/j.cell.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Shin YH, Hou L, Huang X, Wei Z, Klann E, Zhang P. The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nat Cell Biol. 2008;10:1083–9. doi: 10.1038/ncb1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Higuera I, Manchado E, Dubus P, Canamero M, Mendez J, Moreno S, Malumbres M. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat Cell Biol. 2008;10:802–11. doi: 10.1038/ncb1742. [DOI] [PubMed] [Google Scholar]
- 21.Almeida A, Bolanos JP, Moreno S. Cdh1/Hct1-APC is essential for the survival of postmitotic neurons. J Neurosci. 2005;25:8115–21. doi: 10.1523/JNEUROSCI.1143-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolanos JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–52. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 23.Kim AH, Puram SV, Bilimoria PM, Ikeuchi Y, Keough S, Wong M, Rowitch D, Bonni A. A centrosomal Cdc20-APC pathway controls dendrite morphogenesis in postmitotic neurons. Cell. 2009;136:322–36. doi: 10.1016/j.cell.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyault C, Gilquin B, Zhang Y, Rybin V, Garman E, Meyer-Klaucke W, Matthias P, Muller CW, Khochbin S. HDAC6-p97/VCP controlled polyubiquitin chain turnover. EMBO J. 2006;25:3357–66. doi: 10.1038/sj.emboj.7601210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–5. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- 26.Stegmeier F, Rape M, Draviam VM, Nalepa G, Sowa ME, Ang XL, McDonald ER, 3rd, Li MZ, Hannon GJ, Sorger PK, Kirschner MW, Harper JW, Elledge SJ. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 2007;446:876–81. doi: 10.1038/nature05694. [DOI] [PubMed] [Google Scholar]
- 27.Gaudilliere B, Konishi Y, de la Iglesia N, Yao G, Bonni A. A CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron. 2004;41:229–41. doi: 10.1016/s0896-6273(03)00841-9. [DOI] [PubMed] [Google Scholar]
- 28.Shalizi A, Bilimoria PM, Stegmuller J, Gaudilliere B, Yang Y, Shuai K, Bonni A. PIASx is a MEF2 SUMO E3 ligase that promotes postsynaptic dendritic morphogenesis. J Neurosci. 2007;27:10037–46. doi: 10.1523/JNEUROSCI.0361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shalizi A, Gaudilliere B, Yuan Z, Stegmuller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311:1012–7. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- 30.Higginbotham HR, Gleeson JG. The centrosome in neuronal development. Trends Neurosci. 2007;30:276–83. doi: 10.1016/j.tins.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–58. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Steward R, Luo L. Drosophila Lis1 is required for neuroblast proliferation, dendritic elaboration and axonal transport. Nat Cell Biol. 2000;2:776–83. doi: 10.1038/35041011. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Kim AH, Yamada T, Wu B, Bilimoria PM, Ikeuchi Y, De la Iglesia N, Shen J, Bonni A. A Cdc20-APC Ubiquitin Signaling Pathway Regulates Presynaptic Differentiation. Science. 2009;326:575–8. doi: 10.1126/science.1177087. [DOI] [PMC free article] [PubMed] [Google Scholar]


