Abstract
Pulmonary arterial hypertension (PAH) is a progressive disease of the pulmonary vasculature, ultimately resulting in right heart failure and death. This disease is strongly predominant in females, although little is known regarding how sex influences disease development. Recent developments highlighting the importance of estrogen metabolites in both animal models and human disease have substantially increased our understanding of PAH in women. This review will focus on general knowledge of PAH, translational and basic science data regarding sex hormones in the pulmonary vasculature and on clinical issues that are particular to women with PAH. Future directions for study include the influence of sex hormones on right ventricular responses, improving the understanding of the influence of estrogen exposure in human disease and the study of dehydroepiandrosterone in basic science and human disease.
Keywords: gender, pulmonary arterial hypertension, pulmonary hypertension, sex, vascular response
Pulmonary hypertension (PH) is a heterogeneous group of disorders characterized by elevated pulmonary artery pressure and right ventricular failure. Vascular injury and remodeling in PH are observed in association with many chronic medical diseases, including connective tissue disease, chronic thromboembolic disease and systolic and diastolic heart failure, as well as in idiopathic and heritable forms of PH. Thus, PH probably represents a common final phenotype stemming from complex interactions between the environment, inflammatory mediators and genetic susceptibility. The spectrum of PH includes pulmonary arterial hypertension (PAH), which has a female predominance and is of special interest to this paper. There have been significant advances in the understanding of the molecular basis, genetics, clinical classification and therapeutic options for PAH in the last decade [1-3]. Despite these advances, PAH continues to be a relentlessly progressive and fatal disease for many patients [4]. This review describes the current nomenclature and classification for PH, the pathophysiology and treatment options of PH, the new insights and understanding of the role of gender and sex hormones in the development of PH and potential future directions for the management and care of patients. We will first focus on PAH in general, and later discuss the data on PAH development in women from basic science and clinical perspectives.
Definition & classification
The preferred nomenclature for this group of diseases has changed with greater knowledge and understanding of the underlying pathogenesis and the role of genetics. The changes in nomenclature of PH over the last two decades have proven to be somewhat confusing for clinicians; however, the recently updated clinical classifications group causes of PH by etiology and pathologic similarity represent the most up-to-date knowledge of this heterogeneous group of diseases (Box 1). In addition, the definition of PH has been simplified following the recent Fourth World Symposium on Pulmonary Hypertension in February 2008. New recommendations have now eliminated the older exercise and pulmonary vascular resistance criteria [5]: the new definition is a resting mean pulmonary artery pressure (mPAP) of greater than or equal to 25 mmHg. An mPAP of 8–20 mmHg should be considered normal, and patients with an mPAP of 21–24 mmHg may be considered an ‘at-risk’ population, but further study is needed in this group.
Box 1. Clinical classification of pulmonary hypertension from the Fourth World Symposium on Pulmonary Hypertension.
- Group 1: PAH:
- – Idiopathic PAH.
- – Heritable PAH.
- – Drug and toxin-induced.
- – Associated with connective tissue disease, HIV, portopulmonary hypertension, schistosomiasis, chronic hemolytic anemia, etc.
Group 1’: Pulmonary veno-occlusive disease and/or pulmonary capillary hemangiomatosis.
Group 2: Pulmonary hypertension owing to left heart disease (including systolic and diastolic dysfunction and mitral or aortic valvular disease).
Group 3: Pulmonary hypertension secondary to lung diseases and/or hypoxia.
Group 4: Chronic thromboembolic pulmonary hypertension.
Group 5: Miscellaneous (e.g., hematologic disorders, sarcoidosis, pulmonary Langerhans cell histocytosis, metabolic disorders).
PAH: Pulmonary arterial hypertension.
Modified from [3].
A subgroup of PH, WHO group 1 PAH, is manifested by vascular remodeling in the pre-capillary arterioles, and it is defined as an mPAP of greater than or equal to 25 mmHg at rest with a pulmonary artery wedge pressure of less than or equal to 15 mmHg. Definitive diagnosis of PH or PAH requires right heart catheterization [6,7] because of the strict hemodynamic criteria in the definition. We will discuss specific evaluation and management in a later section.
Group 1 contains disease processes manifesting with PAH, including idiopathic, heritable, drug- and toxin-induced forms, and PAH associated with connective tissue diseases, HIV, portal hypertension and congenital heart disease, among other causes. With increased recognition and understanding of the specific genetic mutations associated with PAH, the term heritable PAH (HPAH) is now preferred over familial PAH and includes patients with identified mutations in the bone morphogenic protein receptor type 2 (BMPR2) gene, activin receptor-like kinase type 1 (ALK-1) gene and endoglin who may not have a family history of PAH but have the heritable disease (see ‘Genetics & heritable pulmonary arterial hypertension’ section). Two rare causes of PAH, pulmonary veno-occlusive disease (PVOD) and pulmonary capillary hemangiomatosis are incorporated into a new subgroup, Group 1’. PVOD and pulmonary capillary hemangiomatosis share pathologic features of other causes of PAH, frequently coexist on biopsy specimens and are often challenging to distinguish from PAH antemortem [3,8,9]. PVOD in particular has a worse prognosis than PAH, and may potentially have an adverse response to PAH-directed therapy, since increasing flow in the context of venous obstruction may result in pulmonary edema. A lack of response to standard therapy, or worsening of symptoms upon treatment, may be an indication that PVOD could be present.
Group 2 includes patients with PH related to left heart disease, widely accepted to be the most common cause of PH in developed countries. PH in this group is related to elevations in mPAP that occur as a result of passive congestion owing to elevations in pressure in the left atrium and pulmonary veins. This group is often described as having pulmonary venous hypertension. Endothelial dysfunction and pulmonary vascular remodeling are thought to occur in this group with similar underlying pathophysio logy to PAH [10,11]. To date, large long-term studies using therapies approved for PAH in patients with PH related to left heart disease have demonstrated poorer outcomes in the case of endothelin-receptor antagonists [12] and prostacyclins [13], or are as yet unknown in the case of phosphodiesterase type 5 (PDE5) inhibitors. PDE5 inhibitors have demonstrated potential in this population, with case series [14-16] and animal data suggestive of a potential treatment benefit [17]. Until data from a large-scale clinical trial of PDE5 inhibitors in pulmonary venous hypertension are available, treatment goals for this disease are focused on the management of left heart disease.
Groups 3 and 4 include PH due to parenchymal or hypoxemic lung disease for the former and chronic thromboembolic PH for the latter (Box 1). Group 5 continues to contain several forms of PH for which the disease mechanism is not fully understood, including chronic hematologic disorders, sarcoidosis and pulmonary Langerhans cell histiocytosis. The increasing number of diagnoses associated with this group highlights the diversity of the PH spectrum and our ongoing need for further research and understanding. The remainder of this review will focus on PAH alone.
Epidemiology
Pulmonary artery hypertension was once thought to be exceedingly rare; however, a recent large French cohort of PAH patients demonstrates a prevalence of 15 cases per million [4]. The median age of those affected is 37–50 years, although recent reports suggest identification of significant PAH even in elderly patients [4,18,19]. Idiopathic PAH (IPAH) is the most common form, with PAH associated with connective tissue disease, congenital heart disease, portal hypertension and anorexigens following in decreasing order of frequency [4]. PAH is observed more commonly in females than males, in a ratio of 1.9:1 to 4.1:1 in larger mixed trials [18,20] and nearly 2.7:1 in HPAH [21]. It is this female predominance that has led to further studies in the role of gender and sex hormones in the development of pulmonary vascular remodel ing, a topic discussed in depth in this review. A recent case–control study in patients with cirrhosis and portal hypertension demonstrated that women were significantly more likely than men to develop portopulmonary hypertension, a subgroup of PAH [22], further suggesting a potential role for estrogens or other gender-specific factors having a role in the pathogenesis of PH.
Genetics & heritable pulmonary arterial hypertension
In 2000, mutations in BMPR2, which participates in signal transduction in the TGF-β family, were described in cases of familial PAH [23,24]. Mutations in BMPR2 are observed in 70% of cases of familial PAH and up to 20% of IPAH cases [25,26]. HPAH in hereditary hemorrhagic telangiectasia is associated with mutations in other TGF-β signaling participants, including ALK-I, the accessory receptor endoglin and Smad 4 [27]. HPAH is inherited as an autosomal dominant trait with incomplete penetrance and female predominance in adulthood, although there is earlier onset in males and increased male fetal wastage [21,28]. The vascular remodeling seen in HPAH is identical to that seen in IPAH cases; however, patients with HPAH are younger at age of disease onset, have more severe hemodynamic impairment and are less likely to respond to vasodilator therapy [29,30].
Pathophysiology of pulmonary arterial hypertension
Pulmonary arterial hypertension is primarily a disease of the small arterioles characterized by intimal hyperplasia and fibrosis, medial hypertrophy, vascular occlusion and smooth muscle and endothelial dysfunction. In advanced stages, endothelial cells, vascular smooth muscle cells and fibroblasts aggregate to form ‘plexiform lesions’, contributing to arteriopathy and loss of vascular cross sectional area, ultimately manifesting as increased pulmonary vascular resistance and right heart failure [31]. The underlying mechanism for development of these derangements in PAH is not fully elucidated; however, there is an increasing understanding of the complex interplay between vasoconstricting and vasodilating substances (e.g., thromboxane, endothelin and nitric oxide [NO]), aberrations in cell-signaling and antiproliferative pathways (i.e., TGF-β family), vascular thrombosis and inflammation. As described later in this article, our current therapies for PAH are directed at each of these various mechanisms.
In PAH, an imbalance of the arachidonic acid pathway metabolites thromboxane A2 (a potent vasoconstictor) and prostacyclin (a potent vasodilator) favors thromboxane A2 [32], leading to vasoconstriction, thrombosis and platelet activation. Patients with PAH have decreased prostacyclin synthase in their small and medium sized pulmonary arteries, potentiating vasoconstriction and platelet activation [33]. NO (a potent vasodilator and inhibitor of platelet activation and smooth muscle cell proliferation) is also involved in the pathogenesis of PAH. There are several isoforms of NO synthase, which catalyzes the production of NO on the vascular endothelium, and patients with PAH have decreased levels of endothelial NO synthase [34]. Patients with PAH also have higher plasma levels of endothelin-1, a potent vasoconstrictor that also stimulates proliferation of smooth muscle cells, contributing to the vascular remodeling observed in PAH [35]. Additional vasoactive mediators including serotonin, vasoactive intestinal peptide and VEGF, which may also be involved [36]. A complex interplay between local cellular effects, sex hormones, genetics and environmental exposures (i.e., hypoxia and drug/toxins) probably contributes to the diversity of disease seen in PH.
Evaluation & treatment
Evaluation of patients with PH is focused on characterizing the etiology and ascertaining the severity of disease by noninvasive and invasive testing. When a diagnosis of PH is suspected based on the presence of disease conditions that are known to be associated with PH or clinical symptoms (most commonly dyspnea or signs of right heart failure), echocardiography is often employed as the first screening tool. In addition to providing an estimation of pulmonary artery pressure, echocardiography is useful in determining the presence of left-sided systolic and diastolic dysfunction, as well as valvular disease and right ventricular function [7]. A detailed discussion of the evaluation of PH is outside the realm of this article, but should include a thorough history and physical examination, pulmonary function testing, chest imaging and a ventilation perfusion lung scan in order to evaluate for chronic thromboembolic disease [5]. Right heart catheterization is the gold standard for definitive diagnosis of PAH, prognostication and therapeutic decision-making when carried out in concert with a vasodilator challenge [5,7].
At present, there are seven US FDA-approved agents for the treatment of PAH (including IPAH and associated PAH, WHO Group 1). The selection of therapy in PAH is a complex decision and is frequently determined by centers with interdisciplinary teams of physicians, case managers and highly-subspecialized nursing staff. Patients with significant responses to vasodilator trial during right heart catheterization (defined as a decrease in mPAP by 10 mmHg to a mPAP of ≤40 mmHg with unchanged or increased cardiac output by consensus [37]) and no evidence of right heart failure may be placed on calcium-channel blocker therapy [2]. This therapy is available for a minority of patients since only approximately 5% of patients with PAH exhibit true vasodilator response [38].
Table 1 outlines the drug classes and current FDA-approved agents for PAH. In 1995, intravenous epoprostenol was introduced as the first specific therapy for PAH and, despite the complexities of prostacyclin therapy, this group of agents continues to be a valuable therapy for patients with advanced disease (primarily NYHA functional class III and IV). Epoprostenol has a short half-life, requiring continuous intravenous administration, and therapy is complicated by catheter-related infections. Two newer prostacyclin analogs allow alternatives to intravenous administration: treprostinil is available as subcutaneous and intravenous infusion as well as an inhalational formulation, and iloprost is available as an inhaled therapy. Within the last 7 years, several oral therapies have surfaced with success in PAH. The endothelin-receptor antagonists bosentan and ambrisentan are oral therapies approved for use in patients with PAH and functional class II–III, and they have been demonstrated to improve exercise capacity and clinical functional class in several trials [39-43]. Sitaxsentan is an endothelin-receptor antagonist that is not currently FDA-approved but is in use in many countries with properties similar to ambrisentan. Phosphodiesterase inhibitors, including sildenafil and more recently tadalafil, have been approved for use in PAH [44,45]. Combination therapy is often used, especially with the oral agents; recent data from the Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) trial of tadalafil suggested that the addition of tadalafil to bosentan provided further improvement in 6-min walk distance, although the trial was not designed to determine the benefit of combination therapy [45]. Further data are needed before combination therapy can be recommended.
Table 1. US FDA-approved pharmacologic therapies available for the treatment of pulmonary arterial hypertension.
| Drug | US FDA-approved class: route |
|---|---|
| Prostacyclin analogs | |
| Epoprostenol (generic, Flolan™) | PAH functional class III and IV; intravenous use |
| Treprostinil (Remodulin™) | PAH functional class II, III and IV; subcutaneous, inhaled or intravenous formations |
| Iloprost (Ventavis™) | PAH functional class III and IV; inhalational use |
| Endothelin-receptor antagonists | |
| Bosentan (Tracleer™) | PAH functional class II, III and IV; oral therapy |
| Ambrisentan (Letairis™) | PAH functional class II, III and IV; oral therapy |
| Phosphodiesterase inhibitors | |
| Sildenafil (Revatio™) | PAH functional class II, III and IV; oral therapy |
| Tadalafil (Adcirca™) | PAH functional class II, III and IV; oral therapy |
PAH: Pulmonary arterial hypertension.
Anticoagulation with warfarin is frequently employed in PAH on the basis of several retrospective reviews demonstrating favorable effect; however, there have been no prospective or randomized trials of anticoagulation for PAH [46,47]. Supportive care, including diuretics for right heart failure symptoms and oxygen for hypoxemic patients, can be helpful as symptoms develop. Very occasionally, patients require lung transplantation or atrial septostomy for end-stage PAH.
Pulmonary arterial hypertension development in women
Basic science
Despite clear epidemiologic data demonstrating a female predominance in the development of many forms of PAH, the mechanisms of gender disparities remain largely unknown. There are several potential reasons why women may develop PAH at a higher frequency than men: estrogens are damaging agents in the pulmonary vasculature; testosterone is beneficial for the pulmonary vasculature; women have an environmental exposure that men do not (e.g., anorexigen exposure and environmental exposure); and other factors associated with being female put patients at risk for PAH (e.g., exposure to fetal cells and increased autoimmunity). Currently, scant literature exists concerning the differing environmental exposures that put women at risk for PH, and while autoimmune disorders are well-documented to be more frequent in women [48-50], how this may specifically lead to pulmonary vascular disease has not been examined. Much basic science investigation has focused on the effects of sex hormones in the pulmonary vasculature and on animal models of PH. Recent findings in human patients are beginning to translate the basic science into knowledge regarding human disease. These findings will be the focus of our discussion, with a particular emphasis on estrogen, since it is the best-characterized sex hormone in the pulmonary vasculature.
Sex hormone metabolism
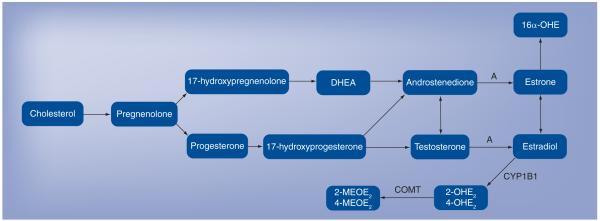
Knowledge of sex hormone synthesis and metabolism is critical to understanding modern thought on the role these compounds play in the pulmonary vasculature. Briefly, all steroid hormones are derived from cholesterol and are primarily synthesized in the gonads, adrenal glands and, in the case of pregnancy, the fetoplacental unit. The pathways for conversion to sex hormones are depicted in Figure 1. Cholesterol is converted to pregnenolone, which in turn is either metabolized through 17-hydroxy-pregnenolone to dehydroepiandrosterone (DHEA) and then androstenedione, or to progesterone and further downstream to testosterone and, via the enzyme aromatase, to estradiol. Estradiol is the primary active sex hormone in women. Estradiol can be converted to estrone, a weaker sex hormone, or metabolized through several different pathways. CYP1B1 and CYP1A1 convert estradiol to the catechol estradiols, 2-hydroxyestradiol (OHE2) or 4-OHE2, which can then be converted via catechol-O-methyltransferase to the methoxyestradiols, 2-methoxyestradiol (MEOE2) and 4-MEOE2 [51,52]. Estrone is metabolized to 16α-hydroxyestrogen (OHE). There is increasing literature that these metabolites are not simply byproducts of estrogen but rather have independent and important actions of their own [28,53,54].
Figure 1. Sex hormone synthetic pathway.
A: Aromatase; COMT: Catechol-O-methyltransferase; DHEA: Dehydroepiandrosterone; MEOE2: Methoxyestradiol; OHE: Hydroxyestrogen; OHE2: Hydroxyestradiol. Adapted from [51,52].
Estrogen exerts its effects through the receptors α and β, which may be either cytosolic or membrane-bound and, classically, estrogen alters gene transcription [55], although recent literature suggests that there may be alternative, non-classical signaling through G-protein-coupled receptors [56].
Estrogens
Animal models have explored the acute and chronic effects of estrogen administration; however, the applicability of these data to humans is questionable since, until recently, there was no true animal model of PAH [57]. The bulk of knowledge is from hypoxia and monocrotaline models that will be explained later in this article. Hypoxia is one of the most potent vasoconstrictive stimuli known in the pulmonary arteries and is widely used as a model of both acute vasoconstriction and chronically to induce irreversible PH. In studies of isolated pulmonary vascular rings acutely challenged with hypoxia or phenylephrine, vascular rings from both female and male mice that were coadministered estrogen displayed vasodilation, demonstrating the acute vasodilator effects of estrogen in vascular rings from both sexes [58]. The same group has found that even physiologic estrogen level fluctuations observed with menstruation attenuate pulmonary artery vasoconstriction in isolated vascular rings, with less vasodilation found prior to ovulation when estrogen levels are lower [58]. Both estrogen receptors α and β appear to mediate this effect through an NO-dependent mechanism [59]. Thus, estrogen appears to acutely promote pulmonary artery vasorelaxation.
In rodent models of PH, such as chronic hypoxia or the vinca alkaloid monocrotaline, estrogen similarly appears to attenuate disease [60-63]. It has been understood for some time that female rodents and swine do not develop PH of comparable severity to males when exposed to chronic hypoxia [60-62]. More recently, in chronically hypoxic rodents who have had ovariectomies, PH has been found to be more severe than in rodents with intact ovaries and return of estrogens allows for a return to baseline levels of PH in female rodents [64]. These findings have been replicated in monocrotaline-associated PH, an inflammatory model of PH resulting from the injection of this toxic plant alkaloid [63]. Interestingly, the nonestrogenic metabolite of estrogen, 2-MEOE2, attenuates monocrotalineinduced PH and also bleomycin-induced PH and fibrosis in both male and female rats [53,63]. Thus, it appears that nonestrogenic estrogen metabolites are potentially the mediators of the beneficial effects of estrogens. In summary, basic science has repeatedly demonstrated that estrogen and its metabolites are beneficial for the pulmonary vasculature in both acute and chronic models of PH. This clearly contradicts the well-documented female predominance of human PAH.
Testosterone
Testosterone is a potential answer to the paradox of the beneficial effects of estrogen in animal models of disease, since the female predominance in PAH might be is explained by the absence of testosterone. There are quite limited data on testosterone in the pulmonary vasculature. Testosterone has been demonstrated to induce vasodilation in the isolated rat pulmonary artery; in fact, it has been demonstrated to be a more potent vasodilator in this bed than estradiol [65,66]. The action of testosterone in the pulmonary artery appears not to be dependent on classic androgen-receptor signaling, release of NO or prostaglandins and is, therefore, thought to have a calcium-antagonistic action [66]. Studies in human pulmonary arteries isolated from elective lung resections for lung nodules have confirmed the vasodilatory action of testosterone and found that this effect is independent of sex (i.e., both male and female isolated pulmonary vascular rings dilated equally to testosterone) [67]. However, it has been clearly demonstrated that male rodents develop more severe pulmonary vascular lesions and physiologic markers of PH in experimental models such as hypoxia and monocrotaline; thus, to date, experiments into testosterone deprivation and supraphysiologic dosing have not been performed [60-64]. Despite the limitations of animal models of PH, the worse phenotype in both commonly used rodent models of PH makes testosterone deprivation unlikely to be at the root of female predominance of PAH.
Dehydroepiandrosterone
Recently, DHEA has been considered as a potentially important mediator of female predominance in human PAH. It serves as a precursor to both estrogen and its metabolites, as well as testosterone, and is synthesized in the adrenal glands, compared with the primarily gonad-synthesized sex hormones. Moreover, DHEA and its sulfated ester, DHEAS, are produced at the highest levels of all circulating steroid hormones. DHEAS serves as a circulating reservoir for DHEA. DHEA inhibits acute hypoxic vasoconstriction [68], has recently been demonstrated to protect against the develop ment of PH due to chronic hypoxia in male rats [69] and reverses monocrotaline-associated PH in a male rat model [70]. This animal model suggests a beneficial effect of DHEA on PH, both acute and chronic, in males. Data regarding female animal models are lacking.
Taken together, the basic science evidence supports a beneficial effect of estrogens and perhaps DHEA with a probable negative effect of testosterone. These data are challenging to reconcile with the clinical observation that females develop more PAH compared with males. The salutary effect of estrogen metabolites in pulmonary vascular disease suggests that altered estrogen metabolism with accumulation of ‘bad’ metabolites may play an important role in PAH.
Translational data
Although the genetic disorder of familial PAH has been known for many years, the female predominance of this somatic gene mutation and the low penetrance of disease at approximately 20% of mutation carriers has not been explained [21]. Gene arrays in both affected and unaffected BMPR2 mutation carriers pointed to CYP1B1 as a potential underlying reason for why females are more affected by HPAH, as this gene was expressed at approximately ten-times lower levels in affected female patients than unaffected female mutation carriers [71]. CYP1B1 is a cytochrome P450 enzyme that catalyzes oxidation of estrogens to 2-OHE2 and 4-OHE2 (Figure 1) [52]. CYP1B1 has been implicated in a number of cancers [72-76], is expressed in the lungs at high levels [77] and is known to metabolize environmental toxins and tobacco smoke [78]. If the activity of CYP1B1 were lower, there would be lower levels of the beneficial 2-OHE2 and 16α-OHE would accumulate. This metabolite constitutively activates the estrogen receptor and thus stimulates cellular proliferation [79]. If CYP1B1 expression was reduced in affected mutation carriers, the ratio of beneficial estrogen metabolites to stimulatory metabolites would be lower. Recently, Austin and colleagues demonstrated that there was indeed a greater penetrance of PAH in female BMPR2 mutation carriers with wild-type CYP1B1, and that, in affected mutation carriers, the urinary ratio of 2-OHE2:16α-OHE was substantially lower in affected mutation carriers compared with unaffected carriers [28]. These findings demonstrated that altered estrogen metabolism through polymorphisms in CYP1B1 may account for the female predominance of HPAH and modify the risk of development of this disease in mutation carriers.
Other clues pointing to underlying mechanisms in human PAH have come from the portopulmonary hypertension literature. It is well known that patients with cirrhotic liver disease are at risk for developing PAH; female sex and autoimmune hepatitis are risk factors for the development of this rare complication [22]. Subsequent data have demonstrated that aromatase polymorphisms explain this observation and, moreover, that these polymorphisms translate into higher estrogen levels in patients with portopulmonary hypertension [80]. Estrogen metabolite levels in this cohort are unknown, but this research clearly indicates that higher levels of estrogen leads to higher levels of, and perhaps imbalance in, estrogen metabolites, which may cause pulmonary vascular disease in susceptible individuals.
Human data
There are scant data in humans regarding the role of environmental and pharmacologic estrogens in PAH. Many women are exposed to estrogens through either HRT or oral contraceptive use. In a small retrospective study, HRT was found to be associated with a lower rate of PAH development in a cohort of patients with scleroderma [81]. Conversely, one case report describes the rapid development of PAH after HRT in a patient with a family history of HPAH that had been followed serially for the development of disease [82]. Thus, the role of pharmaceutical estrogens in PAH is not yet known.
As the right ventricle is the primary determinant of survival in PAH [83] and gender influences on right ventricular function in animal models of disease have been found in our laboratory [Hemnes AR, Unpublished Data], recent research has focused on the influence of gender on right ventricular function in humans. In a study of PAH patients who underwent equilibrium radionuclide angiography to measure right ventricular ejection fraction, male sex was associated with lower right ventricular ejection fraction on multivariate analysis [84]. In normal individuals, right ventricular ejection fraction is lower in males compared with females [85], and these observations should lead to further study of the underlying mechanisms of right ventricular hypertrophic differences in males and females with PAH. Table 2 provides a summary of established data regarding the role of estrogens in PAH subtypes.
Table 2. Summary of data regarding estrogens in pulmonary arterial hypertension subtypes.
| Disease | Female predominance (ratio) |
Animal data | Human data | Ref. |
|---|---|---|---|---|
| Idiopathic PAH | Yes (1.9:1) | Weak and conflicting, estrogen metabolites may be involved |
None | [4,52-54,56] |
| Scleroderma PAH | Possibly, female predominance in scleroderma makes the epidemiology difficult to interpret |
None | Weak, hormone replacement is possibly protective |
[4,81] |
| Portopulmonary PAH | Yes (ratio unknown) | None | Yes, aromatase polymorphisms increase estrogen levels |
[22,80] |
| Heritable PAH | Yes (3:1) | None | Yes, CYP1B1 expression alters estrogenic compound exposure |
[21,28,71] |
PAH: Pulmonary arterial hypertension.
Recommendations for pulmonary arterial hypertension care in women
Most issues related to the care of women with PAH relate to issues of hormone manipulation and avoidance, whether as part of birth control, menopause or pregnancy. Pregnancy in the setting of PH carries significant risk for maternal death, although most of the data reported are in the setting of PAH. The physiologic changes that occur with pregnancy, including increased cardiac output and demand on the right ventricle in the setting of poorly compliant pulmonary vasculature, are not well-tolerated in PAH patients. Before the advent of PAH-specific therapy, the maternal mortality with pregnancy in PAH ranged from 30 to 56% [86]. A recent review of the published literature from 1997 to 2007 by Bedard and colleagues, since the development of PAH-specific therapies (primarily prostacyclin and NO) demonstrated significant decreases in maternal mortality compared with the prior era (1978–1996), with a maternal mortality of 17% in IPAH, 28% in PAH related to congenital heart disease and 33% in other causes of PAH [87]. Most of the maternal deaths occurred within the first month postpartum and several factors were found to be associated with higher mortality, including usage of general anesthesia and primagravid status [38]. Thus, even with advances in the medical treatment of PAH and in the interdisciplinary care of high-risk pregnancy, pregnancy in PAH carries a prohibitive risk. It is our practice to counsel all women of childbearing age with PAH to avoid pregnancy and recommend sterilization in those patients who are willing. Pregnant PAH patients should be referred to a PH center for specialized management with high-risk specialist obstetricians.
Because of the conflicting data regarding the use of HRT in PAH, we do not recommend its routine use unless there is a compelling reason to continue. Similarly, routine use of oral contraceptives is not recommended. Given the risk of pregnancy and the teratogenic potential of endothelin receptor antagonists, we recommend that all of our female patients use two forms of birth control unless they have postmenopausal or sterilized. Sterilization can be accomplished safely by hysteroscopy; alternatively, patients can have IUDs inserted into the uterus [88].
Future perspective
The well-documented female predominance in both idiopathic, heritable and many associated causes of PAH provides a basis for further study into the influence of sex on the pulmonary vasculature. The discussion in this article has highlighted the knowledge of sex hormones in both animal and human PH, but these data are currently quite limited. Future questions on this topic include the influence of exogenous hormones in PAH, the role of pregnancy and hormone fluctuations on PAH development, progesterone in the pulmonary vasculature and the study of DHEA and testosterone levels in human PAH and animal models of disease. As our understanding of sex hormones in PAH expands, it is quite possible that we will see trials of hormonal manipulation in human disease. We may also be able to determine whether one gender is more likely to respond to a particular therapy and so better tailor our pharmaceutical interventions. In addition, the central role of the right ventricle in PAH, and the absence of data regarding the influence of sex on right ventricular function, point to fertile research ground for the future, both in animal models of PH and in human disease. Lastly, this review has focused on sex hormones in the pulmonary vasculature, but it is clear that although this is the most studied aspect of sex and gender, other hypotheses deserve further investigation as well. Perhaps the inflammation induced by autoimmunity is a major contributor to disease and females are at a higher risk because of their propensity to develop autoimmune disease.
In conclusion, PAH is a devastating disease with a clear female predominance. There are limited and conflicting data from basic science on the influence of sex hormones on the pulmonary vasculature, but recent evidence strongly points to altered estrogen levels and metabolism in PAH leading to the development of disease. Future study in human disease focusing on sex hormones, gender-specific response to therapy, autoimmunity and sexual dimorphism in right ventricular function and adaptation will greatly improve our ability to understand and treat this devastating disease.
Executive summary.
Clinical pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is characterized by elevated pulmonary artery pressure with normal left-sided filling pressure.
PAH can be idiopathic, heritable or associated with other conditions.
Many subtypes of PAH, especially idiopathic, heritable and portopulmonary hypertension, are stongly predominant in females.
Treatment with endothelin-receptor blockers, phosphodiesterase 5 inhibitors, prostaglandins or calcium-channel blockers has substantially reduced mortality rates.
Knowledge regarding mechanisms of female predominance in pulmonary arterial hypertension
Basic data suggest that estrogens have a salutary effect on acute and chronic models of pulmonary hypertension. This beneficial effect might occur through particular estrogen metabolites.
Testosterone and dehydroepiandrosterone may also play a role in determining pulmonary vascular disease.
CYP1B1 expression is low in heritable PAH, and subsequent altered estrogen metabolism results in lower levels of 2-hydroxyestradiol, which is thought to be a positive agent in the pulmonary vasculature.
Increased estrogen levels in patients with portopulmonary hypertension may be explained by aromatase polymorphisms with higher activity levels, explaining the female predominance in this subtype of PAH.
Right ventricular function is affected by sex, but little is known concerning how this affects outcomes in PAH.
Future perspective
Human data on estrogen exposures, both pharmaceutical and environmental, will help elucidate mechanisms of PAH development in women.
Further research on right ventricular function, autoimmunity and pregnancy will allow for better understanding of sex hormone modification of disease development and prognosis, and possibly produce new targets for treatment.
Trials of estrogen manipulation are needed before recommendations can be made regarding their use or avoidance in PAH therapy.
Acknowledgments
Financial & competing interests disclosure
Dr Anna R Hemnes is supported by NIH grant K08 HL93363–01. She has received grant support from Actelion and research support from Gilead. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Meredith E Pugh, Division of Allergy, Pulmonary & Critical, Care Medicine, T1218 Medical Center, North, 1161 21st Avenue South Nashville, TN 37232, USA, Tel.: +1 615 322 3412, Fax: +1 615 343 7448, meredith.e.pugh@vanderbilt.edu.
Anna R Hemnes, Division of Allergy, Pulmonary & Critical, Care Medicine, T1218 Medical Center, North, 1161 21st Avenue South Nashville, TN 37232, USA, Tel.: +1 615 343 8227, Fax: +1 615 343 7448, anna.r.hemnes@vanderbilt.edu.
Bibliography
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Invest. 2008;118(7):2372–2379. doi: 10.1172/JCI33452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barst RJ, Gibbs JS, Ghofrani HA, et al. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009;54(Suppl. 1):S78–S84. doi: 10.1016/j.jacc.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2009;54(Suppl. 1):S43–S54. doi: 10.1016/j.jacc.2009.04.012. •• The most up-to-date clinical classification of pulmonary hypertension (PH) as established by the 4th World Symposium on Pulmonary Hypertension.
- 4.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am. J. Respir. Crit. Care Med. 2006;173(9):1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 5.Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009;54(Suppl. 1):S55–S66. doi: 10.1016/j.jacc.2009.04.011. •• Highlights the new consensus definition of PH and provides an in-depth review of the evaluation of PH for clinicians.
- 6.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2009;179(7):615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemnes AR, Forfia PR, Champion HC. Assessment of pulmonary vasculature and right heart by invasive haemodynamics and echocardiography. Int. J. Clin. Pract. Suppl. 2009;162:4–19. doi: 10.1111/j.1742-1241.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 8.Pietra GG, Capron F, Stewart S, et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J. Am. Coll. Cardiol. 2004;43(12 Suppl. S):S25–S32. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 9.Lantuejoul S, Sheppard MN, Corrin B, Burke MM, Nicholson AG. Pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis: a clinicopathologic study of 35 cases. Am. J. Surg. Pathol. 2006;30(7):850–857. doi: 10.1097/01.pas.0000209834.69972.e5. [DOI] [PubMed] [Google Scholar]
- 10.Oudiz RJ. Pulmonary hypertension associated with left-sided heart disease. Clin. Chest Med. 2007;28(1):233–241. X. doi: 10.1016/j.ccm.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Moraes DL, Colucci WS, Givertz MM. Secondary pulmonary hypertension in chronic heart failure: the role of the endothelium in pathophysiology and management. Circulation. 2000;102(14):1718–1723. doi: 10.1161/01.cir.102.14.1718. [DOI] [PubMed] [Google Scholar]
- 12.Packer M, McMurray J, Massie BM, et al. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: results of a pilot study. J. Card. Fail. 2005;11(1):12–20. doi: 10.1016/j.cardfail.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Califf RM, Adams KF, McKenna WJ, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: the Flolan International Randomized Survival Trial (FIRST) Am. Heart J. 1997;134(1):44–54. doi: 10.1016/s0002-8703(97)70105-4. [DOI] [PubMed] [Google Scholar]
- 14.Lewis GD, Lachmann J, Camuso J, et al. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115(1):59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 15.Lewis GD, Shah R, Shahzad K, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116(14):1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 16.Tedford RJ, Hemnes AR, Russell SD, et al. PDE5A inhibitor treatment of persistent pulmonary hypertension after mechanical circulatory support. Circ. Heart Fail. 2008;1(4):213–219. doi: 10.1161/CIRCHEARTFAILURE.108.796789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takimoto E, Champion HC, Li M, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat. Med. 2005;11(2):214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 18.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Ann. Intern. Med. 1987;107(2):216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 19.Yigla M, Kramer MR, Bendayan D, Reisner SA, Solomonov A. Unexplained severe pulmonary hypertension in the elderly: report on 14 patients. Isr. Med. Assoc. J. 2004;6(2):78–81. [PubMed] [Google Scholar]
- 20.Badesch DB, Raskob GE, Elliot CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest. 2010;137(2):376–387. doi: 10.1378/chest.09-1140. •• Details the characteristics of the REVEAL registry, a large prospective cohort of a US patient population with WHO Group I pulmonary arterial hypertension (PAH). This study demonstrated a high female predominance of disease with a 4.1:1 female-to-male ratio among patients with idiopathic PAH.
- 21.Loyd JE, Butler MG, Foroud TM, Conneally PM, Phillips JA, 3rd, Newman JH. Genetic anticipation and abnormal gender ratio at birth in familial primary pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1995;152(1):93–97. doi: 10.1164/ajrccm.152.1.7599869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawut SM, Krowka MJ, Trotter JF, et al. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008;48(1):196–203. doi: 10.1002/hep.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng Z, Morse JH, Slager SL, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am. J. Hum. Genet. 2000;67(3):737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman JH, Wheeler L, Lane KB, et al. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. N. Engl. J. Med. 2001;345(5):319–324. doi: 10.1056/NEJM200108023450502. [DOI] [PubMed] [Google Scholar]
- 25.Aldred MA, Vijayakrishnan J, James V, et al. BMPR2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension. Hum. Mutat. 2006;27(2):212–213. doi: 10.1002/humu.9398. [DOI] [PubMed] [Google Scholar]
- 26.Thomson JR, Machado RD, Pauciulo MW, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-β family. J. Med. Genet. 2000;37(10):741–745. doi: 10.1136/jmg.37.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machado RD, Eickelberg O, Elliott CG, et al. Genetics and genomics of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009;54(Suppl. 1):S32–S42. doi: 10.1016/j.jacc.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin ED, Cogan JD, West JD, et al. Alterations in estrogen metabolism: implications for higher penetrance of FPAH in females. Eur. Respir. J. 2009;34(5):1093–1099. doi: 10.1183/09031936.00010409. • Demonstrates that altered levels of an estrogen-metabolizing enzyme, CYP1B1, may modify risk of developing PAH in female patients with BMPR2 mutation. This highlights the potential role of estrogen metabolites in disease pathobiology.
- 29.Elliott CG, Glissmeyer EW, Havlena GT, et al. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation. 2006;113(21):2509–2515. doi: 10.1161/CIRCULATIONAHA.105.601930. [DOI] [PubMed] [Google Scholar]
- 30.Sztrymf B, Coulet F, Girerd B, et al. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am. J. Respir. Crit. Care Med. 2008;177(12):1377–1383. doi: 10.1164/rccm.200712-1807OC. [DOI] [PubMed] [Google Scholar]
- 31.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin. Chest Med. 2007;28(1):23–42. VII. doi: 10.1016/j.ccm.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christman BW, McPherson CD, Newman JH, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N. Engl. J. Med. 1992;327(2):70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- 33.Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1999;159(6):1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 34.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1995;333(4):214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 35.Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1993;328(24):1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 36.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N. Engl. J. Med. 2004;351(16):1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 37.McLaughlin VV, Archer SL, Badesch DB, et al. American College of Cardiology Foundation Task Force on Expert Consensus Documents. American Heart Association. American College of Chest Physicians. American Thoracic Society, Inc. Pulmonary Hypertension Association ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J. Am. Coll. Cardiol. 2009;53(17):1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Sitbon O, Humbert M, Jais X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111(23):3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 39.Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358(9288):1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 40.Galie N, Rubin L, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371(9630):2093–2100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 41.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2002;346(12):896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 42.Galie N, Badesch D, Oudiz R, et al. Ambrisentan therapy for pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2005;46(3):529–535. doi: 10.1016/j.jacc.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 43.Galie N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117(23):3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 44.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2005;353(20):2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 45.Galie N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119(22):2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 46.Frank H, Mlczoch J, Huber K, Schuster E, Gurtner HP, Kneussl M. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112(3):714–721. doi: 10.1378/chest.112.3.714. [DOI] [PubMed] [Google Scholar]
- 47.Johnson SR, Mehta S, Granton JT. Anticoagulation in pulmonary arterial hypertension: a qualitative systematic review. Eur. Respir. J. 2006;28(5):999–1004. doi: 10.1183/09031936.06.00015206. [DOI] [PubMed] [Google Scholar]
- 48.Rahman A, Isenberg DA. Systemic lupus erythematosus. N. Engl. J. Med. 2008;358(9):929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 49.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N. Engl. J. Med. 2009;360(19):1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 50.Shinomiya F, Mima N, Nanba K, et al. Life expectancies of Japanese patients with rheumatoid arthritis: a review of deaths over a 20-year period. Mod. Rheumatol. 2008;18(2):165–169. doi: 10.1007/s10165-008-0031-6. [DOI] [PubMed] [Google Scholar]
- 51.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004;25(6):947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 52.Lahm T, Crisostomo PR, Markel TA, et al. The effects of estrogen on pulmonary artery vasoreactivity and hypoxic pulmonary vasoconstriction: potential new clinical implications for an old hormone. Crit. Care Med. 2008;36(7):2174–2183. doi: 10.1097/CCM.0b013e31817d1a92. [DOI] [PubMed] [Google Scholar]
- 53.Tofovic SP, Zhang X, Jackson EK, Zhu H, Petrusevska G. 2-methoxyestradiol attenuates bleomycin-induced pulmonary hypertension and fibrosis in estrogen-deficient rats. Vascul. Pharmacol. 2009;51(2–3):190–197. doi: 10.1016/j.vph.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tofovic SP, Zhang X, Zhu H, Jackson EK, Rafikova O, Petrusevska G. 2-ethoxyestradiol is antimitogenic and attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling. Vascul. Pharmacol. 2008;48(4–6):174–183. doi: 10.1016/j.vph.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19(1):1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Meldrum DR. G-protein-coupled receptor 30 mediates estrogen’s nongenomic effects after hemorrhagic shock and trauma. Am. J. Pathol. 2007;170(4):1148–1151. doi: 10.2353/ajpath.2007.070025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.West J, Harral J, Deng Y, et al. Mice expressing BMPR2r899x transgene in smooth muscle develop pulmonary vascular lesions. Circ. Res. 2007;295(5):L744–L755. doi: 10.1152/ajplung.90255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lahm T, Patel KM, Crisostomo PR, et al. Endogenous estrogen attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction: the effects of sex and menstrual cycle. Am. J. Physiol. Endocrinol. Metab. 2007;293(3):E865–E871. doi: 10.1152/ajpendo.00201.2007. [DOI] [PubMed] [Google Scholar]
- 59.Lahm T, Crisostomo PR, Markel TA, et al. Selective estrogen receptor-α and estrogen receptor-β agonists rapidly decrease pulmonary artery vasoconstriction by a nitric oxide dependent mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295(5):R1486–R1493. doi: 10.1152/ajpregu.90667.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore LG, McMurtry IF, Reeves JT. Effects of sex hormones on cardiovascular and hematologic responses to chronic hypoxia in rats. Proc. Soc. Exp. Biol. Med. 1978;158(4):658–662. doi: 10.3181/00379727-158-40268. [DOI] [PubMed] [Google Scholar]
- 61.Rabinovitch M, Gamble WJ, Miettinen OS, Reid L. Age and sex influence on pulmonary hypertension of chronic hypoxia and on recovery. Am. J. Physiol. 1981;240(1):H62–H72. doi: 10.1152/ajpheart.1981.240.1.H62. [DOI] [PubMed] [Google Scholar]
- 62.McMurtry IF, Frith CH, Will DH. Cardiopulmonary responses of male and female swine to simulated high altitude. J. Appl. Physiol. 1973;35(4):459–462. doi: 10.1152/jappl.1973.35.4.459. [DOI] [PubMed] [Google Scholar]
- 63.Tofovic SP, Zhang X, Jackson EK, Dacic S, Petrusevska G. 2-methoxyestradiol mediates the protective effects of estradiol in monocrotaline-induced pulmonary hypertension. Vascul. Pharmacol. 2006;45(6):358–367. doi: 10.1016/j.vph.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Resta TC, Kanagy NL, Walker BR. Estradiol-induced attenuation of pulmonary hypertension is not associated with altered eNOS expression. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280(1):L88–L97. doi: 10.1152/ajplung.2001.280.1.L88. [DOI] [PubMed] [Google Scholar]
- 65.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Horm. Metab. Res. 2001;33(11):645–652. doi: 10.1055/s-2001-18689. [DOI] [PubMed] [Google Scholar]
- 66.Jones RD, English KM, Pugh PJ, Morice AH, Jones TH, Channer KS. Pulmonary vasodilatory action of testosterone: evidence of a calcium antagonistic action. J. Cardiovasc. Pharmacol. 2002;39(6):814–823. doi: 10.1097/00005344-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Smith AM, Bennett RT, Jones TH, Cowen ME, Channer KS, Jones RD. Characterization of the vasodilatory action of testosterone in the human pulmonary circulation. Vasc. Health Risk Manag. 2008;4(6):1459–1466. doi: 10.2147/vhrm.s3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farrukh IS, Peng W, Orlinska U, Hoidal JR. Effect of dehydroepiandrosterone on hypoxic pulmonary vasoconstriction: a Ca2+-activated K+-channel opener. Am. J. Physiol. 1998;274(2 Pt 1):L186–L195. doi: 10.1152/ajplung.1998.274.2.L186. [DOI] [PubMed] [Google Scholar]
- 69.Oka M, Karoor V, Homma N, et al. Dehydroepiandrosterone upregulates soluble guanylate cyclase and inhibits hypoxic pulmonary hypertension. Cardiovasc. Res. 2007;74(3):377–387. doi: 10.1016/j.cardiores.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Homma N, Nagaoka T, Karoor V, et al. Involvement of RhoA/Rho kinase signaling in protection against monocrotaline-induced pulmonary hypertension in pneumonectomized rats by dehydroepiandrosterone. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295(1):L71–L78. doi: 10.1152/ajplung.90251.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.West J, Cogan J, Geraci M, et al. Gene expression in BMPR2 mutation carriers with and without evidence of pulmonary arterial hypertension suggests pathways relevant to disease penetrance. BMC Med. Genomics. 2008;1:45. doi: 10.1186/1755-8794-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diergaarde B, Potter JD, Jupe ER, et al. Polymorphisms in genes involved in sex hormone metabolism, estrogen plus progestin hormone therapy use, and risk of postmenopausal breast cancer. Cancer Epidemiol. Biomarkers Prev. 2008;17(7):1751–1759. doi: 10.1158/1055-9965.EPI-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roos PH, Bolt HM. Cytochrome P450 interactions in human cancers: new aspects considering CYP1B1. Expert Opin. Drug Metab. Toxicol. 2005;(2):187–202. doi: 10.1517/17425255.1.2.187. [DOI] [PubMed] [Google Scholar]
- 74.Sorensen M, Autrup H, Tjonneland A, Overvad K, Raaschou-Nielsen O. Genetic polymorphisms in CYP1B1, GSTA1, NQO1 and NAT2 and the risk of lung cancer. Cancer Lett. 2005;221(2):185–190. doi: 10.1016/j.canlet.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 75.Wenzlaff AS, Cote ML, Bock CH, et al. CYP1A1 and CYP1B1 polymorphisms and risk of lung cancer among never smokers: a population-based study. Carcinogenesis. 2005;26(12):2207–2212. doi: 10.1093/carcin/bgi191. [DOI] [PubMed] [Google Scholar]
- 76.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006;354(3):270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 77.Spivack SD, Hurteau GJ, Reilly AA, Aldous KM, Ding X, Kaminsky LS. CYP1B1 expression in human lung. Drug Metab. Dispos. 2001;29(6):916–922. [PubMed] [Google Scholar]
- 78.Kim JH, Sherman ME, Curriero FC, Guengerich FP, Strickland PT, Sutter TR. Expression of cytochromes P450 1A1 and 1B1 in human lung from smokers, non-smokers, and ex-smokers. Toxicol. Appl. Pharmacol. 2004;199(3):210–219. doi: 10.1016/j.taap.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 79.Muti P, Bradlow HL, Micheli A, et al. Estrogen metabolism and risk of breast cancer: a prospective study of the 2:16α-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology. 2000;11(6):635–640. doi: 10.1097/00001648-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 80.Roberts KE, Fallon MB, Krowka MJ, et al. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am. J. Respir. Crit. Care Med. 2009;179(9):835–842. doi: 10.1164/rccm.200809-1472OC. • Demonstrates that certain genetic polymorphisms in estrogen signaling and cell growth are associated with the development of PH in patients with chronic liver disease.
- 81.Beretta L, Caronni M, Origgi L, Ponti A, Santaniello A, Scorza R. Hormone replacement therapy may prevent the development of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Scand. J. Rheumatol. 2006;35(6):468–471. doi: 10.1080/03009740600844498. [DOI] [PubMed] [Google Scholar]
- 82.Morse JH, Horn EM, Barst RJ. Hormone replacement therapy: a possible risk factor in carriers of familial primary pulmonary hypertension. Chest. 1999;116(3):847. doi: 10.1378/chest.116.3.847. [DOI] [PubMed] [Google Scholar]
- 83.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann. Intern. Med. 1991;115(5):343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 84.Kawut SM, Al-Naamani N, Agerstrand C, et al. Determinants of right ventricular ejection fraction in pulmonary arterial hypertension. Chest. 2009;135(3):752–759. doi: 10.1378/chest.08-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tandri H, Daya SK, Nasir K, et al. Normal reference values for the adult right ventricle by magnetic resonance imaging. Am. J. Cardiol. 2006;98(12):1660–1664. doi: 10.1016/j.amjcard.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 86.Weiss BM, Zemp L, Seifert B, Hess OM. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. J. Am. Coll. Cardiol. 1998;31(7):1650–1657. doi: 10.1016/s0735-1097(98)00162-4. [DOI] [PubMed] [Google Scholar]
- 87.Bedard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur. Heart J. 2009;30(3):256–265. doi: 10.1093/eurheartj/ehn597. [DOI] [PubMed] [Google Scholar]
- 88.Hemnes AR, Robbins IM. Hysteroscopic sterilization in women with pulmonary vascular disease. Mayo Clin. Proc. 2008;83(10):1188–1189. doi: 10.4065/83.10.1188-a. author reply 1189. [DOI] [PubMed] [Google Scholar]