Abstract
Neuropeptidomic data were collected on the mosquito Ae. aegypti which is considered the most tractable mosquito species for physiological and endocrine studies. The data were solely obtained by direct mass spectrometric profiling, including tandem fragmentation, of selected tissues from single specimens which yielded a largely complete accounting of the putative bioactive neuropeptides; truncated neuropeptides with low abundance were not counted as mature peptides. Differential processing within the CNS was detected for the CAPA-precursor and differential post-translational processing (pyroglutamate formation) was detected for AST-C and CAPA-PVK-2. For the first time in insects, we succeeded in the direct mass spectrometric profiling of midgut tissue which yielded a comprehensive and immediate overview of the peptides involved in the endocrine system of the gut. Head peptides which were earlier identified as the most abundant RFamides of Ae. aegypti, were not detected in any part of the CNS or midgut. This study provides a framework for future investigations on mosquito endocrinology and neurobiology. Given the high sequence similarity of neuropeptide precursors identified in other medically important mosquitoes, conclusions regarding the peptidome of Ae. aegypti likely are applicable to these mosquitoes.
Keywords: Aedes aegypti, Culicidae, peptidomics, insect neuropeptides, midgut, MALDI-TOF, CAPA-peptides
Introduction
Mosquitoes require blood for egg maturation and thereby act as vectors to transmit disease-causing viruses and parasites by subsequent feeding on human and animal hosts. Mosquito - pathogen associations are generally species specific: Anopheles spp. – Plasmodium spp. (malaria), Culex spp. – encephalitis viruses and nematodes (lymphatic filariasis) and Aedes spp. – yellow fever and dengue viruses and nematodes (heartworm). There is an urgent need to improve the control of these diseases and their vectors, and the availability of the genome and EST databases for Anopheles gambiae, Aedes aegypti, and Culex quinquefasciatus at a central site (http://www.vectorbase.org/index.php) has accelerated research to develop new mosquito control strategies. In this context it is noteworthy that no peptidomic study of neuropeptide expression in mosquitoes has been reported. This is surprising since neuropeptides regulate many key processes in the physiology and behavior of mosquitoes.1
Neuropeptides and protein hormones are produced by endocrine cells or neurons as larger precursors (prepropeptides). Subsequently, these prepropeptides are processed, stored and released within the nervous system as neurotransmitters or neuromodulators and from the midgut endocrine system, neurosecretory cells of the central nervous system (CNS), and peripheral neurosecretory cells as circulating hormones. These peptide messengers exert their action by binding to membrane receptors, most often to G-protein coupled receptors (GPCRs) and, to a lesser extent, to receptor tyrosine kinases. With bioinformatic approaches, 35 genes encoding putative neuropeptides and protein hormones were annotated from the An. gambiae genome.1 Cloned products confirmed the transcription and expression of only a few genes in different life stages and tissues of An. gambiae: neuropeptide F (NPF) and its GPCR,2 short NPF (sNPF) and its GPCR,3 adipokinetic hormone 1 (AKH-1), AKH-2, and the AKH-1 GPCR,4,5 and seven insulin-like peptide (ILP) genes.6
This is somewhat different for Ae. aegypti, which is considered the most tractable mosquito species for physiological and endocrine studies. Many neuropeptides and protein hormones in this species are characterized at the transcript, processed peptide, and function levels: head peptides,7–9 insect kinins,10 allatostatins (AST-A),11 ovary ecdysteroidogenic hormone (OEH),12 NPF,13 allatotropin (AT),14 AST-C,15 eight different ILPs,16,17 ecdysis triggering hormones (ETHs),18 and AKHs.19
In this study, a comprehensive peptidomic analysis of processed mature neuropeptides from Ae. aegypti was performed using mass spectrometry (MS). In earlier investigations of other insect species with complete genome information, predominantly LC/ESI-QTOF MS analysis of extracts from brain or other parts of the CNS was used to reveal the peptidome as a list of expressed neuropeptides.20–22 In the current study neuropeptidomic data were collected on the mosquito Ae. aegypti entirely via direct profiling of portions of the nervous system or of midgut tissue by MALDI-TOF MS. In insects, this type of approach (termed “mass spectrometric morphology”) was first introduced using the nervous system of the American cockroach Periplaneta americana.23 Such strategy made it possible to focus, step by step, on processed products of specific neuropeptide genes that are expressed in well-defined compartments. The proper dissection of these tissues may yield mass spectra that predominantly contain products of specific neuropeptide genes. It also enhanced the completeness of the collected data set and enabled an estimation of the relative abundances of the mature peptides from single neuropeptide genes. Owing to the rapid development of sensitive MALDI-TOF mass spectrometers with TOF/TOF optics, mass fingerprints of Ae. aegypti tissues could be corroborated by the fragmentation data that were obtained from the same samples. This effort resulted in a final list of detected neuropeptides that covers almost all of the predictable neuropeptides and a number of unexpected processing products.
Experimental section
Animals
Adult Ae. aegypti were kindly provided by Prof. Craig Coates from a colony maintained at the Department of Entomology, Texas A&M University. In addition, eggs were obtained from Bayer CropScience (Monheim, Germany). Hatched larvae were reared in small pans containing 2 liter of water until adulthood.
Gene and transcript identification
To identify genomic sequences encoding Ae. aegypti neuropeptides and peptide hormones, trace DNA sequences generated by TIGR and the Broad Institute were first searched by TBLASTN 24 using amino acid sequences of candidate peptides from An. gambiae, D. melanogaster, other insects, invertebrates, and vertebrates. Nucleotide sequences were downloaded, assembled and analyzed as previously described.1 TBLASTN searches, using the amino acid sequences of putative Ae. aegypti neuropeptide and peptide hormone orthologs identified in the trace data, were performed on the assembled Ae. aegypti genome and transcript DNA sequences deposited in the GenBank database.25 Analyses of the putative prepropeptides included signal peptide identification using both the Neural networks and Hidden Markov models contained in the SignalP program (http://www.cbs.dtu.dk/)26 and determination of proteolytic processing sites.27
Dissection and sample preparation for mass spectrometry
Males or females (non-blood fed) were fixed with needles and the body cavity opened with scissors. The different parts of the CNS, corpora cardiaca (CC; see for terminology28), and midgut were dissected and transferred with a stainless steel insect pin or a glass capillary into a drop of distilled water on the sample plate for MALDI-TOF MS. The water was subsequently removed using a glass capillary. Approximately 50 nl of matrix solution (saturated α-cyano-4-hydroxycinnamic acid dissolved in methanol/water [1:1]) was pumped onto the dried tissue over a period of about 5 sec using a Nanoliter injector (World Precision Instruments, Berlin, Germany). Each preparation was air-dried and covered with pure water for a few seconds, which was removed by cellulose paper. At least 10 preparations of each tissue sample were prepared. For the analysis of ETHs, tracheal trunks of larvae were dissected in an analogous manner.
MALDI-TOF MS
MALDI-TOF analyses were performed on an ABI 4800 Proteomics Analyzer (Applied Biosystems, Framingham, MA). Due to the nature of the samples all acquisitions were taken manually. Initially the instrument was operated in reflectron mode, in order to determine the parent masses. A laser intensity of 3800 was typically employed for ionizing the neuropeptides. For the tandem MS experiments (performed in gas on and gas off mode), a CID acceleration of 1 kV was used in all cases. The number of laser shots used to obtain a spectrum varied from 800–4000, depending on signal quality. The fragmentation patterns were used to manually determine the sequence of the peptides by using the Data ExplorerT software package.
Results and discussion
Homology-based searches of the Ae. aegypti genome and EST databases resulted in the identification of 43 neuropeptide and protein hormone genes listed in Table 1 along with the homolog genes of Drosophila melanogaster 29,30 and An. gambiae.1 For practical reasons, processed peptides are classified as neuropeptides if their mass is < 3000 Da and protein hormones, if >3000 Da. Calcitonin-like diuretic hormone (DH 31), with a mass just above 3000 Da, was included in the list of neuropeptides. Accordingly, 22 neuropeptide genes exist in Ae. aegypti, and the purpose of this study was to identify the products of these genes in the nervous system and midgut of adults.
Table 1.
Neuropeptides and protein hormones identified in genome databases for the mosquitoes, Aedes aegypti and Anopheles gambiae, and the fly Drosophila melanogaster.
| Peptide name | Ae. aegypti1 | Ae. aegypti transcript | D. melanogaster2 | An. gambiae3 |
|---|---|---|---|---|
| Neuropeptides | ||||
| Adipokinetic hormone 1 (AKH) | gb|AAGE02022019.1| | gb|AY431300.1| | CG1171 | XP_001238167 |
| AKH-2 | gb|AAGE02019657.1| | gb|XM_001661147.1| | ||
| Allatostatin-A | gb|AAGE02032617.1| | gb|U66841.1| | CG13633 | XP_313511 |
| Allatostatin-B (MIP) | gb|AAGE02022351.1| | gb|XM_001655823.1| | CG6456 | XP_316799 |
| Allatostatin-C | gb|AAGE02009205.1 | gb|XM_001651331.1| | CG14919 | XP_001238143 |
| Allatotropin | gb|AAGE02016767.1| | gb|U65314.1| | ND | XM_320402 |
| Calcitonin-like diuretic hormone (DH-31) | gb|AAGE02013728.1| | gb|XM_001658818.1| | CG13094 | XP_321755.2 |
| CAPA | gb|AAGE02008523.1| | gb|XM_001650839.1| | CG15520 | XP_566030 |
| Crustacean cardioactive peptide (CCAP) | gb|AAGE02000952.1| | gb|XM_001649143.1| | CG4910 | XP_318812 |
| Corazonin | gb|AAGE02008171.1| | gb|XM_001650517.1| | CG3302 | XP_001238800 |
| Diuretic hormone (DH-44) | gb|AAGE02030775.1| gb|AAGE02017550.1 |
ND | CG8348 |
XP_001230569 XM_001230568 |
| Ecdysis triggering hormone (ETH) | gb|AAGE02002869.1| | gb|DQ864499.1| | CG18105 | XP_308702 |
| Extended FMRFamide | gb|AAGE02026154.1| | gb|XM_001663782.1| | CG2346 | XP_556154 |
| Insecxt kinin | gb|AAGE02018083.1| | gb|U66832.1| | CG13480 | Leucokinins |
| Myosuppressin (MS) | gb|AAGE02012008.1| | gb|XM_001652600.1| | CG6440 | XP_321650 |
| Neuropeptide F (NPF) | gb|AAGE02004205.1| gb|AAGE02017729.1| |
gb|AF474405.1| | CG13968 | XP_315165 |
| Proctolin | ND | ND | CG7105 | AgPT |
| Pyrokinin (PK) | gb|AAGE02022205.1| | gb|XM_001662162.1| | CG6371 | XP_307885 |
| Short Neuropeptide F (sNPF) | gb|AAGE02023256.1| gb|AAGE02023254.1| |
gb|DQ459411.1| | CG13968 | ABD96048 |
| SIFamide | gb|AAGE02017476.1| | gb|XM_001654001.1| | CG4681 | XP_308708 |
| Sulfakinin (SK) | gb|AAGE02010444.1| | gb|DV383998.1| | CG18090 | AAR03495 |
| Tachykinin related peptide (TKRP) | gb|AAGE02010775.1| | Not found | CG14734 | XP_319161 |
| Orcokinin | gb|AAGE02012230.1| | Not found | - | XP_320317 |
| Pigment dispersing hormone (PDH) | gb|AAGE02002858.1| | gb|XM_001653921.1| | CG6496 | XP_315791 |
| Neuropeptide-like precursor-1(NPLP) | gb|AAGE02023487.1| gb|AAGE02030041.1| |
gb|XM_001649319.1| | CG3441 | XP_311578 |
| Protein hormones (>3000 Da) | ||||
| Bursicon-alpha subunit | gb|AAGE02007773.1| gb|AAGE02011233.1| |
ND | CG13419 | AY735443 |
| Bursicon-beta subunit | gb|AAGE02026399.1| | gb|XM_001663860.1| | CG15284 | XP_313804 |
| Eclosion hormone 1 (EH) | gb|AAGE02020363.1| | gb|XM_001661458.1| | CG5400 | XP_001230805 |
| Eclosion hormone 2 | gb|AAGE02020363.1| | gb|XM_001661457.1| | ||
| Eclosion hormone 3 | gb|AAGE02034655.1| | gb|XM_001647603.1| | ||
| Eclosion hormone 4 | gb|AAGE02028822.1| | gb|XM_001648672.1| | ||
| Eclosion hormone 5 | gb|AAGE02010971.1| | gb|XM_001652153.1| | ||
| Glycoprotein hormone alpha 2 (GPA2) | gb|AAGE02002350.1| | gb|BN001241.1| | AAX38184.1 | XP_317164 |
| Glycoprotein hormone beta-5 (GPB5) | gb|AAGE02002350.1| | gb|XM_001653331.1| | CG40041 | XP_555160 |
| Insulin-like peptide 1 | gb|AAGE02001358.1| | gb|DQ845750.1| | CG14173 | AY324307 |
| Insulin-like peptide 2 | gb|AAGE02001358.1| | gb|DQ845751.1| | CG8167 | AY324308 |
| Insulin-like peptide 3 | gb|AAGE02001358.1| | gb|DQ845752.1| | CG14167 | AY324309 |
| Insulin-like peptide 4 | gb|AAGE02001358.1| | gb|DQ845753.1| | CG6736 | AY324310 |
| Insulin-like peptide 5 | gb|AAGE02004597.1| gb|AAGE02004598.1| |
gb|DQ845758.1| | CG33273 | AY324311–12 |
| Insulin-like peptide 6a | gb|AAGE02007124.1| | gb|DQ845755.1| | CG14049 | AY324313 |
| Insulin-like peptide 6b | gb|AAGE02007124.1| | gb|DQ845756.1| | ||
| Insulin-like peptide 7 | gb|AAGE02001358.1| | gb|DQ845757.1| | CG13317 | AY324314–15 |
| Insulin-like peptide 8 | gb|AAGE02001358.1| | gb|DQ845754.1| | ||
| Ion transport peptide (ITP) | gb|AAGE02002845.1| | gb|AY950506.1| | CG13586 | XP_313928 |
| gb|AAGE02002848.1| | ||||
| Ovary Ecdysteroidogenic Hormone (OEH; Neuroparsin A homolog) | gb|AAGE02006435.1| | gb|U69542.1| | ND | XP_311039 |
| Prothoracicotropic hormone (PTTH) | gb|AAGE02026723.1| gb|AAGE02034932.1| |
gb|DV370510.1| | CG13687 | XP_555854 |
Aedes aegypti neuropeptide and protein hormone names are assigned to encoding GenBank genes and ESTs as identified.
Drosophila melanogaster names are assigned to CG numbers or encoding GenBank gene as available.
Anopheles gambiae names are assigned to encoding GenBank genes based on Riehle.1
As in other insects, the predicted amino acid sequences for prepropeptides encoded by neuropeptide genes in Ae. aegypti often contain multiple repeated peptide sequences that begin and end with protease cleavage sites (Supplemental table 1). Genes with this feature include AST-A, myoinhibitory peptides (MIPs), CAPA-peptides, ETHs, extended FMRFamides, head peptides, kinins, neuropeptide-like precursor 1 (NPLP-1) peptides, orcokinins, pyrokinins (PKs), sNPF, sulfakinins (SKs), and tachykinin-related peptides (TKRPs).
Profiling of the central nervous system (CNS)
For an overview of neuropeptides present in the Ae. aegypti CNS, the following distinct regions were dissected from single specimens, prepared and directly profiled using MALDI-TOF MS: abdominal ganglia with attached perisympathetic organs (PSOs), postero-lateral edges of thoracic ganglia, medio-ventral parts of the subesophageal ganglion (SEG), pars lateralis and pars intercerebralis (protocerebrum). As with other insects, these compartments contain neurosecretory neurons which store expressed neuropeptides that are easily identified by MS.31 This concept is best illustrated by the detection and sequence elucidation of CAPA peptides, which are typically found in such cells in the abdominal ganglia.32 In mosquitoes, two neurosecretory neurons in each abdominal ganglion express CAPA peptides which are transported via the median nerve to neurohemal release sites along the body wall (abdominal PSOs, see inset Fig. 1A and OEH I).33 Six shorter neuropeptides were predicted to be processed from the CAPA-prepropeptide, namely two CAPA-periviscerokinins (PVKs), two CAPA-PKs, and two precursor peptides (PPs), which precede the PVK-1 and CAPA-PK-1 sequences. Long precursor peptides (> 3000 Da), such as those located between PVK-1 and PVK-2 sequences, as well as between the CAPA-PK-2 sequence and the stop codon, were generally not detected in this study. For D. melanogaster, it was confirmed that CAPA-PVKs and CAPA-PK interact with their own specific receptor.34–36 The physiological relevance of the CAPA-PPs is not known.
Fig. 1.
Detection of putative CAPA-peptides from Ae. aegypti by means of MALDI-TOF MS. A) MALDI-TOF mass spectrum typical of an abdominal ganglion preparation with attached perisympathetic organ (aPSO). Only CAPA-peptides are labelled, and the most prominent mature products are given in bold. Note the complete absence of the ion signal of putative CAPA-PK-2 ([M+H]: 1920.0). The inset shows an abdominal ganglion immunostained with an anti-CAPA-PVK serum (Neupert, unpublished). B) Sequence of the CAPA-precursor with the putative cleavage sites are labelled in bold. No signal peptide was predicted. Neuropeptides with mass match in A) are underlined.
Mass spectra of abdominal ganglia with attached abdominal PSOs (n=10) revealed prominent ion signals that were assigned, by mass-match, to putative cleavage products of the CAPA-precursor (Fig. 1). Altogether, the mass fingerprints provided evidence for five of the six predicted CAPA-peptides, although the ion signal of CAPA-PP-1 had very low intensity. CAPA-PP-1 has an uncertain N-terminal extension because no signal peptide was identified for the CAPA-precursor. By using the same preparations, the putative CAPA-peptides were subsequently fragmented to confirm their sequences (see Fig. 2 for CAPA-PVK-2). Remarkably, no signal was observed for CAPA-PK-2, although this putative neuropeptide is flanked by a dibasic and a monobasic cleavage site common to other PVKs. A predicted CAPA-PK ortholog also was not detected in Tribolium castaneum,22 and a homologous sequence is missing in the capa gene of An. gambiae and D. melanogaster.
Fig. 2.
CID mass spectrum of the CAPA-peptide at [M+H]+: 994.58 Da. The fragments were analyzed manually and the resulting sequence is given in the inset. A number of fragment ions are labelled which confirmed the amino acid sequence of N-terminally blocked (pGlu) CAPA-PVK-2.
In a final experimental step, all remaining ion signals from preparations of abdominal ganglia that had sufficient intensity for tandem MS analyses were screened for other processed peptides in the CAPA prepropeptide. This effort resulted in the identification of the N-terminally blocked (pGlu) form of CAPA-PVK-2. The blocked form showed higher signal intensity than the non-blocked form. In addition, truncated forms of CAPA-PVK-1 with low signal intensity and an extended form of CAPA-PP-1 with high signal intensity were found (Table 2). Signal intensity of the truncated forms was increased by using a higher laser power during the acquisition of the fingerprints. For that reason, these peptides and other truncated peptides with low abundance were classified as artefacts in this study and generally not considered to be mature peptides. The extended form of CAPA-PP-1 did not match with the annotated N-terminus of the CAPA-precursor, differing in the first two amino acids of the N-terminus. In summary, peptidomic analysis of CAPA processing in abdominal ganglia identified five predicted peptides, confirmed a posttranslationally modified form of CAPA-PVK-2, and detected an inaccurately annotated N-terminus of the precursor sequence. Mass spectra also led to the conclusion that CAPA-PK-2 is not processed as expected.
Table 2.
List of mature neuropeptides of Ae. aegypti that were identified by mass spectrometry. PP, precursor peptide
| peptide name | peptide sequence | [M+H]+, m/z |
|---|---|---|
| Adipokinetic hormones | ||
| AKH-1 | pQLTFTPSWa | 961.48 |
| Allatostatin-A | ||
| AST-1 | SPKYNFGLa | 924.49 |
| AST-2 | LPHYNFGLa | 959.51 |
| AST-3 | ASAYRYHFGLa | 1183.60 |
| AST-4 | RVYDFGLa | 868.47 |
| AST-5 | LPNRYNFGLa | 1092.59 |
| ext. AST-5 | VYEDKRLPNRYNFGLa | 1882.99 |
| PP-1 | RYIIEDVPGA-OH | 1132.59 |
| Allatostatin-C | ||
| AST-C | QIRYRQCYFNPISCF-OH | 1935.91 |
| pQIRYRQCYFNPISCF-OH | 1918.91 | |
| Allatotropin | ||
| AT | APFRNSEMMTARGFa | 1613.77 |
| CAPA | ||
| CAPA-PVK-1 | GPTVGLFAFPRVa | 1259.73 |
| CAPA-PVK-2 (pGln) | pQGLVPFPRVa | 994.58 |
| CAPA-PVK-2 (Gln) | QGLVPFPRVa | 1011.61 |
| CAPA-PK | AGNSGANSGMWFGPRLa | 1620.77 |
| PP-1 | SDLDSVSEGRH-OH | 1201.54 |
| ext. PP-1 | DASDLDSVSEGRH-OH | 1387.61 |
| PP-2 | SGMNAARFYWPKTMMPQQQ-OH | 2272.05 |
| Corazonin | pQTFQYSRGWTNa | 1369.63 |
| Diuretic hormones | ||
| TVDFGLSRGYSGAQEAKHRMAMAVANFAGG | ||
| DH31 | Pa | 3195.56 |
| Ecdysis triggering hormones | ||
| ETH-1* | DETPGFFIKLSKSVPRIa | 1933.09 |
| ETH-2* | GDFENFFLKQSKSVPRIa | 2011.08 |
| Extended FMRFamides | ||
| FMRFa-1 | SALDKNFMRFa | 1227.63 |
| FMRFa-2 | ASKQANLMRFa | 1164.63 |
| FMRFa-3 | AGQGFMRFa | 912.45 |
| FMRFa-4 | DSPKNLMRFa | 1106.58 |
| FMRFa-5 | DDTNKFLRLS-OH | 1208.64 |
| FMRFa-6 | ANLMRFa | 750.41 |
| FMRFa-7 | AGSEAGGNLQRTNFLRFa | 1836.95 |
| FMRFa-8 | GSGNLMRFa | 880.45 |
| FMRFa-9 | AKGNLMRFa | 935.52 |
| FMRFa-10 | SDPRFLRLV-OH | 1102.64 |
| FMRFa-11 | MDNNFMRFa | 1073.43 |
| SIFamide peptide | ||
| SIFa | GYRKPPFNGSIFa | 1381.74 |
| ext. SIFa | GYRKPPFNGSIFG-OH | 1439.74 |
| Kinins | ||
| Kin-1** | NSKYVSKQKFYSWGa | 1720.88 |
| Kin-2** | NNPNVFYPWGa | 1206.57 |
| Kin-3** | NTGRVHRQPKVVIRNPFHAWGa | 2468.37 |
| Myoinhibitory-like peptide | ||
| MIP-1 | TWKNLQGGWa | 1088.56 |
| MIP-2 | AWNKINGGWa | 1044.54 |
| MIP-3 | VNAGPAQWNKFRGSWa | 1716.87 |
| MIP-4* | EPGWNNLKGLWa | 1312.68 |
| MIP-5 | SEKWNKLSSSWa | 1350.68 |
| Myosuppressin | ||
| MS | TDVDHVFLRFa | 1247.65 |
| Short Neuropeptide F | ||
| sNPF-1 | KAVRSPSLRLRFa | 1428.89 |
| sNPF-14–11 | SPSLRLRFa | 974.59 |
| sNPF-2+4 | APQLRLRFa | 999.62 |
| sNPF-3 | APSQRLRWa | 1012.58 |
| PP-2* | SDPSVPVEPEDDDMVDQRSI-OH | 2229.98 |
| PP-4* | SGGGMFSTNDVMQQKAI-OH | 1770.81 |
| Neuropeptide-like precursor 1 | ||
| NPLP-1-1 | SYRSLLRDGATFa | 1384.73 |
| NPLP-1-2 | NLGSLARAGLLRTPSTDYL-OH | 2018.10 |
| NPLP-1-4 | NLASARASGYMLNa | 1366.69 |
| NPLP-1-5 | NIASLARKYELPa | 1373.79 |
| NPLP-1-6 | NIQSLLRTGMLPSIAP-OH | 1710.96 |
| ext. NPLP-1-6 | NIQSLLRTGMLPSIAPK-OH | 1839.05 |
| NPLP-1-7 | NMQSLARDNSLPHFAGAAAQES-OH | 2315.08 |
| NPLP-1-8 | NIQTLVRDWNLPRQQSMAADNE-OH | 2599.27 |
| NPLP-1-9 | NIQSLKNAQGQGGGSSSGa | 1688.83 |
| Pigment-dispersing hormone | ||
| PDH | NSELINSLLSLPKKLNDAa | 1968.11 |
| Pyrokinins | ||
| PK-1 | AAAMWFGPRLa | 1118.59 |
| PK-2 | DASSSNENNSRPPFAPRLa | 1957.95 |
| PK-3 | NLPFSPRLa | 942.55 |
| PP-1 | GEVPDATEQKINNFLASGKDSEDLS | 2664.25 |
| PP-2A* | TIASELHDEMMDEIDDNPLYYSa | 2600.12 |
| Sulfakinin | ||
| SK-1 | FDDY(SO3)GHMRFa | 1186.51*** |
| SK-2 | GGGGEGEQFDDY(SO3)GHMRFa | 1857.76*** |
| Tachykinin-related peptides | ||
| TKRP-1 and 4 | APSGFLGLRa | 916.54 |
| TKRP-2 | VPSGFTGMRa | 950.49 |
| TKRP-3 | APSGFLGMRa | 934.49 |
| TKRP-5 | VPNGFLGVRa | 957.56 |
| PP-1 | NIPFYPLRLYP-OH | 1392.77 |
| Orcokinins | ||
| Orc-1 | NFDEIDRYSTFGa | 1462.66 |
| PP-1 | NYEFMTPQERHSTI-OH | 1752.80 |
| Orc. not assigned | NFDEIDRYXXXX | 1517.69 |
mass match only
identified in Veenstra (1994)10
sulfation only detectable in the negative mode
The same approach was performed with other neural compartments of the CNS which contain neurosecretory cell clusters (see above) and the following gene products were identified: extended FMRFamides (postero-lateral edges of thoracic ganglia), PKs (medio-ventral part of SEG), corazonin (pars lateralis), SIFamide (pars intercerebralis), and myosuppressin (pars intercerebralis). Most of these peptides, except for corazonin, were also found in other parts of the CNS but with lower signal intensity. The signal intensity of extended FMRFamides was generally low in mass spectra, even in samples from postero-lateral edges of thoracic ganglia, where neurosecretory FMRFamide producing cells are present.37 This resulted in a limited number of fragments obtained during tandem fragmentation of these peptides. The co-occurrence of multiple forms of extended FMRFamides in single mass spectra was therefore taken as an additional confirmation for their presence. The other neuropeptide precursors were also processed as expected, including the N-terminal pyroglutamate in corazonin (a non-blocked form was not detected). In the pars intercerebralis cells of a few mosquitoes, an increased amount of a C-terminal extended SIFamide peptide (GYRKPPFNGSIFG-OH) was observed. In these mosquitoes, this peptide was present throughout the CNS, although it was always less abundant than the mature peptide.
The following processed peptides from other neuropeptide precursors were mainly identified in preparations of neuropil regions, such as optic lobes, antennal lobes, and dorso-caudal neuropil (DCN) of the terminal ganglion and in samples of brain tissue: AST-A, AST-C, AT, DH-31, NPLP-1 peptides, SKs, sNPFs, MIPs, pigment dispersing hormone (PDH), TKRPs, and orcokinins. With the exception of the putative orcokinin-2 (NFDEIDQWAamide), all predicted neuropeptides with homologs in other insects were identified from these precursors. The two SKs of Ae. aegypti, identified only in brain tissue, were confirmed to be sulfated by re-analyzing samples in the negative mode (Fig. 3).38 Due to low signal intensity, one of the MIPs (MIP-4) could not be fragmented successfully, though a correct mass match together with the co-occurrence of the other peptides from the MIP-precursor suggested that this peptide is cleaved as expected.
Fig. 3.
MALDI-TOF mass spectra of a preparation of brain tissue with fairly high concentrations of sulfakinins. The comparison of mass spectra in the positive and negative mode indicates the occurrence of the sulfate groups (mass difference of 80 Da). As already shown for other sulfakinins,38 ions with sulfation are detectable in the negative mode only. The generally low amount of sulfakinins in the brain of Ae. aegypti limited the quality (signal-to-noise) of the mass spectra in the negative mode.
A number of additional peptides with at least moderate signal intensity could be fragmented from samples of the nervous system, and subsequent fragment analysis yielded other peptides not initially predicted. These neuropeptides are listed in Table 2 together with the other identified products of the respective neuropeptide precursors. One of these peptides was an orcokinin (monoisotopic mass [M+H]+ 1517.6; NFDEIDRY…) with high sequence similarity to orcokinin-1, but it could not be assigned to the annotated orcokinin-precursor. The most unusual peptide identified was a C-terminally extended form of NPLP-6 which contains the Lys from the common Lys-Arg cleavage site and was always much more abundant than the predicted NPLP-6 (see Fig. 4). No trace of a similarly extended form of the other NPLPs or any other neuropeptide from Ae. aegypti was found. To our knowledge, only one similarly extended peptide was identified from insects so far; namely an intermediate product of AKH-processing that was found in D. melanogaster.31 However, this intermediate product from D. melanogaster was less abundant than the mature peptide. The complete processing of all other NPLP-1 peptides of Ae. aegypti supports the idea that the C-terminally extended form of NPLP-6 is the mature peptide rather than an intermediate product. A single peptide from the AST-A precursor (AST-5) was identified as an N-terminally extended form that incorporates a predicted Lys-Arg cleavage site, but the extended form was much less abundant (approx. 10%) than the completely processed AST-5. Similarly extended forms of ASTs were also reported from the honey bee, A. mellifera.39
Fig. 4.
MALDI-TOF mass spectrum of a preparation of the tritocerebrum demonstrating the abundance of the C-terminal extended NPLP-1-6 in comparison to the predicted NPLP-1-6 sequence (for better visibility only a limited mass range of 1650-2100 is shown). The NPLP-1 peptides are designated with numbers. SK, sulfakinin; PK, pyrokinin; AST, allatostatin.
The peptidomic analysis of the CNS resulted in the unambiguous and nearly complete identification of processed peptides encoded by 17 neuropeptide genes. Aedes kinins were not sequenced in this study but were in an earlier study.10 Of the identified neuropeptide genes listed in Table 1, no peptides encoded by the akh, eth, ccap (crustacean cardioactive peptide) genes were detected in the CNS, but AKH-1 and ETHs were detected in other tissues (see below). Thus, (CCAP) was the only neuropeptide not detected in any tissues from single adults, most likely because of its low abundance. For all peptides with a mass-match ([M+H]+: 956.5) predicted for CCAP, subsequent MS/MS analyses revealed either fragments from sodium adduct ions of TKRP-3 ([M+Na]+: 956.5), extended FMRFa-9 ([M+Na]+: 957.5), or fragments of mass-related TKRP-5 ([M+H]+: 957.5). Analysis of CNS extracts from other holometabolous insects by MS (MALDI-TOF or LC-ESI-QTOF) also failed to identify a CCAP (A. mellifera;21,39 D. melanogaster;20,31 T. castaneum22).
Differential distribution of neuropeptide-precursor products in the CNS
In addition to the detection of Aedes neuropeptides, comprehensive profiling of the CNS revealed a different processing of products for the CAPA-precursor and the AST-C precursor in different parts of the CNS. In abdominal ganglia as well as in the brain, two CAPA-PVKs, CAPA-PK-1 and the CAPA-PP-1 and 2 were identified, but mass spectra of the SEG yielded only signals of CAPA-PK-1 and CAPA-PP-2. An identical situation was reported for D. melanogaster31,40 and suggests a different processing of the CAPA precursor in the SEG and abdominal ganglia/brain of these insects. CAPA-PVKs are known to stimulate diuresis by the Malpighian tubules in the abdomen of Diptera41,42 and other insects.32 A recent study confirmed that the CAPA-PVK-receptor of D. melanogaster43 is predominantly expressed in Malpighian tubules. CAPA-PVKs of Ae. aegypti and D. melanogaster are seemingly not expressed in the CAPA cells of the SEG. Instead, the CAPA-PK is processed, which activates a specific receptor with an unknown function. In addition to the differences in the synthesis of CAPA-PVKs and PK in different ganglia, differential posttranslational processing of CAPA-PVK-2 in Ae. aegypti tissues was observed. The N-terminally blocked form (pGlu) of CAPA-PVK-2 was abundant in the abdominal ganglia, but in the brain, the non-blocked form was much more prominent.
A nearly identical situation was found for the N-terminally blocked form (pGlu) of AST-C that was much more abundant in the abdominal and thoracic ganglia than the non-blocked form. This ratio was reversed in the brain and dorso-caudal neuropil of the terminal ganglion. This differential posttranslational processing has not been reported in insects so far, and it may be related to their roles as hormones or neuromodulators within the CNS. When CAPA-PVK-2 or AST-C are likely to be released as hormones into the hemolymph, they are predominantly blocked and therefore less suitable to degradation by hemolymph enzymes.
A third important finding of the peptidomic analysis of the CNS was the astonishing number of different neuropeptides in the DCN of the terminal ganglion of Ae. aegypti (Fig. 5). The number was not surpassed by any other region of the CNS analyzed in this study. The DCN is a distinct neuropil region that links neurons distributed all over the CNS with the proctodeal nerve (Neupert, unpublished). The peptidome of the DCN of Ae. aegypti was very different from that of other parts of the terminal ganglion or unfused abdominal ganglia. It accordingly should be regarded as part of a ‘posterior brain’.
Fig. 5.
MALDI-TOF mass spectrum of a preparation of the dorso-caudal neuropil (DCN) of the terminal ganglion. The number of different neuropeptides in the DCN of Ae. aegypti was not surpassed by any other distinct region of the CNS; in this spectrum mature products of 12 neuropeptide precursors are detectable (due to shortage of space, not all neuropeptide signals are designated). The question marks designate putative peptides which could not be assigned to the neuropeptide precursors of Ae. aegypti. PK, pyrokinin; AST, allatostatin; TKRP, tachykinin-related peptide; sNPF, short neuropeptide F, PVK-periviscerokinin; NPLP, neuropeptide-like precursor; Orc, orcokinin; AT, allatotropin; MIP, myoinhibitory peptide; PDH, pigment dispersing hormone; DH, diuretic hormone.
Profiling of the corpora cardiaca, Inka-cells, and midgut
The direct mass spectrometric profiling of the CNS was complemented by the analysis of the peptidome of the CC, Inka-cells, and midgut. The CC of adult mosquitoes is a neurohemal storage area fused around the aorta from which peptide hormones produced in brain neurosecretory cells are released into the hemolymph.28,44 A cluster of neurosecretory cells (X cells) is separate and posterior to the CC, and axons from these cells extend to the CC. In female An. gambiae and Ae. aegypti, these cells and other brain cells with axons to the CC and along the anterior midgut contain AKH-immunoreactivity.4,19 In this study, mass spectra of the CC from Ae. aegypti yielded distinct signals of the predicted AKH-1 with an N-terminus pGlu (Fig. 6), and its sequence was confirmed by tandem MS.
Fig. 6.
MALDI-TOF mass spectrum from the glandular portions of the corpora cardiaca. Only AKH-1 is detectable, ion signals of the putative AKH-2 are completely missing. AKH, adipokinetic hormone; CCgland, glandular portions of the corpora cardiaca.
Expression of a gene encoding AKH-2 was characterized in Ae. aegypti.19 Native AKH-2 was resolved by HPLC from head extracts of female An. gambiae in parallel with synthetic AKH-2, as detected by radioimmunoassay with the same AKH antiserum as used for immunocytochemistry.4 In this study, we did not detect AKH-2 in the brain or in the CC. Due to the lack of basic amino acids, AKHs are generally not easily ionized and yield low signal intensities in MALDI-TOF mass spectra and therefore low amounts may become undetectable.
In preparations of the CC, corazonin, orcokinins, sNPFs, myosuppressin, and two CAPA-peptides (CAPA-PK-1, CAPA-PP-2) were identified and their sequences confirmed by MS/MS analyses. The occurrence of these peptides in the CC indicates a role as classical neurohormones. In addition to these neuropeptides, two distinct signals at m/z 1161.7 and 1752.8 were observed. These substances were partially sequenced but could not be assigned to any of the annotated precursor sequences.
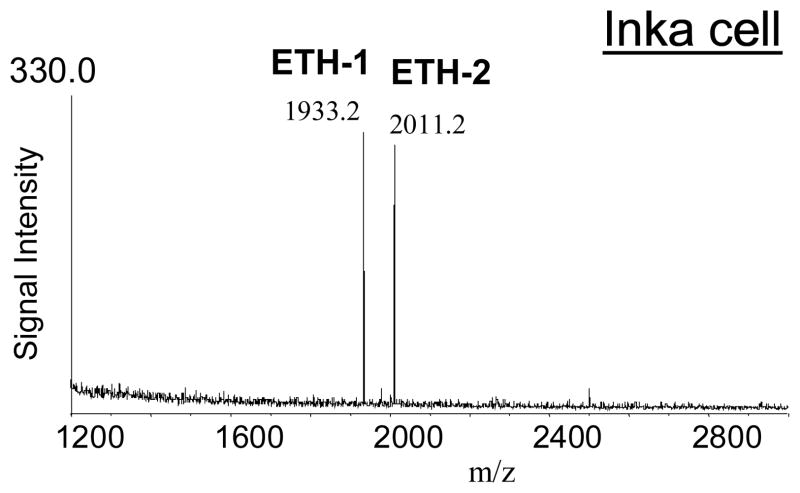
Inka-cells are peripheral endocrine cells attached to the tracheal system of insects that produce and release ecdysis triggering hormones (ETH) at each ecdysis.45 Peptide synthesis in these cells is restricted to larval stages. In Ae. aegypti, single Inka-cells are associated with epitracheal glands located along the lateral tracheal trunks.46,18 Pieces of the tracheal trunks of Ae. aegypti larva revealed the presence of the two ETHs (Fig. 7).
Fig. 7.
MALDI-TOF mass spectrum of a preparation of a lateral tracheal trunk (with Inka-cells) of an Ae. aegypti larvae. Two distinct ion signals representing the ETHs are marked. The synthesis and release of these (neuro)peptides is restricted to Inka-cells of larval stages.
The midgut of all mosquito life stages contains tens to hundreds of dispersed endocrine cells.47,37 It was supposed that these cells may produce many of the same regulatory peptides found in the CNS.48 This feature resembles the brain-gut system of peptides found in vertebrates. In addition to the endocrine cells, peptidergic neurons from the CNS innervate the muscles of the gut, either via the stomatogastric nervous system or the proctodeal nerves. To our knowledge, direct mass spectrometric profiling of midgut endocrine peptides has not been accomplished for any insect to date. Previously, specific peptides were purified by HPLC from gut extracts, based on bioactivity or immunoreactivity, for only a few insects.49–53 It was therefore a surprise that experiments with midgut tissue of freshly emerged male mosquitoes succeeded in the detection of many neuropeptides, including sNPFs, TKRPs, MIPs, ASTs, and CAPA-peptides. As expected from earlier immunocytochemistry studies,54–57 the gut peptides were not evenly distributed along the midgut, which can be divided into a narrow anterior part and expanded posterior part. Pieces from the most anterior section of the midgut contained very abundant ion signals of sNPFs and, with a lower signal intensity, TKRPs, MIPs, and CAPA-peptides (Fig. 8A). As in preparations from SEG and CC, only CAPA-PK-1 and CAPA-PP-2 were detected from the CAPA-prepropeptide. Mass signals of sNPFs and the CAPA-peptides disappeared in preparations of the posterior midgut. Instead, AST-A peptides were abundant (Fig. 8B). Occurrence of AST-A-like immunoreactivity in the most posterior section of the midgut was suggested based on immunocytochemistry.57 Presence of sNPFs in the anterior midgut of adult Ae. aegypti was not detected by antisera specific to RFamide or NPF/pancreatic polypeptide,54,57,58 but such staining was present in hundreds of endocrine cells in the posterior midgut. The occurrence of CAPA-peptides in the midgut has not been previously reported. The peptidome of the hindgut of male Ae. aegypti consisted mainly of AST-A and AST-C peptides and other peptides that could not be assigned to the annotated precursors. Some of the above peptides were tested for effects on ion transport and peristalsis of midguts from Ae. aegypti larvae, including ASTs, allatotropin, head peptide, sNPFs, and NPF.59 Since the hindgut contains no endocrine cells, the allatostatins are likely produced in the CNS and transported via the proctodeal nerve to the surface of the hindgut.
Fig. 8.
MALDI-TOF mass spectra of preparations of midgut tissue from freshly hatched male Ae. aegypti; which demonstrate the uneven distribution of a number of neuropeptides in the midgut. A) Preparation of an anterior portion of the midgut. B) Preparation of a posterior portion of the midgut. AST, allatostatin; TKRP, tachykinin-related peptide; sNPF, short neuropeptide F, MIP, myoinhibitory peptide; PK, pyrokinin.
A final comment is needed for the head peptides of Ae. aegypti that were initially isolated based on their RF-immunoreactivity from extracts of adult heads 7 and other body regions.60 Synthetic head peptides were shown to inhibit host-seeking by female mosquitoes,8 and their hemolymph titer was profiled in females post blood meal. Molecular characterization of the head peptides gene revealed a precursor with three copies of head peptide and its expression in many female and male tissues.9 We were unable to identify the head peptide gene in the completed Ae. aegypti genome by bioinformatics, but it was found in trace sequences obtained from the Broad Institute excluded reads database (identifier: G719P616810RC2. T0). Interestingly, our mass spectrometric analyses failed to detect head peptides in any tissue, even though large amounts were reported to be present in adult extracts.7,60 No head peptide gene homologs are known for An. gambiae, D. melanogaster, or any other insect, but it does share limited similarity to the snpf gene identified in Ae. aegypti and many other insects. In this study, the products of the snpf gene of Ae. aegypti were found to be abundant in different parts of the CNS and also in the gut.
Conclusions
The first comprehensive peptidomic analysis of a mosquito species resulted in an almost complete list of mature neuropeptides of Ae. aegypti. The data were solely obtained by direct mass spectrometric profiling of selected tissues from single specimens which yielded a largely complete accounting of the putative bioactive neuropeptides. Other methods such as ESI-QTOF MS, which consume more material, may provide more complete fragmentation spectra, but the time for consecutive fragmentations is sometimes not sufficient to obtain all peptides with an intensity to identify the neuropeptides. In this study, the main focus was not the counting of observed peptides (including breakdown products) but the generation of data that can be used for future peptidomic studies. As shown, single specimens are sufficient to obtain the complete neuropeptidome on a tissue-specific level once the identity of neuropeptides has been confirmed by fragmentation data. Hence, only fingerprints are necessary to obtain differences in the time- or site-specific expression/processing of certain neuropeptides during development or following genetic manipulation. Particularly important in this context is the finding that the midgut tissue can be included in such an approach. Altogether, this study provides a framework for future investigations on mosquito endocrinology and neurobiology. Given the high sequence similarity of neuropeptide precursors identified in the other medically important mosquitoes, An. gambiae and C. quinquefasciatus, conclusions regarding the peptidome of Ae. aegypti likely are applicable to these mosquitoes.
Acknowledgments
This work was supported in part by a grant from the USDA/DOD DWFP Initiative (#0500-32000-001-01R)(RJN), grants from the US-Israel Binational Agricultural Research and Development Fund (BARD) (IS-4205-09C)(RJN), NIH (R01AI033108 to MRB) and USDA/CREES (GEO00607 to MRB). We thank Prof. Craig Coates (Texas A&M University) and Dr. Günther Nentwig (Bayer CropScience Monheim, Germany) for kindly providing Ae. aegypti mosquitoes for this study.
Supporting Information Supplement 1, Fasta file for putative transcriptions of annotated neuropeptide and protein hormone genes.
References
- 1.Riehle MA, Garczynski SF, Crim JW, Hill CA, Brown MR. Neuropeptides and peptide hormones in Anopheles gambiae. Science. 2002;298 (5591):172–175. doi: 10.1126/science.1076827. [DOI] [PubMed] [Google Scholar]
- 2.Garczynski SF, Crim JW, Brown MR. Characterization of neuropeptide F and its receptor from the African malaria mosquito, Anopheles gambiae. Peptides. 2005;26 (1):99–107. doi: 10.1016/j.peptides.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Garczynski SF, Crim JW, Brown MR. Characterization and expression of the short neuropeptide F receptor in the African malaria mosquito, Anopheles gambiae. Peptides. 2007;28 (1):109–118. doi: 10.1016/j.peptides.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufmann C, Brown MR. Adipokinetic hormones in the African malaria mosquito, Anopheles gambiae: Identification and expression of genes for two peptides and a putative receptor. Insect Biochem Molec Biol. 2006;36 (6):466–481. doi: 10.1016/j.ibmb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann C, Brown MR. Regulation of carbohydrate metabolism and flight performance by a hypertrehalosaemic hormone in the mosquito Anopheles gambiae. J Insect Physiol. 2008;54 (2):367–377. doi: 10.1016/j.jinsphys.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krieger MJB, Jahan N, Riehle MA, Cao C, Brown MR. Molecular characterization of insulin-like peptide genes and their expression in the African malaria mosquito, Anopheles gambiae. Insect Mol Biol. 2004;13 (3):305–315. doi: 10.1111/j.0962-1075.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto S, Brown MR, Crim JW, Vigna SR, Lea AO. Isolation and primary structure of neuropeptides fron the mosquito, Aedes aegypti, immunoreactive to FMRFamide antiserum. Insect Biochem. 1989;19:277–283. [Google Scholar]
- 8.Brown MR, Klowden MJ, Crim JW, Young L, Shrouder LA, Lea AO. Endogenous regulation of mosquito host-seeking behavior by a neuropeptide. J Insect Physiol. 1994;40 (5):399–406. [Google Scholar]
- 9.Stracker TH, Thompson S, Grossman GL, Riehle MA, Brown MR. Characterization of the AeaHP gene and its expression in the mosquito Aedes aegypti (Diptera : Culicidae) J Med Entomol. 2002;39 (2):331–342. doi: 10.1603/0022-2585-39.2.331. [DOI] [PubMed] [Google Scholar]
- 10.Veenstra JA. Isolation and identification of three leucokinins from the mosquito Aedes aegypti. Biochem Biophys Res Commun. 1994;202:715–719. doi: 10.1006/bbrc.1994.1989. [DOI] [PubMed] [Google Scholar]
- 11.Veenstra JA, Noriega FG, Graf R, Feyereisen R. Identification of three allatostatins and their cDNA from the mosquito Aedes aegypti. Peptides. 1997;18:937–942. doi: 10.1016/s0196-9781(97)00032-6. [DOI] [PubMed] [Google Scholar]
- 12.Brown MR, Graf R, Swiderek KM, Fendley D, Stracker TH, Champagne DE, Lea AO. Identification of a steroidogenic neurohormone in female mosquitoes. J Biol Chem. 1998;273 (7):3967–3971. doi: 10.1074/jbc.273.7.3967. [DOI] [PubMed] [Google Scholar]
- 13.Brown MR, Crim JW, Arata RC, Cai HN, Chun C, Shen P. Identification of a Drosophila brain-gut peptide related to the neuropeptide Y family. Peptides. 1999;20 (9):1035–1042. doi: 10.1016/s0196-9781(99)00097-2. [DOI] [PubMed] [Google Scholar]
- 14.Veenstra JA, Costes L. Isolation and identification of a peptide and its cDNA from the mosquito Aedes aegypti related to Manduca sexta allatotropin. Peptides. 1999;20:1145–1151. doi: 10.1016/s0196-9781(99)00117-5. [DOI] [PubMed] [Google Scholar]
- 15.Li YP, Hernandez-Martinez S, Fernandez F, Mayoral JG, Topalis P, Priestap H, Perez M, Navare A, Noriega FG. Biochemical, molecular, and functional characterization of PISCF-allatostatin, a regulator of juvenile hormone biosynthesis in the mosquito Aedes aegypti. J Biol Chem. 2006;281 (45):34048–34055. doi: 10.1074/jbc.M606341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riehle MA, Fan YL, Cao C, Brown MR. Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: Expression, cellular localization, and phylogeny. Peptides. 2006;27 (11):2547–2560. doi: 10.1016/j.peptides.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, Sulderman RJ, Strand MR. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2008;105 (15):5716–5721. doi: 10.1073/pnas.0800478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai L, Adams ME. Ecdysis triggering hormone signaling in the yellow fever mosquito Aedes aegypti. Gen Comp Endocr. 2009;162 (1):43–51. doi: 10.1016/j.ygcen.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufmann C, Merzendorfer H, Gäde G. The adipokinetic hormone system in Culicinae (Diptera: Culicidae): Molecular identification and characterization of two adipokinetic hormone (AKH) precursors from Aedes aegypti and Culex pipiens and two putative AKH receptor variants from A. aegypti. Insect Biochem Molec Biol. 39(11):770–781. doi: 10.1016/j.ibmb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Baggerman G, Cerstiaens A, De Loof A, Schoofs L. Peptidomics of the larval Drosophila melanogaster central nervous system. J Biol Chem. 2002;277 (43):40368–40374. doi: 10.1074/jbc.M206257200. [DOI] [PubMed] [Google Scholar]
- 21.Hummon AB, Richmond TA, Verleyen P, Baggerman G, Huybrechts J, Ewing MA, Vierstraete E, Rodriguez-Zas SL, Schoofs L, Robinson GE, Sweedler JV. From the genome to the proteome: Uncovering peptides in the Apis brain. Science. 2006;314 (5799):647–649. doi: 10.1126/science.1124128. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Predel R, Neupert S, Hauser F, Tanaka Y, Cazzamali G, Williamson M, Verleyen P, Schoofs L, Schachtner J, Grimmelikhuijzen CJP, Park Y. Genomics, Transcriptomics, and Peptidomics of Neuropeptides and Protein Hormones in the Red Flour Beetle Tribolium castaneum. Genome Res. 2008;18:113–122. doi: 10.1101/gr.6714008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Predel R. Peptidergic neurohemal system of an insect: Mass spectrometric morphology. J Comp Neurol. 2001;436 (3):363–375. [PubMed] [Google Scholar]
- 24.Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25 (17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. GenBank. Nucleic Acids Res. 2008;36:D25–D30. doi: 10.1093/nar/gkm929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340 (4):783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Veenstra JA. Mono- and dibasic proteolytic cleavage sites in insect neuroendocrine peptide precursors. Arch Insect Biochem Physiol. 2000;43 (2):49–63. doi: 10.1002/(SICI)1520-6327(200002)43:2<49::AID-ARCH1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 28.Meola SM, Lea AO. The ultrastructure of the corpus cardiacum of Aedes sollicitans and the histology of the cerebral neurosecretory system of mosquitoes. Gen Comp Endocr. 1972;18 (2):210–234. doi: 10.1016/0016-6480(72)90208-0. [DOI] [PubMed] [Google Scholar]
- 29.Hewes RS, Taghert PH. Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res. 2001;11 (6):1126–1142. doi: 10.1101/gr.169901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broeck JV. Neuropeptides and their precursors in the fruitfly, Drosophila melanogaster. Peptides. 2001;22 (2):241–254. doi: 10.1016/s0196-9781(00)00376-4. [DOI] [PubMed] [Google Scholar]
- 31.Predel R, Wegener C, Russell WK, Tichy SE, Russell DH, Nachman RJ. Peptidomics of CNS-associated neurohemal systems of adult Drosophila melanogaster: A mass spectrometric survey of peptides from individual flies. J Comp Neurol. 2004;474 (3):379–392. doi: 10.1002/cne.20145. [DOI] [PubMed] [Google Scholar]
- 32.Predel R, Wegener C. Biology of the CAPA peptides in insects. Cell Mol LIFE Sci. 2006;63 (21):2477–2490. doi: 10.1007/s00018-006-6187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown MR, Cao C. Distribution of ovary ecdysteroidogenic hormone I in the nervous system and gut of mosquitoes. J Insect Sci. 2001:1.3. doi: 10.1093/jis/1.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iversen A, Cazzamali G, Williamson M, Hauser F, Grimmelikhuijzen CJP. Molecular cloning and functional expression of a Drosophila receptor for the neuropeptides capa-1 and-2. Biochem Biophys Res Commun. 2002;299 (4):628–633. doi: 10.1016/s0006-291x(02)02709-2. [DOI] [PubMed] [Google Scholar]
- 35.Park Y, Kim YJ, Adams ME. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc Natl Acad Sci U S A. 2002;99 (17):11423–1128. doi: 10.1073/pnas.162276199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cazzamali G, Torp M, Hauser F, Williamson M, Grimmelikhuijzen CJP. The Drosophila gene CG9918 codes for a pyrokinin-1 receptor. Biochem Biophys Res Commun. 2005;335 (1):14–19. doi: 10.1016/j.bbrc.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 37.Brown MR, Lea AO. FMRFamide- and adipokinetic hormone-like immunoreactivity in the nervous system of the mosquito, Aedes aegypti. J Comp Neurol. 1988;270 (4):606–614. doi: 10.1002/cne.902700413. [DOI] [PubMed] [Google Scholar]
- 38.Predel R, Brandt W, Kellner R, Rapus J, Nachman RJ, Gade G. Post-translational modifications of the insect sulfakinins - Sulfation, pyroglutamate-formation and O-methylation of glutamic acid. Eur J Biochem. 1999;263 (2):552–560. doi: 10.1046/j.1432-1327.1999.00532.x. [DOI] [PubMed] [Google Scholar]
- 39.Boerjan B, Cardoen D, Bogaerts A, Landuyt B, Schoofs L, Verleyen P. Mass spectrometric profiling of (neuro)-peptides in the worker honeybee, Apis mellifera. Neuropharmacology. 2010;58 (1):248–258. doi: 10.1016/j.neuropharm.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 40.Wegener C, Reinl T, Jansch L, Predel R. Direct mass spectrometric peptide profiling and fragmentation of larval peptide hormone release sites in Drosophila melanogaster reveals tagma-specific peptide expression and differential processing. J Neurochem. 2006;96 (5):1362–1374. doi: 10.1111/j.1471-4159.2005.03634.x. [DOI] [PubMed] [Google Scholar]
- 41.Pollock VP, McGettigan J, Cabrero P, Maudlin IM, Dow JAT, Davies SA. Conservation of capa peptide-induced nitric oxide signalling in Diptera. J Exp Biol. 2004;207 (23):4135–4145. doi: 10.1242/jeb.01255. [DOI] [PubMed] [Google Scholar]
- 42.Coast GM. Neuroendocrine control of ionic homeostasis in blood-sucking insects. J Exp Biol. 2009;212 (3):378–386. doi: 10.1242/jeb.024109. [DOI] [PubMed] [Google Scholar]
- 43.Chintapalli VR, Wang J, Dow JAT. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39 (6):715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 44.Clements AN, Potter SA, Scales MDC. The cardiacal neurosecretory system and associated organs of an adult mosquito, Aedes aegypti. J Insect Physiol. 1985;31 (10):821–830. [Google Scholar]
- 45.Zitnan D, Kingan TG, Hermesman JL, Adams ME. Identification of ecdysis-triggering hormone from an epitracheal endocrine system. Science. 1996;271 (5245):88–91. doi: 10.1126/science.271.5245.88. [DOI] [PubMed] [Google Scholar]
- 46.Zitnan D, Zitnanova I, Spalovska I, Takac P, Park Y, Adams ME. Conservation of ecdysis-triggering hormone signalling in insects. J Exp Biol. 2003;206 (8):1275–1289. doi: 10.1242/jeb.00261. [DOI] [PubMed] [Google Scholar]
- 47.Brown MR, Raikhel AS, Lea AO. Ultrastructure of midgut endocrine cells in the adult mosquito, Aedes aegypti. Tissue & Cell. 1985;17 (5):709–721. doi: 10.1016/0040-8166(85)90006-0. [DOI] [PubMed] [Google Scholar]
- 48.Brown MR, Lea AO. Neuroendocrine and midgut endocrine systems in the adult mosquito. Adv Dis Vector Res. 1989;6:29–58. [Google Scholar]
- 49.Reichwald K, Unnithan GC, Davis NT, Agricola H, Feyereisen R. Expression of the allatostatin gene in endocrine cells of the cockroach midgut. Proc Natl Acad Sci U S A. 1994;91 (25):11894–11898. doi: 10.1073/pnas.91.25.11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veenstra JA, Lambrou G. Isolation of a novel RFamide peptide from the midgut of the American cockroach, Periplaneta americana. Biochem Biophys Res Commun. 1995;213 (2):519–524. doi: 10.1006/bbrc.1995.2162. [DOI] [PubMed] [Google Scholar]
- 51.Huang YQ, Brown MR, Lee TD, Crim JW. RF-amide peptides isolated from the midgut of the corn earworm, Helicoverpa zea, resemble pancreatic polypeptide. Insect Biochem Molec Biol. 1998;28 (5–6):345–356. doi: 10.1016/s0965-1748(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 52.Muren JE, Nassel DR. Isolation of five tachykinin-related peptides from the midgut of the cockroach Leucophaea maderae: Existence of N-terminally extended isoforms. Regulatory Peptides. 1996;65 (3):185–196. doi: 10.1016/0167-0115(96)00092-4. [DOI] [PubMed] [Google Scholar]
- 53.Sakai T, Satake H, Minakata H, Takeda M. Characterization of crustacean cardioactive peptide as a novel insect midgut factor: Isolation, localization, and stimulation of alpha-amylase activity and gut contraction. Endocrinology. 2004;145 (12):5671–5678. doi: 10.1210/en.2004-0722. [DOI] [PubMed] [Google Scholar]
- 54.Brown MR, Crim JW, Lea AO. FMRFamide- and pancreatic polypeptide-like immunoreactivity of endocrine cells in the midgut of a mosquito. Tissue & Cell. 1986;18 (3):419–428. doi: 10.1016/0040-8166(86)90061-3. [DOI] [PubMed] [Google Scholar]
- 55.Hernandez-Martinez S, Li YP, Lanz-Mendoza H, Rodriguez MH, Noriega FG. Immunostaining for allatotropin and allatostatin-A and -C in the mosquitoes Aedes aegypti and Anopheles albimanus. Cell Tissue Res. 2005;321 (1):105–113. doi: 10.1007/s00441-005-1133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moffett SB, Moffett DF. Comparison of immunoreactivity to serotonin, FMRFamide and SCPb in the gut and visceral nervous system of larvae, pupae and adults of the yellow fever mosquito Aedes aegypti. J Insect Sci. 2005;5:20. doi: 10.1093/jis/5.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veenstra JA, Lau GW, Agricola HJ, Petzel DH. Immunohistological localization of regulatory peptides in the midgut of the female mosquito Aedes aegypti. Histochem Cell Biol. 1995;104:337–347. doi: 10.1007/BF01458127. [DOI] [PubMed] [Google Scholar]
- 58.Stanek DM, Pohl J, Crim JW, Brown MR. Neuropeptide F and its expression in the yellow fever mosquito, Aedes aegypti. Peptides. 2002;23 (8):1367–1378. doi: 10.1016/s0196-9781(02)00074-8. [DOI] [PubMed] [Google Scholar]
- 59.Onken H, Moffett SB, Moffett DE. The anterior stomach of larval mosquitoes (Aedes aegypti): effects of neuropeptides on transepithelial ion transport and muscular motility. J Exp Biol. 2004;207 (21):3731–3739. doi: 10.1242/jeb.01208. [DOI] [PubMed] [Google Scholar]
- 60.Veenstra JA. Isolation and identification of three RFamide-immunoreactive peptides from the mosquito Aedes aegypti. Peptides. 1999;20:31–38. doi: 10.1016/s0196-9781(98)00153-3. [DOI] [PubMed] [Google Scholar]