Abstract
Recent studies from our laboratory and others have shown that increases in cytoplasmic superoxide (O2·−) levels and Akt activation play a key role in agonist-stimulated NF-κB activation and cardiomyocyte hypertrophy in vitro. In this study, we tested the hypothesis that adenovirus (Ad)-mediated intramyocardial gene transfer of cytoplasmic superoxide dismutase (AdCu/ZnSOD) or a dominant-negative form of Akt (AdDNAkt) in mice would attenuate pressure overload-induced increases in activation of the redox-sensitive transcription factor NF-κB and cardiac hypertrophy. Adult C57BL/6 mice were subjected to thoracic aortic banding (TAB) or sham surgery, and intramyocardial injections of viral vectors (AdCu/ZnSOD, AdDNAkt, or control) were performed. There was robust transgene expression in the heart, which peaked 6–7 days after injection and then declined to undetectable levels by 12–14 days. In mice injected with AdBgL II, TAB caused a significant increase in O2·− generation and cardiac mass at 1 wk, and these responses were markedly attenuated by AdCu/ZnSOD. In addition, TAB induced time-dependent activation of NF-κB in the myocardium as measured longitudinally by in vivo bioluminescent imaging of NF-κB-dependent luciferase expression. This was also abolished by intracardiac AdCu/ZnSOD or AdDNAkt, but not the control vector. The inhibition of Akt and O2·−-mediated NF-κB activation in TAB hearts was associated with an attenuation of cardiac hypertrophy. Since a direct cause-and-effect relationship between NF-κB activation and cardiomyocyte hypertrophy has been established previously, our data support the hypothesis that increased O2·− generation and Akt activation are key signaling intermediates in pressure overload-induced activation of NF-κB and cardiac hypertrophy.
Keywords: reactive oxygen species, superoxide dismutase, dominant-negative Akt bioluminescent imaging, gene therapy
cardiac hypertrophy is a common compensatory response to a variety of myocardial stresses, including pressure overload, and is a strong predictor of cardiovascular morbidity and mortality (15, 35). In patients with ischemic or dilated cardiomyopathy, reactive oxygen species (ROS) production in the myocardium is increased (2, 49). Extensive in vitro evidence from our laboratory (24) and others (36, 49) has demonstrated that hypertrophic stimuli such as angiotensin II (ANG II) and tumor necrosis factor-α (TNF-α), factors that are increased in hypertrophic and failing myocardium, produce significant increases in ROS in cardiomyocytes with concomitant increases in cardiomyocyte size. Moreover, increased ROS signaling has been shown to play an important role in hypertrophy and remodeling in the failing human heart and in animal models of hypertrophy and heart failure (2, 47).
Investigations into the source of ROS in the myocardium have identified the superoxide (O2·−)-generating NADPH oxidase as a key source of agonist-induced ROS generation (2, 24, 47). We (24) and others (36, 42, 49) have recently shown that ANG II stimulation of primary neonatal rat cardiomyocytes increases cytoplasmic O2·− generation from a Rac1-regulated NADPH oxidase, and inhibition of this oxidase or increased scavenging of O2·− by genetic or pharmacological means abolishes cardiomyocyte hypertrophy. Similarly, in aortic constriction models of cardiac hypertrophy, NADPH oxidase expression is increased in the myocardium, with a parallel increase in ROS generation and cardiac hypertrophy (2). However, a direct causative role of myocardium-derived O2·− in cardiac hypertrophy in vivo has yet to be established. Furthermore, downstream redox signaling pathways have not been fully elucidated in vivo.
The pathological hypertrophic program requires the coordinated upregulation of several ROS-activated signaling cascades (2, 4, 22, 49). Evidence from in vitro and in vivo studies suggests that the serine-threonine kinase Akt/protein kinase B (Akt) and the transcription factor NF-κB are regulated by ROS in cardiovascular cells and are important downstream mediators of various agonists that cause cellular hypertrophy (18, 21, 22, 49). Overexpression of NF-κB in primary cardiomyocytes increases levels of the hypertrophic marker atrial natriuretic factor (ANF) and cardiomyocyte surface area (16, 44). Furthermore, inhibition of NF-κB by dominant-negative strategies or ROS scavenging enzymes attenuates cardiomyocyte hypertrophy induced by ANG II (17, 18, 24, 28). In vivo, pressure overload leads to rapid activation of NF-κB in the myocardium, and this increase is correlated with increased cardiac hypertrophy (14, 30). Similarly, expression of a constitutively active mutant of Akt has been shown to increase cell surface area and expression of the cardiac hypertrophy marker ANF (10, 16). Transgenic mice overexpressing Akt from a cardiomyocyte-specific promoter exhibit cardiac hypertrophy and enhanced myocardial contractility (37). Emerging evidence indicates that Akt can activate NF-κB via multiple cell type-specific pathways (8, 9), suggesting a possible cross talk between these important signaling intermediates in cardiomyocyte hypertrophy.
The goal of this study was to establish a definitive causal role of increased myocardial O2·− and Akt activation in cardiac NF-κB activation and hypertrophy in vivo. Using selective genetic approaches to target expression of cytoplasmic superoxide dismutase (AdCu/ZnSOD) or a dominant-negative Akt mutant (AdDNAkt) to the myocardium in mice with thoracic aortic banding (TAB)-induced hypertrophy, in combination with in vivo bioluminescent imaging, we provide evidence that 1) pressure overload activates NF-κB through a O2·−-dependent mechanism in myocardium; 2) Akt is an essential signaling intermediate in the activation of NF-κB by pressure overload; and 3) increased O2·− production and Akt activation are key regulators of pressure overload-induced cardiomyocyte hypertrophy. As such, increased scavenging of O2·− and/or inhibition of Akt/NF-κB activation in the myocardium may represent a novel therapeutic strategy in the prevention of cardiac hypertrophy-related heart disease.
MATERIALS AND METHODS
Animals.
Adult male C57BL/6 mice (8–10 wk, Harlan, Indianapolis, IN) were used for all experiments. All experimental protocols were approved by the Animal Care and Use Committees at the University of Iowa and Cornell University, and care of the mice was in accordance with the standards set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals, USDA regulations, and the American Veterinary Medical Association.
Recombinant adenoviral vectors.
Construction and characterization of E1-deleted adenoviral (Ad) vectors encoding 1) firefly luciferase (AdLuc); 2) NF-κB-driven luciferase containing four copies of the consensus NF-κB binding site cloned upstream of firefly luciferase (AdNF-κB-Luc); 3) human Cu/ZnSOD (AdCu/ZnSOD); 4) dominant-negative Akt in which alanine has been substituted for threonine 308 and serine 473 (AdDNAkt); and 5) control/reporter vectors AdBgL II (empty vector), enhanced green fluorescent protein (AdGFP), and bacterial β-galactosidase (AdLacZ) have been described previously (24, 33, 38, 56).
TAB and Ad-mediated gene transfer to myocardium.
TAB or sham surgery was performed to increase proximal aortic pressure as described previously (23). At the time of TAB or sham surgery, Ad-mediated intramyocardial gene transfer was carried out as described in detail previously (6, 11). Briefly, mice were anesthetized with pentobarbital (1 mg/kg ip), endotracheally intubated, and ventilated with a mixture of room air and methoxyflurane (Harvard Apparatus; 200-ml tidal volume, 120 cycles/min). The anterior chest was shaved and an incision made for access to the thorax. For TAB, the transverse aortic arch between the innominate and left common carotid arteries was ligated with 7-0 Prolene and an overlying 27-gauge needle, which was removed immediately after constriction to generate stenosis. Sham surgery was identical except for ligation. Next, the ascending aorta and pulmonary arteries were clamped and appropriate adenoviral vectors (1 × 109 total particles, 80 μl) diluted in phosphate-buffered saline (PBS) were rapidly injected into the left ventricular (LV) cavity via an apically inserted 33-gauge needle. The clamp was maintained for 30 s to allow the virus to perfuse the endocardium. Transient blanching of the heart is a hallmark of efficient gene delivery (11) and was confirmed after every injection. A droplet of Vetbond (3M, St. Paul, MN) was applied to seal the injection site. The chest was closed, and the remaining air and blood were removed with a chest tube by applying negative pressure as described previously (32). Mice were ventilated for 60 min on a heating pad before being extubated and returned to their home cages. Approximately 90% of all mice subjected to TAB and intracardiac gene transfer survived until completion of the experimental protocols.
Assessment of cardiac gene transfer in mice: time course, distribution, and efficiency.
To thoroughly characterize Ad-mediated intracardiac gene transfer, we used a variety of reporter genes. First, to monitor Ad-mediated gene transfer to the myocardium over time in vivo, mice were injected with AdLuc (or control vector AdLacZ) as described above and in vivo bioluminescent imaging (IVIS-200 System, Xenogen, Alameda, CA) was used to longitudinally track photon flux (luciferase activity) as previously described (38). Briefly, mice were anesthetized with isoflurane, and the substrate d-luciferin (150 mg/kg, Xenogen) was injected intraperitoneally 15–20 min before imaging. Mice were imaged every other day for 14 days to determine the time course of luciferase expression in the myocardium. Bioluminescence was measured as photon emission per unit area, and signal intensity was quantified with Living Image software (Xenogen) as described previously (38).
In a separate group of mice, AdGFP was used to determine the distribution of Ad-mediated transgene expression in the myocardium. Mice received intramyocardial injections of AdGFP or AdBgL II as described above, and hearts were harvested after 7 days. Brightfield and fluorescent images of the hearts were captured to examine GFP fluorescence with an Olympus Fluorescent Stereoscope (Olympus, Melville, NY).
Finally, to quantify efficiency of Ad-mediated gene transfer, mice received intramyocardial injections of AdLacZ or AdBgL II and were killed after 1 wk. Hearts were cryosectioned (coronal, 20 μm), and β-galactosidase (β-gal) activity was examined by X-gal staining as described previously (31). In addition, β-gal activity in heart homogenates was assessed with the β-gal Assay Kit (Promega, Madison, WI) as described previously (31). Additional studies were carried out in a subset of AdLacZ-injected hearts in which cardiomyocytes were isolated and plated in culture dishes and stained with X-gal in vitro, and the percentage of β-gal-positive cells was determined by counting blue-stained cells compared with all cardiomyocytes as described previously (31).
Ad-mediated Cu/ZnSOD expression in myocardium.
Human Cu/ZnSOD expression in AdCu/ZnSOD-infected TAB hearts was assessed by two different methods. First, to quantify human Cu/ZnSOD levels, we performed Western blot analysis as previously described (55). Briefly, 5 μg of LV protein from sham-operated hearts and AdCu/ZnSOD- or AdBgL II-injected TAB hearts 7 days after gene transfer was resolved by SDS-PAGE, transferred to nitrocellulose membranes, and incubated with anti-human Cu/ZnSOD antibody (1:1,000, The Binding Site) at 4°C for 24 h. After washing, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:1,200, Santa Cruz), and bands were visualized with enhanced chemiluminescence (ECL) reagents (Amersham, Piscataway, NJ). Band intensities were quantified with the Fluor-S MultiImager (Bio-Rad, Hercules, CA).
To demonstrate that Ad-mediated Cu/ZnSOD gene transfer caused increased levels of functional Cu/ZnSOD protein, Cu/ZnSOD activity was measured 1 wk after gene transfer in LV tissue homogenates from sham-operated and TAB hearts injected with AdCu/ZnSOD or AdBgL II as described previously (46). Data are expressed in units of Cu/ZnSOD activity per milligram of protein, where 1 unit is defined as the amount of enzyme needed to reduce product formation by 50% with purified Cu/ZnSOD in the absence of CN− as the standard.
Measurement of O2·− production in myocardium.
O2·− levels were analyzed in the LV of mice 1 wk after sham or TAB procedure and intracardiac injections of AdBgL II or AdCu/ZnSOD as previously described (32, 33). Briefly, hearts were collected, snap-frozen, and embedded in OCT medium. Cryosections (20 μm, coronal) were rehydrated with PBS on microscope slides, incubated with the fluoroprobe dihydroethidium (DHE, 5 μM; Molecular Probes, Eugene, OR) for 5 min in the dark, and imaged by confocal microscopy (Zeiss LSM 510). Ethidium fluorescence was quantified with Image Pro-Plus 4.5 software (Media Cybernetics, Silver Spring, MD). Since measurement of O2·− levels by DHE in tissue sections is a semiquantitative approach and may be confounded because of its oxidation by cytochrome c and other heme-containing proteins, it is important to employ SOD treatment groups as a control (5, 13, 54). As such, here we compared ethidium fluorescence in LV sections from mice subjected to sham surgery and TAB combined with intramyocardial AdCu/ZnSOD (or AdLacZ) gene transfer. It is important to note that identical microscope settings were used during acquisition of data from each of these groups.
Bioluminescent detection of TAB-induced NF-κB activation.
Bioluminescent imaging was used to longitudinally track NF-κB activation in vivo in mice that had undergone intramyocardial injections of a cocktail containing equal parts AdNF-κB-Luc and either AdLacZ, AdDNAkt, or AdCu/ZnSOD (1 × 109 total particles, 80 μl total) immediately after sham or TAB procedure. Imaging was performed with the IVIS-200 (Xenogen) as described above and by Peterson et al. (38). Mice were imaged daily for 2 wk, and data are averaged over 3- to 4-day segments. Data analyses were performed as described above.
Analysis of TAB-induced NF-κB binding activity by electrophoretic mobility shift assay.
In a separate cohort of TAB hearts transduced with AdLacZ or AdCu/ZnSOD, bioluminescence data were verified by using electrophoretic mobility shift assay (EMSA) to measure NF-κB activity at 7 days. Nuclear protein was extracted from LV with a NE-PER nuclear extraction kit (Pierce, Rockford, IL) according to manufacturer's instructions, and protein concentration was quantified by the Bradford method. EMSA was performed with 6 μg of nuclear protein incubated with a γ-32P-labeled NF-κB consensus oligonucleotide probe 5′-AGTTGAGGGGACTTTCCCAGGC-3′ (Promega, Madison, WI) as described earlier (52). DNA-protein complexes were analyzed by autoradiography, and band intensities were quantified with the Bio-Rad Molecular Imager Gel Doc system.
Measurement of cardiac hypertrophy.
On day 7 after sham or TAB procedure, mice injected with AdCu/ZnSOD, AdDNAkt, or AdLacZ were weighed and euthanized. Hearts were removed, blotted to remove excess blood, and weighed. Tibias were excised and digested with proteinase K at 50°C for 24 h, and tibia lengths were measured. Cardiac hypertrophy was expressed as a ratio of heart weight (mg) to body weight (g) and heart weight (mg) to tibia length (mm) as previously described (55). LVs were dissected and saved for immunohistochemical and biochemical assays.
Statistical analysis.
All data are expressed as means ± SE and were analyzed by one-way ANOVA, followed by Newman-Keuls correction for multiple comparisons; a value of P < 0.05 was considered to be significant.
RESULTS
Ad-mediated reporter gene expression in murine myocardium.
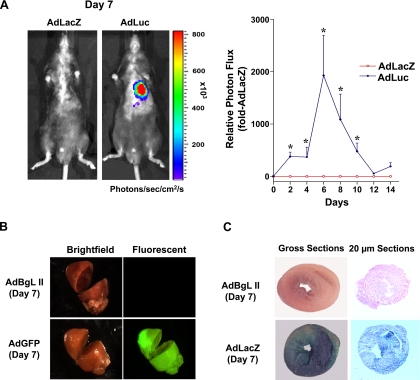
Virus-based vectors for myocardial gene transfer have emerged as an important strategy for studying molecular and cellular mechanisms of cardiac function (6, 11). Here we performed several studies to verify the effectiveness of this approach in our hands. First, to examine the time course of Ad-mediated transgene expression in the myocardium, we used a firefly luciferase reporter gene in conjunction with in vivo real-time bioluminescent imaging to longitudinally track transgene expression within the same animal. As shown in the representative images (day 7) and summary data of Fig. 1A, intramyocardial injection of AdLuc induced a time-dependent increase in photon emission—which reflects luciferase activity—in the myocardium as early as day 2. Little or no bioluminescence was detected in control AdLacZ-injected mice throughout the imaging period. Luciferase activity peaked at day 6 in AdLuc-treated mice, remained significantly elevated at days 8 and 10, and then gradually declined to undetectable levels by days 12–14.
Fig. 1.
Adenovirus (Ad)-mediated reporter gene expression in the murine myocardium. A: representative images (day 7, left) and summary data (right) showing the time course of photon flux as measured by in vivo bioluminescent imaging in mice that received intramyocardial injections of AdLuc or AdLacZ. In the pseudocolored scale, areas of high photon emission are displayed as red and areas of low photon emission are displayed as blue. Summary data (right) are means ± SE (n = 4 or 5/group) expressed relative to AdLacZ-injected mice; *P < 0.05 vs. day 0. Luc, luciferase; LacZ, control vector (β-galactosidase). B: representative brightfield and fluorescent images of hearts 7 days after gene transfer showing robust myocardial green fluorescent protein (GFP) expression in mice injected with AdGFP (n = 3) but not in mice injected with the control vector AdBgL II (n = 3). C: representative gross sections (left) and 20-μm cryosections (right) showing β-galactosidase expression in myocardium from AdBgL II (n = 3)- and AdLacZ (n = 3)-injected mice 7 days after injection.
Because of the relative lack of spatial resolution associated with bioluminescent imaging, we next investigated the distribution and efficiency of Ad-mediated transgene expression in the myocardium with adenoviruses encoding GFP and LacZ, respectively. As shown in the representative images in Fig. 1B, Ad-mediated GFP expression was robust and distributed throughout the myocardium at day 7 after injection, with particularly high levels in the LV. As expected, no fluorescence was observed in hearts injected with AdBgL II. The representative images provided in Fig. 1C from AdLacZ-injected hearts confirm and extend the findings in Fig. 1, A and B, showing widespread β-gal expression in gross samples (left) and in cryostat sections (right) 1 wk after gene transfer. Occasionally, we observed low levels of β-gal expression in liver or lungs of AdLacZ-injected mice. Importantly, β-gal staining in AdLacZ-injected hearts correlated with a fivefold increase in β-gal activity (RLU/mg protein) compared with AdBgL II-infected hearts [AdLacZ: 43.8 ± 10.6 (n = 3); AdBgL II: 8.5 ± 1.2 (n = 3); P < 0.05]. We also analyzed isolated single cardiomyocytes from two AdLacZ-injected hearts, and 54% ± 8% of cells (n = 1,000 cells counted) were β-gal positive. As such, these studies set the stage for performing intracardiac gene transfer of Cu/ZnSOD and dominant-negative Akt to examine the molecular mechanisms underlying TAB-induced cardiac hypertrophy.
Efficient expression of human Cu/ZnSOD in TAB hearts.
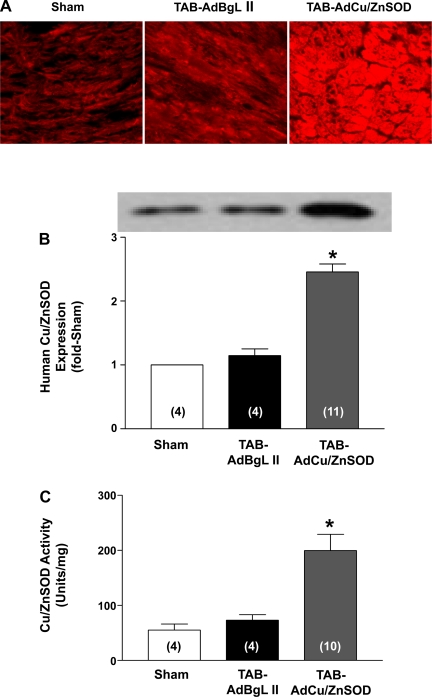
As shown in representative photomicrographs from heart sections stained with anti-human Cu/ZnSOD (Fig. 2A) and Western blot analysis of LV homogenates (Fig. 2B), AdCu/ZnSOD injection at the time of TAB surgery caused widespread and robust Cu/ZnSOD expression at 1 wk after surgery. The low levels of Cu/ZnSOD detected in sham-operated and AdBgL II-infected hearts are most likely due to cross-reactivity of the human Cu/ZnSOD antibody with the endogenous mouse enzyme.
Fig. 2.
Ad-mediated Cu/ZnSOD expression and activity in the myocardium. A: representative images of coronal sections of left ventricular (LV) tissue showing human Cu/ZnSOD staining 7 days after sham surgery (n = 4) or thoracic aortic banding (TAB) surgery combined with injection of AdBgL II (n = 4) or AdCu/ZnSOD (n = 9). B: representative Western blot and summary data from mice that underwent sham or TAB procedure along with intracardiac injections of AdBgL II or AdCu/ZnSOD 7 days earlier. Data are means ± SE (n = 4–11/group) expressed relative to sham-operated hearts. C: summary data demonstrating an increase in Cu/ZnSOD activity in heart homogenates from AdCu/ZnSOD-injected TAB mice compared with those treated with AdBgL II. Data are means ± SE (n = 4–10/group). *P < 0.05 vs. sham surgery and TAB-AdBgL II.
Next, to verify that virally expressed human Cu/ZnSOD was functional, and to examine the extent of increase in Cu/ZnSOD activity in the AdCu/ZnSOD-injected hearts, we measured Cu/ZnSOD activity in LV homogenates. As shown in the summary data in Fig. 2C, injection of AdCu/ZnSOD into the LV cavity at the time of TAB surgery resulted in an ∼3.5-fold increase in total Cu/ZnSOD activity 1 wk later compared with sham-operated hearts injected with vehicle or TAB hearts injected with the control vector AdBgL II. Together, these results demonstrate that the intracardiac gene transfer protocol results in robust transduction of murine hearts with functional Cu/ZnSOD, and validate the use of this method to examine the effect of increased O2·− scavenging in TAB-induced cardiac hypertrophy and O2·−-dependent signaling pathways in the myocardium.
AdCu/ZnSOD inhibits TAB-induced increases in myocardial O2·−.
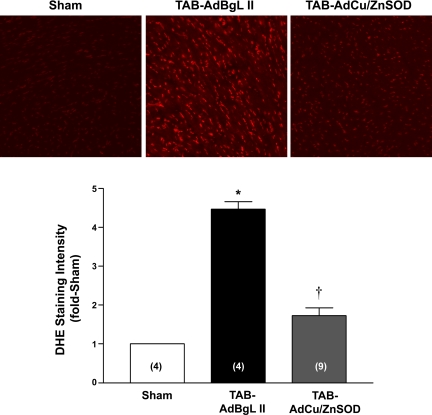
Having verified efficient delivery and expression of AdCu/ZnSOD in the myocardium, we next investigated O2·− production in myocardial sections from sham-operated and TAB mice injected with AdBgL II or AdCu/ZnSOD, using the O2·−-sensitive fluoroprobe DHE. As shown in the representative photomicrographs and summary data in Fig. 3, TAB caused a marked increase in O2·− production at 1 wk, as indicated by the ∼4.5-fold increase in ethidium fluorescence in AdBgL II-treated mice compared with sham-treated mice. However, in mice injected with AdCu/ZnSOD, the increase in fluorescence was significantly attenuated. These results demonstrate that pressure overload increases O2·− formation in the myocardium. Furthermore, given that AdCu/ZnSOD efficiently and selectively targets the cytoplasm of cardiac cells (24), the results suggest that this subcellular compartment is a key source of O2·− in this pressure-overload model.
Fig. 3.
AdCu/ZnSOD attenuates TAB-induced increases in superoxide (O2·−) production in the myocardium: representative confocal images and summary data showing changes in ethidium fluorescence in coronal sections of LV from mice 7 days after sham surgery or TAB surgery combined with injection of AdBgL II or AdCu/ZnSOD. Data are means ± SE (n = 4–9/group) expressed relative to sham-operated hearts. *P < 0.05 vs. sham surgery; †P < 0.05 vs. TAB-AdBgL II. DHE, dihydroethidium.
TAB-induced NF-κB activation is regulated by O2·− and Akt.
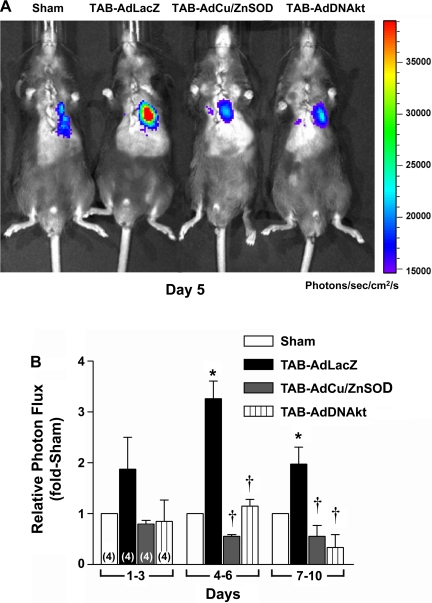
Recent studies in rat neonatal cardiomyocytes have suggested that Akt is required for NF-κB activation and cardiomyocyte hypertrophy (8, 9). Here we sought to extend these in vitro studies to examine the role of Akt in NF-κB activation and cardiac hypertrophy in vivo. Using bioluminescent imaging in conjunction with intracardiac gene transfer of a NF-κB-driven firefly luciferase reporter gene, we longitudinally monitored NF-κB activation following TAB or sham surgery in the presence of intracardiac AdLacZ, AdCu/ZnSOD, or AdDNAkt to determine whether TAB activates NF-κB via a ROS-sensitive Akt-dependent mechanism in the myocardium. As shown in the representative image (day 5) in Fig. 4A and summary data in Fig. 4B, TAB induced a marked time-dependent increase in cardiac photon emission—reflecting NF-κB activation—compared with sham-operated mice. NF-κB activity began to rise 1–3 days after TAB, reached a maximum ∼3.5-fold increase after 4–6 days, and started to decline but remained elevated above sham-operated levels through days 7–10. Interestingly, TAB-induced NF-κB activity was abolished in mice coinjected with AdCu/ZnSOD at all time points. Similarly, mice that were coinfected with AdDNAkt showed marked reductions in cardiac NF-κB transcription after TAB at all time points.
Fig. 4.
TAB-induced NF-κB activation is attenuated by AdCu/ZnSOD and AdDNAkt. Representative images at day 5 (A) and summary data (B) show the effects of AdLacZ, AdCu/ZnSOD, or AdDNAkt on NF-κB-driven luciferase activity (photon emission) over time in TAB mice compared with sham-operated mice. NF-κB activation was measured by in vivo bioluminescent imaging using Xenogen IVIS-200. In the pseudocolored scale, areas of high photon emission are displayed as red and areas of low photon emission are displayed as blue. Data are means ± SE (n = 4/group) expressed relative to sham-operated mice. *P < 0.05 vs. sham surgery; †P < 0.05 vs. TAB-AdLacZ. DNAkt, dominant-negative form of Akt.
To verify a subset of the in vivo bioluminescence data, we also measured cardiac NF-κB binding activity by EMSA on day 7 in separate cohorts of the AdCu/ZnSOD and AdLacZ groups. TAB caused a significant increase in NF-κB binding activity in LVs of AdLacZ-injected mice (2.9 ± 0.2-fold vs. sham surgery, n = 4; P < 0.05), but this was significantly blunted in mice pretreated with AdCu/ZnSOD (1.4 ± 0.2 fold vs. sham surgery, n = 7; P < 0.05). Together, these data suggest that O2·− and Akt are key upstream mediators of cardiac NF-κB activation in response to pressure overload.
AdCu/ZnSOD and AdDNAkt attenuate TAB-induced cardiac hypertrophy.
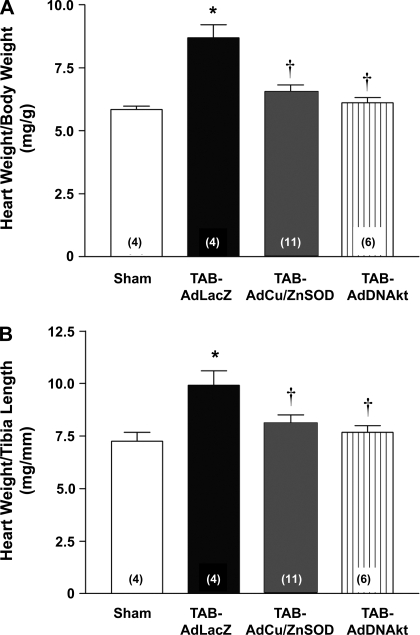
Given our recent findings (24) that increased O2·− scavenging and inhibition of Akt attenuated ANG II-dependent cell hypertrophy in cultured cardiomyocytes, we next examined the effects of intracardiac gene transfer of AdCu/ZnSOD and AdDNAkt on TAB-induced cardiac hypertrophy in vivo. These studies were performed 1 wk after TAB and gene transfer surgery because of our earlier results showing maximal hypertrophy (23) and Ad-mediated gene expression at this time (see above). Similar to our previous results (23), TAB produced significant cardiac hypertrophy in AdLacZ mice compared with sham-operated mice as evaluated by both heart weight-to-body weight (Fig. 5A) and heart weight-to-tibia length ratios (Fig. 5B). Importantly, both AdCu/ZnSOD and AdDNAkt treatment significantly reduced TAB-induced cardiac hypertrophy compared with AdLacZ-treated mice (Fig. 5). These results establish a direct causal link between both myocardial O2·− generation and Akt activation and the development of cardiac hypertrophy in this model.
Fig. 5.
Intracardiac injection of AdCu/ZnSOD or AdDNAkt attenuate TAB-induced cardiac hypertrophy. Summary data show heart weight/body weight (A) and heart weight/tibia length (B) 7 days after sham or TAB procedure and intracardiac injection of AdLacZ, AdCu/ZnSOD, or AdDNAkt. Data are means ± SE (n = 4–11/group). *P < 0.05 vs. sham surgery, †P < 0.05 vs. TAB-LacZ.
DISCUSSION
Recent evidence suggests that O2·− radicals are essential signaling intermediates in the development of cardiomyocyte hypertrophy (2, 24, 49). Additional evidence in various cardiovascular cell types suggests that NF-κB and Akt are targets of increased ROS, and are also key regulators of cell growth and survival (10, 22, 24). Studies using TAB to recapitulate pressure overload-induced cardiac hypertrophy have shown a correlation between increased ROS formation and upregulation of various signaling intermediates involved in cardiac hypertrophy in vivo (2, 49). However, a causal link between pressure overload-induced increases in myocardial ROS and activation of Akt and NF-κB, as well as potential cross talk between these pathways in the hypertrophic myocardium, has not been well elucidated. In this study, using Ad-mediated cardiac gene transfer to alter the redox state and Akt activation selectively in myocardium, we provide direct evidence that O2·− generation and Akt activation in myocardium are essential mediators of pressure overload-induced cardiac hypertrophy and activation of the hypertrophic transcription factor NF-κB. This conclusion is based on the following evidence: 1) TAB induced a significant increase in O2·− levels in the LV, and this was abolished by intracardiac injection of AdCu/ZnSOD; 2) TAB induced a time-dependent increase in NF-κB activation in the myocardium, and this was attenuated in mice injected with AdCu/ZnSOD or AdDNAkt; and 3) intramyocardial injection of AdDNAkt or AdCu/ZnSOD at the time of TAB surgery blunted cardiac hypertrophy.
Gene transfer to murine myocardium.
A number of different intramyocardial gene transfer strategies have emerged for rodents, with varying routes of delivery, times for aortic/pulmonary artery clamping, and cooling protocols (6, 11, 12, 29). Here we performed studies to first verify the effectiveness of the strategy that we adopted. Using a 30-s occlusion, direct injection into the LV cavity, and no cooling of animals, we detected robust reporter gene expression in murine myocardium as early as 2–3 days after virus injection. This reached a maximum at 6–7 days and then gradually declined to undetectable levels by 12–14 days after injection, a time course that is similar to what others have observed (11). In addition, we observed that 50–60% of cardiomyocytes isolated from AdLacZ-injected hearts expressed high levels of β-gal, a finding that is consistent with previous studies showing 50–70% infection efficiency in rat hearts with a similar method of gene delivery (11, 34, 50). This level of transduction was shown to be sufficient to induce morphological and physiological changes in the myocardium (11, 50). Similar to Del Monte et al. (11), we did not observe significant cytotoxicity in cardiac tissue in response to Ad-mediated gene transfer, and only low levels of sporadic gene expression were observed in liver and lungs. Thus our results confirm and extend the findings of other investigators, demonstrating efficient and widespread transgene expression in murine myocardium, with a time course compatible with studying the molecular mechanisms underlying TAB-induced cardiac hypertrophy.
ROS play an important role in pressure overload-induced cardiac hypertrophy.
In cultured cardiomyocytes, we (24) and others (2, 49, 53) have demonstrated that ROS, in particular O2·−, play a key signaling role in response to a variety of hypertrophic stimuli such as ANG II. While in vivo evidence is limited, a few studies using nonspecific ROS scavengers have shown that antioxidant therapy decreases pathological cardiac hypertrophy (2, 53). However, the lack of specificity and global (i.e., extracardiac) effects of the antioxidant protocols in these studies make it difficult to interpret the results. To overcome these limitations, we used an Ad-mediated gene delivery approach targeted selectively to the myocardium. With this strategy, we observed that increased expression of Cu/ZnSOD in the myocardium abolished TAB-induced increases in O2·− levels in the LV, with a parallel inhibition in cardiac hypertrophy and NF-κB activation. Although we used microscopic measurements of ethidium fluorescence in frozen sections to measure O2·− levels, and there certainly are other more quantitative methods of ROS detection available, our results showing a marked increase in ethidium fluorescence in LV tissue from TAB compared with sham-operated mice, along with almost complete normalization of this fluorescent signal in TAB mice administered intracardiac AdCu/ZnSOD, confirm the specificity of O2·− measurements by this method. Recent studies suggest that measurements of 2-hydroxyethidium (which contains an additional oxygen atom in its molecular structure) by HPLC may provide an excellent quantitative method to measure O2·− levels in intact tissue (13, 54). Although future studies utilizing this method will be required to confirm the findings presented here, our studies do support and extend in vitro studies described above (2, 24, 49, 53), and also support earlier studies suggesting involvement of O2·−-generating NADPH oxidase in the development of cardiac hypertrophy in response to pressure overload or ANG II (2, 4, 42, 53). Together, these studies clearly implicate increased production of O2·− in the myocardium in pressure-overload hypertrophy.
Akt is required for TAB-induced cardiac hypertrophy.
One of the primary mechanisms by which ROS influence cellular function is through activation of redox-sensitive kinases and their downstream targets. In particular, phosphoinositide 3-kinase (PI3K), p38 MAPK, ERKs, and JNK are activated by both oxidative stress and pressure overload in the myocardium (16, 22, 49). Recently, Akt has emerged as a key signaling molecule regulating cardiomyocyte survival and hypertrophy in vitro (10, 22, 43), and mice with cardiac-specific expression of constitutively active Akt spontaneously develop cardiac hypertrophy (37). On the other hand, inactivation of Akt by dominant-negative Akt inhibits cardiomyocyte hypertrophy in vitro induced by growth-promoting molecules such as TNF-α (8) and ANG II (24) and constitutively active PI3K-induced cardiac hypertrophy in vivo (37). Previous results from our laboratory (24) have demonstrated that Akt activation in cardiomyocytes in response to hypertrophic stimuli is inhibited by SOD overexpression. Similarly, several other groups have demonstrated that ROS-mediated activation of Akt is required for hypertrophic responses in cardiomyocytes and cardiac endothelial cells (7, 48). Although we did not directly measure Akt activity in LV tissue from TAB mice, our data showing that transduction of the myocardium with either AdCu/ZnSOD or AdDNAkt significantly attenuates TAB-induced cardiac hypertrophy extend these earlier findings, providing a direct causal link between increased O2·− generation as well as Akt activation in pressure overload-induced hypertrophy. In future studies, direct measurements of Akt activation in TAB hearts, both with and without AdCu/ZnSOD, would provide useful additional data in support of ROS-mediated Akt activation as a key component of the hypertrophic signaling cascade in this model.
Akt- and ROS-dependent activation of NF-κB are involved in pressure-overload hypertrophy.
Previous studies have suggested that Akt regulates cardiomyocyte hypertrophy by modulating several signaling pathways. One of the many targets of Akt is glycogen synthetase kinase 3β (GSK3β), which is active in myocardium under normal conditions and serves to antagonize cardiac hypertrophic signaling cascades through interaction with multiple transcription factors (22). Akt-mediated phosphorylation of GSK3β inhibits its activity and as such abrogates its antihypertrophic effects (16, 22, 48). Akt also influences protein synthesis by activating the small S6 ribosomal subunit through induction of p70S6 kinase (16, 22). In this study, we identify NF-κB as an additional pathway through which activated Akt regulates cardiac hypertrophy. NF-κB is a well-established mediator of cardiomyocyte hypertrophy both in cultured cardiomyocytes and in experimental models (16–18, 44). In vitro, overexpression of the p65 or c-rel subunits of NF-κB increased cell surface and ANF expression in cultured cardiomyocytes (39), while inactivation of NF-κB attenuated ANG II-induced ANF expression and increased protein content (27, 39). In vivo, Li et al. (30) showed that intracardiac expression of a NF-κB suppressor was associated with attenuation of TAB-induced increases in cardiac mass and ANF secretion and altered myocardial morphology. However, the upstream pathways linking pressure overload to NF-κB-mediated hypertrophy were poorly defined. Results here showing that AdCu/ZnSOD as well as AdDNAkt significantly inhibit TAB-stimulated activation of NF-κB place O2·− and Akt upstream of NF-κB in this signaling cascade. Coupled with our results demonstrating that AdDNAkt decreases TAB-mediated hypertrophy, these data implicate both Akt and NF-κB in the cardiac hypertrophic program in vivo.
Although the mechanisms underlying Akt-dependent activation of NF-κB are incompletely understood, recent evidence suggests that the extent of cross talk between these molecules and the mechanisms involved are cell type- and agonist specific (19, 20). In addition to regulating translocation of NF-κB to the nucleus via IKK-dependent degradation of IκB proteins in various cell types (8, 20), Akt has been shown to increase the transactivation potential of the RelA/p65 subunit of NF-κB via p38-dependent phosphorylation of serine 529 and 536 in the NF-κB transactivation domain (9). Further studies will be required to fully understand the molecular mechanisms underlying O2·−-mediated regulation of Akt, and whether O2·− and Akt activate NF-κB independently or in succession in response to pressure overload.
Although the present study clearly demonstrates important cross talk between Akt and NF-κB downstream of TAB-induced O2·− generation, the hypertrophic agonists that stimulate Akt and NF-κB activation in pressure-overload models remain to be identified. Since an increase in ANG II levels in TAB hearts has been observed (1), an important study in the future will be to determine the effects of ANG II receptor antagonism on the TAB-induced increases in NF-κB activation and cardiac mass. In addition, TAB has also been shown to increase levels of the prohypertrophic peptide TNF-α, and an interaction between Akt and NF-κB is required for the development of TNF-α-induced cardiomyocyte hypertrophy (8). Recently, ROS-mediated activation of apoptosis signal-regulating kinase-1 (ASK-1) has been shown as an additional mechanism in cardiac hypertrophy and cytokine release (25). In this cascade, ROS-mediated oxidation of thioredoxin-1 leads to its dissociation from the NH2-terminal domain of the ASK-1 complex, leading to its activation. Activated ASK-1 then regulates NF-κB and other transcription factors via MKK and MAP kinase pathways (25). Therefore, the ROS-mediated activation of ASK-1 and Akt and the resulting increase in NF-κB activation may be an important mediator downstream of several hypertrophic agonists. Future studies investigating the role of ASK-1 in NF-κB activation and cardiac hypertrophy will be necessary to determine whether this signaling molecule also figures in the complex signaling pathways mediating pressure overload-induced cardiac hypertrophy.
Conclusions.
Using a modified intramyocardial gene transfer protocol in mice, we have demonstrated that pressure overload-induced increases in O2·− levels and Akt activation in the myocardium play a key role in NF-κB activation and cardiac hypertrophy. As such, we propose that dysregulation of cardiac O2·−-scavenging enzymes and/or increased production of O2·− in the myocardium results in activation of the Akt/NF-κB signaling pathways, leading to cardiac hypertrophy in response to pressure overload. These data suggest, like many other reports in experimental animal models, that antioxidant treatment would have a beneficiary effect in the treatment of cardiac hypertrophy. However, we are cognizant that previous clinical studies using antioxidant supplements have shown contradictory results in terms of prevention and management of cardiovascular disease (3, 26, 45, 51). In fact, a recent study has demonstrated that antioxidant supplements actually prevent the health-promoting effects of physical exercise in humans (41). Possible explanations for the lack of beneficial effects of antioxidant therapy in humans include inadequate levels of antioxidant molecules reaching target cells/subcellular compartments and/or the use of antioxidants that are not as specific and effective as those used in experimental settings (3, 40). As such, we speculate that increased scavenging of ROS levels with new antioxidant strategies and/or inhibition of Akt/NF-κB activation in the myocardium by gene targeting or pharmacological approaches may be an effective means of interrupting pressure overload-induced cardiac hypertrophy and related sequelae.
GRANTS
This study was supported by grants from the National Heart, Lung, and Blood Institute to R. L. Davisson (HL-55006, HL-63887, HL-84624). R. L. Davisson is an Established Investigator of the American Heart Association (054011N). S. D. Hingtgen was supported by a Pre-Doctoral Minority Graduate Research Supplement to HL-63887.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank the University of Iowa Gene Transfer Vector Core and the University of Iowa and Cornell University Microscopy and Imaging Core Facilities. We also thank Robert Doran for expert assistance with the images.
REFERENCES
- 1.Amedeo Modesti P, Zecchi-Orlandini S, Vanni S, Polidori G, Bertolozzi I, Perna AM, Formigli L, Cecioni I, Coppo M, Boddi M, Serneri GG. Release of preformed Ang II from myocytes mediates angiotensinogen and ET-1 gene overexpression in vivo via AT1 receptor. J Mol Cell Cardiol 34: 1491–1500, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Anilkumar N, Sirker A, Shah AM. Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci 14: 3168–3187, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Briasoulis A, Tousoulis D, Antoniades C, Stefanadis C. The oxidative stress menace to coronary vasculature: any place for antioxidants? Curr Pharm Des 15: 3078–3090, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res 98: 730–742, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Cai H, Dikalov S, Griendling KK, Harrison DG. Detection of reactive oxygen species and nitric oxide in vascular cells and tissues: comparison of sensitivity and specificity. Methods Mol Med 139: 293–311, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Champion HC, Georgakopoulos D, Haldar S, Wang L, Wang Y, Kass DA. Robust adenoviral and adeno-associated viral gene transfer to the in vivo murine heart: application to study of phospholamban physiology. Circulation 108: 2790–2797, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chen JX, Zeng H, Tuo QH, Yu H, Meyrick B, Aschner JL. NADPH oxidase modulates myocardial Akt, ERK1/2 activation, and angiogenesis after hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol 292: H1664–H1674, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condorelli G, Morisco C, Latronico MV, Claudio PP, Dent P, Tsichlis P, Condorelli G, Frati G, Drusco A, Croce CM, Napoli C. TNF-alpha signal transduction in rat neonatal cardiac myocytes: definition of pathways generating from the TNF-alpha receptor. FASEB J 16: 1732–1737, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-kappaB is controlled by mTOR and Raptor in association with IKK. Genes Dev 22: 1490–1500, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation 113: 2097–2104, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Del Monte F, Butler K, Boecker W, Gwathmey JK, Hajjar RJ. Novel technique of aortic banding followed by gene transfer during hypertrophy and heart failure. Physiol Genomics 9: 49–56, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Del Monte F, Hajjar RJ. Efficient viral gene transfer to rodent hearts in vivo. Methods Mol Biol 219: 179–193, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol 287: C895–C902, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Freund C, Schmidt-Ullrich R, Baurand A, Dunger S, Schneider W, Loser P, El-Jamali A, Dietz R, Scheidereit C, Bergmann MW. Requirement of nuclear factor-kappaB in angiotensin II- and isoproterenol-induced cardiac hypertrophy in vivo. Circulation 111: 2319–2325, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol 65: 45–79, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Gupta S, Das B, Sen S. Cardiac hypertrophy: mechanisms and therapeutic opportunities. Antioxid Redox Signal 9: 623–652, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Young D, Maitra RK, Gupta A, Popovic ZB, Yong SL, Mahajan A, Wang Q, Sen S. Prevention of cardiac hypertrophy and heart failure by silencing of NF-kappaB. J Mol Biol 375: 637–649, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Young D, Sen S. Inhibition of NF-kappaB induces regression of cardiac hypertrophy, independent of blood pressure control, in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 289: H20–H29, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Gustin JA, Korgaonkar CK, Pincheira R, Li Q, Donner DB. Akt regulates basal and induced processing of NF-kappaB2 (p100) to p52. J Biol Chem 281: 16473–16481, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Gustin JA, Ozes ON, Akca H, Pincheira R, Mayo LD, Li Q, Guzman JR, Korgaonkar CK, Donner DB. Cell type-specific expression of the IkappaB kinases determines the significance of phosphatidylinositol 3-kinase/Akt signaling to NF-kappaB activation. J Biol Chem 279: 1615–1620, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Ha T, Li Y, Gao X, McMullen JR, Shioi T, Izumo S, Kelley JL, Zhao A, Haddad GE, Williams DL, Browder IW, Kao RL, Li C. Attenuation of cardiac hypertrophy by inhibiting both mTOR and NFkappaB activation in vivo. Free Radic Biol Med 39: 1570–1580, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, Kerber RE, Weiss RM. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation 101: 2863–2869, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu Y, Sharma RV, Engelhardt JF, Davisson RL. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics 26: 180–191, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res 81: 457–464, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Kang JH, Cook NR, Manson JE, Buring JE, Albert CM, Grodstein F. Vitamin E, vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: the Women's Antioxidant and Cardiovascular Study. Circulation 119: 2772–2780, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawano S, Kubota T, Monden Y, Kawamura N, Tsutsui H, Takeshita A, Sunagawa K. Blockade of NF-kappaB ameliorates myocardial hypertrophy in response to chronic infusion of angiotensin II. Cardiovasc Res 67: 689–698, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Kawano S, Kubota T, Monden Y, Tsutsumi T, Inoue T, Kawamura N, Tsutsui H, Sunagawa K. Blockade of NF-kappaB improves cardiac function and survival after myocardial infarction. Am J Physiol Heart Circ Physiol 291: H1337–H1344, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Lai NC, Roth DM, Gao MH, Tang T, Dalton N, Lai YY, Spellman M, Clopton P, Hammond HK. Intracoronary adenovirus encoding adenylyl cyclase VI increases left ventricular function in heart failure. Circulation 110: 330–336, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Ha T, Gao X, Kelley J, Williams DL, Browder IW, Kao RL, Li C. NF-kappaB activation is required for the development of cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol 287: H1712–H1720, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Sharma RV, Duan D, Davisson RL. Adenovirus-mediated gene transfer to adult mouse cardiomyocytes is selectively influenced by culture medium. J Gene Med 5: 765–772, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Lindley TE, Doobay MF, Sharma RV, Davisson RL. Superoxide is involved in the central nervous system activation and sympathoexcitation of myocardial infarction-induced heart failure. Circ Res 94: 402–409, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Lindley TE, Infanger DW, Rishniw M, Zhou Y, Doobay MF, Sharma RV, Davisson RL. Scavenging superoxide selectively in mouse forebrain is associated with improved cardiac function and survival following myocardial infarction. Am J Physiol Regul Integr Comp Physiol 296: R1–R8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyon AR, Sato M, Hajjar RJ, Samulski RJ, Harding SE. Gene therapy: targeting the myocardium. Heart 94: 89–99, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature 451: 919–928, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Nakagami H, Takemoto M, Liao JK. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol 35: 851–859, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res 82: 250–260, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Peterson JR, Infanger DW, Braga VA, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Longitudinal noninvasive monitoring of transcription factor activation in cardiovascular regulatory nuclei using bioluminescence imaging. Physiol Genomics 33: 292–299, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Purcell NH, Tang G, Yu C, Mercurio F, DiDonato JA, Lin A. Activation of NF-kappaB is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc Natl Acad Sci USA 98: 6668–6673, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riccioni G, Bazzano LA. Antioxidant plasma concentration and supplementation in carotid intima media thickness. Expert Rev Cardiovasc Ther 6: 723–729, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106: 8665–8670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci USA 103: 7432–7437, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiekofer S, Shiojima I, Sato K, Galasso G, Oshima Y, Walsh K. Microarray analysis of Akt1 activation in transgenic mouse hearts reveals transcript expression profiles associated with compensatory hypertrophy and failure. Physiol Genomics 27: 156–170, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Sen CK, Roy S. Relief from a heavy heart: redox-sensitive NF-kappaB as a therapeutic target in managing cardiac hypertrophy. Am J Physiol Heart Circ Physiol 289: H17–H19, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effects of vitamins C and E and beta-carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: a randomized controlled trial. Am J Clin Nutr 90: 429–437, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem 179: 8–18, 1989 [DOI] [PubMed] [Google Scholar]
- 47.Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res 81: 482–490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119: 2758–2771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49: 241–248, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Tsuji T, Del Monte F, Yoshikawa Y, Abe T, Shimizu J, Nakajima-Takenaka C, Taniguchi S, Hajjar RJ, Takaki M. Rescue of Ca2+ overload-induced left ventricular dysfunction by targeted ablation of phospholamban. Am J Physiol Heart Circ Physiol 296: H310–H317, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 361: 2017–2023, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Yang J, Marden JJ, Fan C, Sanlioglu S, Weiss RM, Ritchie TC, Davisson RL, Engelhardt JF. Genetic redox preconditioning differentially modulates AP-1 and NF kappaB responses following cardiac ischemia/reperfusion injury and protects against necrosis and apoptosis. Mol Ther 7: 341–353, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Zhao W, Zhao D, Yan R, Sun Y. Cardiac oxidative stress and remodeling following infarction: role of NADPH oxidase. Cardiovasc Pathol 18: 156–166, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc 3: 8–21, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res 95: 532–539, 2004 [DOI] [PubMed] [Google Scholar]