Abstract
Klotho is a recently identified antiaging gene. Brain endothelin-1 (ET1) is important in the regulation of blood pressure (BP). We hypothesized that silence of brain klotho potentiates cold-induced elevation of BP via the endothelin pathway. To silence brain klotho, we constructed adeno-associated virus (AAV) carrying rat klotho small interference hairpin RNA (KL-shRNA). AAV carrying ET1-shRNA was used to silence brain ET1. Scrambled shRNA was used as Control-shRNA. Three groups of male Sprague-Dawley rats (6 rats/group) received KL-shRNA, KL-shRNA plus ET1-shRNA, and Control-shRNA, respectively, via intracerebroventricular injection. BP was monitored daily using a telemetry system. All animals were exposed to a moderate cold environment (5°C) at 12 days after gene delivery. KL-shRNA significantly increased BP by 9 days of exposure to cold, while BP in the Control-shRNA group remained unchanged. ET1-shRNA abolished KL-shRNA-induced elevation of BP during cold exposure. Interestingly, KL-shRNA increased brain ET1 expression and plasma norepinephrine level, suggesting that silencing of brain klotho increased ET1 production and the sympathetic nervous activity. The KL-shRNA-induced increase in sympathetic nervous activity was mediated by ET1 because it could be abolished by silencing of ET1. These results demonstrated that silencing of brain klotho potentiated and expedited cold-induced elevation of BP by upregulation of ET1 and the subsequent activation of the sympathetic nervous system.
Keywords: endothelin-1, cold exposure, adeno-associated virus, choroid plexus, RNA interference, short hairpin RNA, norepinephrine
cold temperatures have adverse effects on the human cardiovascular system. The prevalence of hypertension and related cardiovascular disease increases in cold regions and in winter (3, 8, 11–12, 17). The cold winter season has the highest mortality and morbidity from cardiovascular diseases (2, 14, 33). Cold temperatures increase severity of hypertension and trigger myocardial infarction and stroke in hypertensive patients (1, 5, 15, 33). Previous studies from our laboratory indicate that chronic exposure to moderate cold (5°C) causes hypertension (10, 36–37). Cold temperatures activate the sympathetic nervous system (SNS), which plays a critical role in the development of cold-induced hypertension (CIH) (29, 37).
Klotho is a recently discovered antiaging gene (41). Genetic mutation of klotho causes extensive premature aging phenotypes and drastically shortens lifespan (21). Overexpression of klotho, however, extends lifespan (22). Our most recent study indicated that in vivo expression of klotho in kidneys prevents progression of spontaneous hypertension and renal damage (43). Klotho gene is composed of five exons and four introns and resides on chromosome 13 with a size >50 kb (21). There are two transcripts generated from alternative RNA splicing, a transmembrane and a secreted form of klotho protein (41). The transmembrane form of klotho has a full-length transcript and encodes 1,014 amino acids. The short arm of the transmembrane domain can be removed and released into the circulation as a secreted form of klotho. An alternative RNA splicing also generates secreted form of klotho, which has a half-length of the transcript and encodes 550 amino acids (41). Klotho is mainly expressed in the kidney tubule epithelial cells. Klotho functions as a circulating hormone (41). Klotho regulates insulin/insulin-like growth factor 1 signaling and suppresses oxidative stress. Klotho acts as a glucuronidase and activates ion channel TRPV5. Klotho protects against endothelial dysfunction and regulates the production of nitric oxide (41). Klotho gene is also expressed in choroid plexus (CP) in the brain (21, 23), and the transmembrane form of klotho protein was found in cerebrospinal fluid (18). However, it is not known if brain klotho is related to the regulation of blood pressure (BP) in cold exposed rats.
Endothelin-1 (ET1) is a potent vasoconstrictor peptide synthesized by vascular endothelium (46). Chronic infusion of ET1 into the cerebral lateral ventricles of conscious rats provokes a progressive increase in arterial BP accompanied by increased urinary excretion of norepinephrine (NE) and epinephrine, reflecting activation of autonomic vasomotor centers. ET produced in the central nervous system may modulate the central control of circulation (31). The pressor action of centrally acting ET is probably mediated by enhancing the efferent sympathetic nerve activity (30). It is not known, however, if brain klotho regulates ET1 expression and the SNS activity in cold-exposed rats.
The objective of this study was to determine the role of brain klotho in the regulation of BP during exposure to cold. We hypothesized that RNA interference (RNAi) inhibition of brain klotho potentiates cold-induced elevation of BP via the ET system.
METHODS
Construction of adeno-associated virus with klotho shRNA and ET1 shRNA.
Two rat klotho shRNAs and two rat ET1 shRNAs were designed using the online shRNA designer software (Gene Link siRNA Explorer) and synthesized by IDT DNA (Coralville, IA). The shRNAs were annealed and ligated into the pAAV.U6 vector as described in our previous study (40). The inhibition efficiency of klotho shRNAs was tested in rat aortic smooth muscle cell cultures. One rat klotho shRNAs (cct tac ttc gag aaa tgc ggg) and one rat ET1 shRNAs (gga gtg tgt cta ctt ctg cca) were chosen based on their higher inhibition efficiency. For details, refer to the Supplemental Methods.1
Packaging of adeno-associated virus with klotho-shRNA and ET1-shRNA.
Adeno-associated virus (AAV)-2 vector carrying klotho shRNA (KL-shRNA) and ET1 shRNA (ET1-shRNA) were packaged as described in our previous studies (35, 40). The titers of recombinant AAVs were determined as described previously (40). For details, refer to the Supplemental Methods.
Quantitative reverse transcription polymerase chain reaction.
The mRNA expression of klotho, ET1, ETA, and ETB was measured using quantitative reverse transcription polymerase chain reaction (qRT-PCR) as described previously (34, 36). Briefly, total RNA was isolated from paraffin-embedded sections in cerebroventricle areas shown choroids plexus. The procedure of RNA isolation was performed according to the manual of RecoverALL Total RNA isolation kit (Ambion, Austin, TX). We used 2 μg of total RNA for reverse transcription, and 2 μl of cDNA was used for PCR. Primers of klotho, ET1, ETA, and ETB used in qRT-PCR were provided in Table 1. For details, refer to the Supplemental Methods.
Table 1.
Sequences of primers used in the experiments
| Primer | Accession # | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|
| Klotho | NM_031336 | caatggcttccctcctttac (nt1533∼nt1542) | agcacaggtttgcgtagtct (nt2044∼nt2025) |
| ET1 | NM_012548 | cacctggacatctctgg (nt386∼nt403) | gtctgtggtctttgtggg (nt 499∼nt 482) |
| ETA | NM_012550 | cgccattgaaattgtctc (nt 898∼nt 915) | ctcccattccttctgttg (nt 1158∼nt 1141) |
| ETB | NM_017333 | acgccacccactaagacc (nt 393∼nt 410) | tgatgcctagcacgaaca (nt 600∼nt 583) |
ET, endothelin.
Western blot.
Klotho protein was measured using western blot described previously (38–39, 43). For details, refer to the Supplemental Methods.
Animals.
This study was carried out according to the guidelines of the National Institute of Health on the care and use of laboratory animals. This project was approved by the Institutional Animal Care and Use Committee.
Eighteen male Sprague-Dawley rats (350–400 g) were randomly divided into three groups (six rats/group). All rats were housed individually and provided with Purina laboratory chow (#5001) and tap water. All rats were handled twice daily to minimize handling stress. Following a 1 wk control period, animals were implanted with the BP devices (TA11PA-C40, DSI) in the abdominal aorta under anesthesia (sodium pentobarbital 65 mg/kg body wt, ip).
Measurement of BP using a telemetry system.
Systolic, diastolic, and mean BP was monitored using a telemetry system (DSI) in conscious freely moving animals. Data were recorded three times before injection and once daily after injection. All reported BP values represented an average of at least 10 repeated measurements for each animal.
Intracerebroventricular injection.
One week after implantation of BP devices, three groups of rats received AAV.KL-shRNA, AAV.KL-shRNA plus AAV.ET1-shRNA, and AAV.Control-shRNA, respectively, via intracerebroventricular (ICV) injection (2 × 108 plaque-forming units/rat, 15 μl) under pentobarbital anesthesia. Preliminary studies indicated that ICV injection of AAV.ET1-shRNA alone did not affect klotho expression nor did it alter BP in cold-exposed rats. Therefore, a group with AAV.ET1-shRNA was not included in this study. For microinjection, a microsyringe was inserted into the left lateral cerebral ventricle (A-P, 1.0 mm caudal to the bregma; L, 1.6 mm lateral to the midline; V, 3.3 mm below the skull surface). The injection speed was maintained at 3 μl/min to prevent leaking.
Cold exposure.
BP was monitored for 12 days before exposure to a moderate cold environment (5°C). BP was recorded daily during exposure to cold. After 12 days of exposure to cold, animals were euthanized (pentobarbital sodium, 100 mg/kg body wt ip) and perfused with saline through the heart. Following perfusion, brains were removed for immunohistochemistry. Before perfusion, blood was collected in EDTA from a small cut in the heart and plasma was separated for measuring plasma level of NE.
Immunohistochemistry.
The paraffin-embedded brain sections (5 μm) from selected brain regions were processed for immunohistochemistry (40, 43). Briefly, sections were stained following standard protocols by using goat-anti-klotho polyclonal antibody (R&D), mouse anti-ET1 monoclonal antibody (Abcam), rabbit anti-ETA polyclonal antibody (Alomone), rabbit anti-ETB polyclonal antibody (Alomone), respectively. Briefly, the tissue sections were incubated with primary antibody at 4°C overnight followed by conjugated secondary antibody for 1 h. The photographs were taken under the Nikon Ti-U microscope (Nikon Instruments, Lewisville, TX) with ×60 oil objective. The staining densities were measured using Nikon Ti-U microscope software. For details, refer to the Supplemental Methods.
Measurement of plasma NE.
Plasma level of NE level was measured using HPLC with an electrochemical detector as described in our previous studies (36–37).
Statistical analysis.
Data for BP was carried out by t-test. The remaining data were analyzed by one-way ANOVA followed by the Tukey procedure to assess the significance of difference between means. Significance was set at a 95% confidence limit.
RESULTS
Inhibition efficiency of AAV.KL-shRNAs and AAV.ET1-shRNAs in cell cultures.
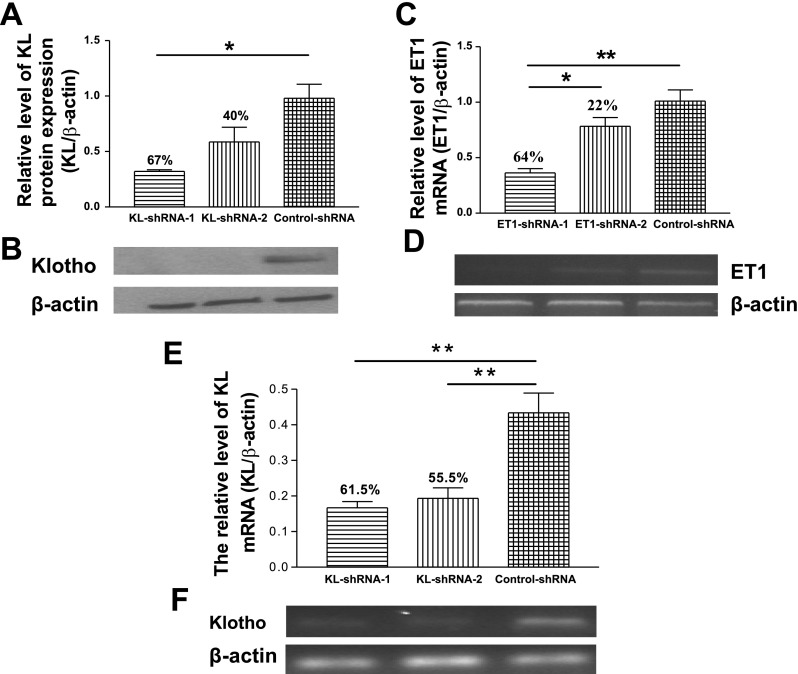
As shown in Fig. 1, two KL-shRNAs and two ET1-shRNAs were tested for their inhibition efficiency. KL-shRNA-1 was chosen for in vivo study because it demonstrated greater inhibition in klotho mRNA and protein expression compared with KL-shRNA-2 (Fig. 1, A, B, E, F). Similarly, ET1-shRNA-1 was selected as it showed greater inhibition in ET1 expression (Fig. 1, C and D).
Fig. 1.
Inhibition efficiency of KL-shRNAs and ET1-shRNAs in cell cultures. Two AAV.KL-shRNAs and two AAV.ET1-shRNAs were tested for inhibition efficiency in cell cultures before packaging of the recombinant AAVs for in vivo study. A: quantitative analysis of klotho protein expression. B: representative Western blot bands of klotho protein expression (130 kDa) and β-actin (42 kDa). C: quantitative analysis of ET1 mRNA expression. D: representative bands of ET1 mRNA expression (113 bp) and β-actin (444 bp) by semiquantification RT-PCR. E: quantitative analysis of klotho mRNA expression. F: representative bands of klotho mRNA expression and β-actin. KL, klotho; ET, endothelin; shRNA, short hairpin RNA; AAV, adeno-associated virus; Data = means ± SE. *P < 0.05, **P < 0.01; n = 3 independent experiments.
Silencing of brain klotho increased BP in cold-exposed rats.
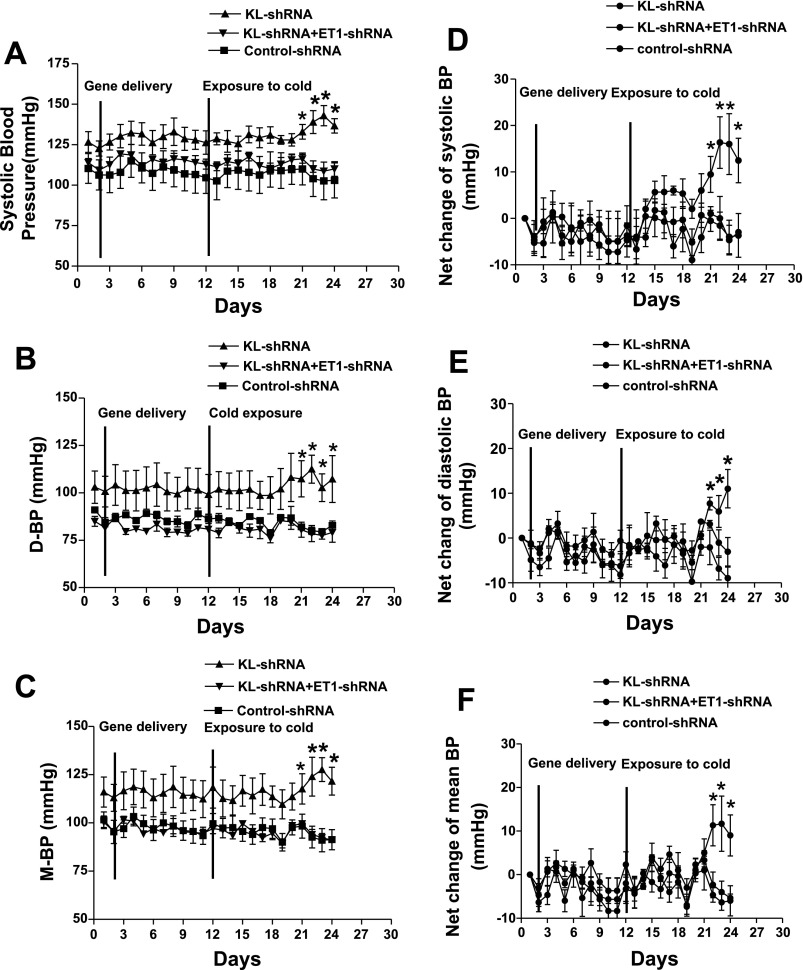
ICV injection of KL-shRNA did not alter BP significantly at room temperature (RT) (Fig. 2). Gene delivery of KL-shRNA increased systolic (Fig. 2, A and D), diastolic (Fig. 2, B and E) and mean BP (Fig. 2, C and F) as early as 9 days of exposure to cold. Thus, silencing of brain klotho resulted in an elevation of BP in cold-exposed rats. In contrast, BP of the KL-shRNA/ET1-shRNA group did not increase and maintained at the level of the Control-shRNA group during exposure to cold. These data suggest that silencing of brain ET1 abolished the KL-shRNA-induced elevation of BP in cold-exposed rats.
Fig. 2.
Effect of silencing of brain klotho on the blood pressure (BP) in cold-exposed rats. BP was monitored using a telemetry system. A, B, C, systolic, diastolic (D-BP), and mean blood pressure (M-BP), respectively. D, E, F, net changes of systolic, D-BP, and M-BP, respectively, compared with preinjection or precold exposure levels. Data = means ± SE. *P < 0.05, n = 6.
Silencing of brain klotho decreased klotho expression but up-regulated ET1 expression in brain CP.
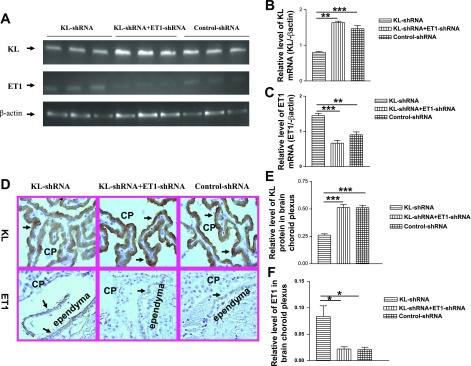
KL-shRNA significantly decreased klotho mRNA expression in brain CP of the ventricular area (Fig. 3, A and B), indicating effective silence of klotho. However, the inhibitory effect of KL-shRNA on klotho was abolished by ET1-shRNA, suggesting that silencing of ET1 may upregulate klotho expression. Notably, KL-shRNA resulted in a significant increase in ET1 mRNA expression (Fig. 3, A and C), indicating that silencing of klotho upregulated ET1 expression. ET1-shRAN effectively blocked the KL-shRNA-induced increase in ET1 mRNA expression (Fig. 3, A and C).
Fig. 3.
Klotho and ET1 expression in choroid plexus (CP) of the brain. A: gel graphs represented klotho and ET1 bands by qRT-PCR. B and C: the relative level of klotho and ET1 mRNA. D: immunohistochemical analysis of klotho (top) and ET1 (bottom) protein expression in CP and ependymal cells of the cerebroventricles. Arrows indicate klotho or ET1 expression in CP and ependymal cells of cerebroventricle. E and F: relative level of klotho and ET1 protein expression, respectively. Microphotos were taken at ×60 (oil) magnification. Data = means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001; n = 4–6.
Figure 3D shows klotho and ET1 protein expression in the CP and ependymal cells of the ventricles. Klotho and ET1 proteins were mainly localized in the apical plasma membrane of the CP epithelial cells and ependymal cells. Klotho expression (Fig. 3D, top) was significantly decreased by ICV injection of KL-shRNA compared with the control-shRNA group (Fig. 3E). This inhibitory effect was abolished by ET1-shRNA. Silencing of brain klotho upregulated ET1 expression (Fig. 3, D bottom and F). ET1-shRNA abolished the KL-shRNA-induced increase in ET1 protein expression (Fig. 3F), confirming effective silencing of brain ET1 expression.
No immunostaining for klotho and ET1 was found in the brain hippocampus (Supplemental Fig. S1) or hypothalamus (Supplemental Fig. S3). KL-shRNA or ET1-shRNA did not affect ETA or ETB expression in these brain regions (Figs. 1–3).
Silencing of brain klotho decreased ETB receptor expression in brain CP.
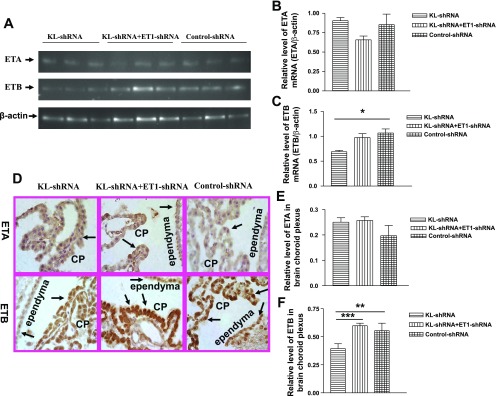
KL-shRNA significantly decreased ETB receptor mRNA expression in brain CP (Fig. 4, A and B), indicating that silencing of klotho down-regulated ETB receptor expression. This effect was abolished by ET1-shRNA (Fig. 4, A and C). In contrast, silencing of brain klotho or ET1 did not affect ETA receptor mRNA expression (Fig. 4, A and B). ETA and ETB receptor proteins were expressed in the CP epithelial cells and ependymal cells (Fig. 4D). ETA receptor expression was not altered significantly by silencing of klotho or ET1 (Fig. 4, D top and E). In contrast, KL-shRNA decreased ETB receptor protein expression (Fig. 4, D bottom and F), confirming that silencing of klotho downregulated ETB receptor expression. The KL-shRNA-induced downregulation of ETB receptor expression was abolished by silencing of ET1 (Fig. 4, D bottom and F).
Fig. 4.
The expression of ET receptors in CP of the brain. A: ETA receptor (260 bp) and ETB receptor (208 bp) mRNA bands in agarose gels. B and C: relative level of ETA receptor and ETB receptor mRNA, respectively, in CP and ependymal cells of the brain. The data shown in the graph were normalized with β-actin. D: immunohistochemical analysis of ETA and ETB receptor protein expression in the CP of the brain. Arrows indicate ETA or ETB expression in CP and ependymal cells of cerebroventricle. E and F: relative levels of ETA and ETB receptor protein, respectively, in CP and ependyma of cerebroventricles. Microphotos were taken at ×60 (oil) magnification. Data = means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001; n = 4–6.
Measurement of plasma norepinephrine.
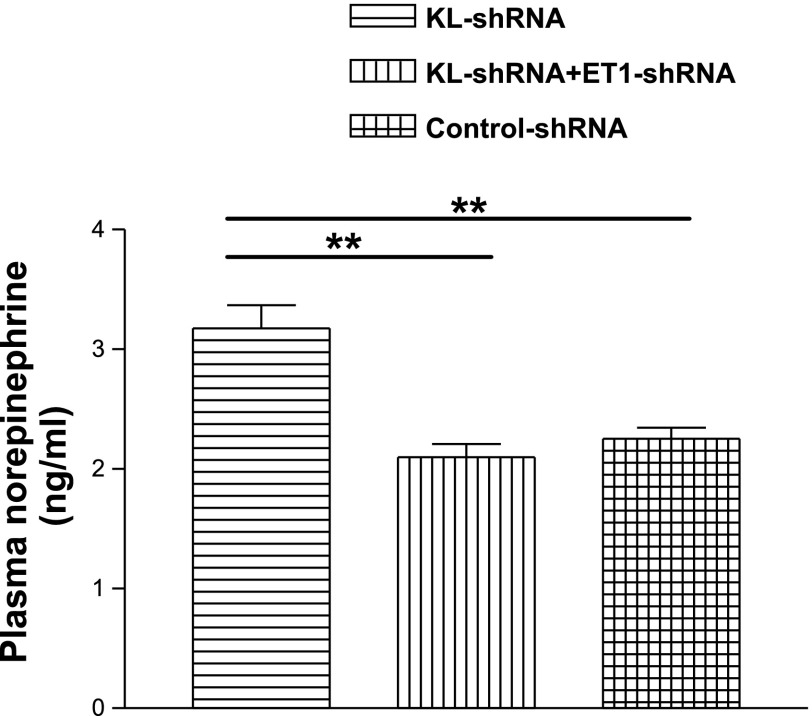
ICV injection of KL-shRNA significantly increased plasma level of NE (Fig. 5), suggesting that silencing of klotho increased the activity of the SNS. This effect was abolished by silencing of ET1.
Fig. 5.
Effects of silencing of brain klotho on plasma level of norepinephrine. Data = means ± SE. **P < 0.01, n = 6.
DISCUSSION
Our previous studies have shown that chronic exposure to cold induces hypertension in rats (10, 36–37, 39). The speed and magnitude of the development of CIH are dependent on the age and body weight at the time when the animals are exposed to cold (32). The development of CIH is delayed in animals with large body weights (32). Indeed, the present study showed that BP did not increase in the Control-shRNA group (body wt ≈400 g) by 12 days of exposure to cold. It is expected that BP in the Control-shRNA group will be elevated by a longer period of exposure to cold. Notably, silencing of brain klotho potentiated and expedited the cold-induced elevation of BP although it did not alter BP at RTs. This finding is both interesting and significant because it discloses a novel role of brain klotho in the stress-induced increase in BP. As indicated in the Introduction, cold temperatures have adverse effects on the human cardiovascular system (2, 6, 14, 33). Cold exposure increases severity of hypertension and triggers stroke and myocardial infarction (2, 14, 33). Klotho is a recently discovered antiaging gene (20–21). Genetic mutation of klotho results in a variety of aging phenotypes and shortens lifespan (21, 41). In humans, the klotho level decreases with age (44), whereas the prevalence of hypertension increases with age (4, 27), suggesting a possible link between klotho and hypertension. Therefore, further studies are warranted to determine if silencing of brain klotho increases the magnitude of CIH following a longer period of exposure to cold.
It is interesting that silencing of klotho in brain CP and ependyma resulted in upregulation of ET1 expression in these regions. This study reveals, for the first time, that there may exist cross talk between klotho and the ET system. Immunohistochemical analysis further demonstrated that klotho and ETA and ETB receptors were localized in the CP, providing a basis for the functional connection of klotho and the ET system. Importantly, the upregulation of ET1 expression may mediate the potentiating effect of KL-shRNA on cold-induced elevation of BP because it could be abolished by silencing of ET1. It is also notable that silencing of ET1 abolished the inhibitory effect of KL-shRNA on klotho expression with the mechanism to be determined. This result is unexpected because KL-shRNA and ET1-shRNA are specific for klotho and ET1, respectively. KL-shRNA and ET1-shRNA should not interfere with each other based on the specific genetic designs. The possible explanation is that silencing of ET1 may increase klotho expression through a mechanism that is independent of the RNAi of klotho. It is known that ET1 increases vascular NADPH oxidase activity and oxidative stress (7), which could suppress klotho expression (41). Knockdown of ET1 decreases the vascular oxidative stress (41) level, which may increase klotho mRNA expression even in the presence of KL-shRNA. Further studies are needed to test this hypothesis. The innovative aspect of this study is that it disclosed a previously unknown relationship between klotho and ET1 in the brain CP.
The present study demonstrated that silencing of klotho in the CP increased the activity of the SNS as evidenced by the increased plasma level of NE. It is known that the sympathetic activation plays a critical role in the development of CIH (29, 36–37). The KL-shRNA-induced activation of the SNS may be mediated by its stimulating effect on ET1 because simultaneous inhibition of ET-1 in the CP abolished KL-shRNA-induced potentiation on sympathetic activation and BP elevation. Indeed, silencing of the CP klotho increased ET1 production from the CP epithelial and ependymal cells. The CP-derived ET1 could reach, via cerebral spinal fluid (CSF), brain areas related to the regulation of SNS and BP. It has been reported that injection of ET1 into the CSF via ICV resulted in significant increases in the SNS activity and BP (25–26, 45), suggesting that ET1 in the CSF may activate the sympathetic and cardiovascular centers. It is known that high levels of brain ET1 are related to the increased sympathetic nerve activity (9, 13, 16, 19, 24, 28). ET1 exerts its function via ETA and ETB receptors. Injection of ET-1 into the CSF increases the SNS activity and BP via ETA receptors but not ETB receptors (26). The potentiating effect of KL-shRNA on the SNS and BP may be due to its stimulating effect on ET1 in the CP because klotho is not expressed in other brain regions or neurons. Further studies are needed to determine what brain areas (specific brain regions) are involved in the effect of CP-derived ET-1. Nevertheless, this study reveals, for the first time, that klotho in the CP via ET-1 may be involved in the regulation of sympathetic and cardiovascular activities in cold-exposed rats, which warrants detailed mechanistic investigation.
Perspectives
The present study demonstrated, for the first time, that silencing of brain klotho potentiated and expedited cold-induced elevation of BP. This study further revealed that the potentiating effect was mediated by upregulation of ET1 expression. This finding suggests that a decrease in brain klotho may facilitate stress-related hypertension. Klotho is an antiaging gene that declines with age, whereas the prevalence of hypertension increases with age. Therefore, it is important to study role of klotho in the pathogenesis of hypertension. A further study is required to evaluate if silencing of brain klotho increases the magnitude of the development of CIH following a longer period of exposure to cold.
GRANTS
This work was supported by National Institutes of Health Grant R01-NHLBI-077490.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Arjona-Castro A, Arjona A. [Cerebrovascular stroke, the cause of the death of the caliph al-Hakam II]. Neurologia 12: 78–81, 1997. [PubMed] [Google Scholar]
- 2. Baker-Blocker A. Winter weather and cardiovascular mortality in Minneapolis-St. Paul. Am J Public Health 72: 261–265, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brennan PJ, Greenberg G, Miall WE, Thompson SG. Seasonal variation in arterial blood pressure. Br Med J (Clin Res Ed) 285: 919–923, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 25: 305–313, 1995. [DOI] [PubMed] [Google Scholar]
- 5. Chen ZY, Chang SF, Su CL. Weather and stroke in a subtropical area: Ilan, Taiwan. Stroke 26: 569–572, 1995. [DOI] [PubMed] [Google Scholar]
- 6. Crawford VL, Stout RW. Seasonal variations in coronary heart disease. QJM 93: 639, 2000. [DOI] [PubMed] [Google Scholar]
- 7. Dammanahalli KJ, Sun Z. Endothelins and NADPH oxidases in the cardiovascular system. Clin Exp Pharmacol Physiol 35: 2–6, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Donaldson GC, Robinson D, Allaway SL. An analysis of arterial disease mortality and BUPA health screening data in men, in relation to outdoor temperature. Clin Sci (Lond) 92: 261–268, 1997. [DOI] [PubMed] [Google Scholar]
- 9. Eguchi S, Hirata Y, Imai T, Kanno K, Akiba T, Sakamoto A, Yanagisawa M, Masaki T, Marumo F. Endothelin receptors in human parathyroid gland. Biochem Biophys Res Commun 184: 1448–1455, 1992. [DOI] [PubMed] [Google Scholar]
- 10. Fregly MJ, Kikta DC, Threatte RM, Torres JL, Barney CC. Development of hypertension in rats during chronic exposure to cold. J Appl Physiol 66: 741–749, 1989. [DOI] [PubMed] [Google Scholar]
- 11. Fu S, Cao Y, Li Y, Li F, Peng Y, Dong L, He Y. Hypertensive epidemiology in Heilongjiang Province in China. Chin Med J (Engl) 115: 498–501, 2002. [PubMed] [Google Scholar]
- 12. Fujiwara T, Kawamura M, Nakajima J, Adachi T, Hiramori K. Seasonal differences in diurnal blood pressure of hypertensive patients living in a stable environmental temperature. J Hypertens 13: 1747–1752, 1995. [PubMed] [Google Scholar]
- 13. Furuya S, Hiroe T, Ogiso N, Ozaki T, Hori S. Localization of endothelin-A and -B receptors during the postnatal development of rat cerebellum. Cell Tissue Res 305: 307–324, 2001. [DOI] [PubMed] [Google Scholar]
- 14. Gyllerup S, Lanke J, Lindholm LH, Schersten B. Cold climate is an important factor in explaining regional differences in coronary mortality even if serum cholesterol and other established risk factors are taken into account. Scott Med J 38: 169–172, 1993. [DOI] [PubMed] [Google Scholar]
- 15. Gyllerup S, Lanke J, Lindholm LH, Schersten B. High coronary mortality in cold regions of Sweden. J Intern Med 230: 479–485, 1991. [DOI] [PubMed] [Google Scholar]
- 16. Hagiwara H, Nagasawa T, Yamamoto T, Lodhi KM, Ito T, Takemura N, Hirose S. Immunochemical characterization and localization of endothelin ETB receptor. Am J Physiol Regul Integr Comp Physiol 264: R777–R783, 1993. [DOI] [PubMed] [Google Scholar]
- 17. Hata T, Ogihara T, Maruyama A, Mikami H, Nakamaru M, Naka T, Kumahara Y, Nugent CA. The seasonal variation of blood pressure in patients with essential hypertension. Clin Exp Hypertens A 4: 341–354, 1982. [DOI] [PubMed] [Google Scholar]
- 18. Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565: 143–147, 2004. [DOI] [PubMed] [Google Scholar]
- 19. Kohzuki M, Chai SY, Paxinos G, Karavas A, Casley DJ, Johnston CI, Mendelsohn FA. Localization and characterization of endothelin receptor binding sites in the rat brain visualized by in vitro autoradiography. Neuroscience 42: 245–260, 1991. [DOI] [PubMed] [Google Scholar]
- 20. Kuro-o M, Hanaoka K, Hiroi Y, Noguchi T, Fujimori Y, Takewaki S, Hayasaka M, Katoh H, Miyagishi A, Nagai R. Salt-sensitive hypertension in transgenic mice overexpressing Na(+)-proton exchanger. Circ Res 76: 148–153, 1995. [DOI] [PubMed] [Google Scholar]
- 21. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997. [DOI] [PubMed] [Google Scholar]
- 22. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct 29: 91–99, 2004. [DOI] [PubMed] [Google Scholar]
- 24. Ling GY, Cao WH, Onodera M, Ju KH, Kurihara H, Kurihara Y, Yazaki Y, Kumada M, Fukuda Y, Kuwaki T. Renal sympathetic nerve activity in mice: comparison between mice and rats and between normal and endothelin-1 deficient mice. Brain Res 808: 238–249, 1998. [DOI] [PubMed] [Google Scholar]
- 25. Matsumura K, Abe I, Tsuchihashi T, Tominaga M, Kobayashi K, Fujishima M. Central effect of endothelin on neurohormonal responses in conscious rabbits. Hypertension 17: 1192–1196, 1991. [DOI] [PubMed] [Google Scholar]
- 26. Nakamura K, Sasaki S, Moriguchi J, Morimoto S, Miki S, Kawa T, Itoh H, Nakata T, Takeda K, Nakagawa M. Central effects of endothelin and its antagonists on sympathetic and cardiovascular regulation in SHR-SP. J Cardiovasc Pharmacol 33: 876–882, 1999. [DOI] [PubMed] [Google Scholar]
- 27. Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension 49: 69–75, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Ouchi Y, Kim S, Souza AC, Iijima S, Hattori A, Orimo H, Yoshizumi M, Kurihara H, Yazaki Y. Central effect of endothelin on blood pressure in conscious rats. Am J Physiol Heart Circ Physiol 256: H1747–H1751, 1989. [DOI] [PubMed] [Google Scholar]
- 29. Papanek PE, Wood CE, Fregly MJ. Role of the sympathetic nervous system in cold-induced hypertension in rats. J Appl Physiol 71: 300–306, 1991. [DOI] [PubMed] [Google Scholar]
- 30. Rossi NF, Maliszewska-Scislo M. Role of paraventricular nucleus vasopressin V1A receptors in the response to endothelin 1 activation of the subfornical organ in the rat. J Physiol Pharmacol 59, Suppl 8: 47–59, 2008. [PMC free article] [PubMed] [Google Scholar]
- 31. Rubanyi GM, Botelho LH. Endothelins. FASEB J 5: 2713–2720, 1991. [DOI] [PubMed] [Google Scholar]
- 32. Shechtman O, Fregly MJ, Papanek PE. Factors affecting cold-induced hypertension in rats. Proc Soc Exp Biol Med 195: 364–368, 1990. [DOI] [PubMed] [Google Scholar]
- 33. Sheth T, Nair C, Muller J, Yusuf S. Increased winter mortality from acute myocardial infarction and stroke: the effect of age. J Am Coll Cardiol 33: 1916–1919, 1999. [DOI] [PubMed] [Google Scholar]
- 34. Sun Z. Genetic AVP deficiency abolishes cold-induced diuresis but does not attenuate cold-induced hypertension. Am J Physiol Renal Physiol 290: F1472–F1477, 2006. [DOI] [PubMed] [Google Scholar]
- 35. Sun Z, Bello-Roufai M, Wang X. RNAi inhibition of mineralocorticoid receptors prevents the development of cold-induced hypertension. Am J Physiol Heart Circ Physiol 294: H1880–H1887, 2008. [DOI] [PubMed] [Google Scholar]
- 36. Sun Z, Cade R, Zhang Z, Alouidor J, Van H. Angiotensinogen gene knockout delays and attenuates cold-induced hypertension. Hypertension 41: 322–327, 2003. [DOI] [PubMed] [Google Scholar]
- 37. Sun Z, Fregly MJ, Cade JR. Effect of renal denervation on elevation of blood pressure in cold-exposed rats. Can J Physiol Pharmacol 73: 72–78, 1995. [DOI] [PubMed] [Google Scholar]
- 38. Wang X, Cade R, Sun Z. Expression of human eNOS in cardiac and endothelial cells. Methods Mol Med 112: 91–107, 2005. [DOI] [PubMed] [Google Scholar]
- 39. Wang X, Cade R, Sun Z. Human eNOS gene delivery attenuates cold-induced elevation of blood pressure in rats. Am J Physiol Heart Circ Physiol 289: H1161–H1168, 2005. [DOI] [PubMed] [Google Scholar]
- 40. Wang X, Skelley L, Cade R, Sun Z. AAV delivery of mineralocorticoid receptor shRNA prevents progression of cold-induced hypertension and attenuates renal damage. Gene Ther 13: 1097–1103, 2006. [DOI] [PubMed] [Google Scholar]
- 41. Wang Y, Sun Z. Current understanding of klotho. Ageing Res Rev 8: 43–51, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 54: 810–817, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xiao NM, Zhang YM, Zheng Q, Gu J. Klotho is a serum factor related to human aging. Chin Med J (Engl) 117: 742–747, 2004. [PubMed] [Google Scholar]
- 45. Yamamoto T, Kimura T, Ota K, Shoji M, Inoue M, Sato K, Ohta M, Yoshinaga K. Central effects of endothelin-1 on vasopressin and atrial natriuretic peptide release and cardiovascular and renal function in conscious rats. J Cardiovasc Pharmacol 17, Suppl 7: S316–318, 1991. [DOI] [PubMed] [Google Scholar]
- 46. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.