To the Editor
Noninvasive monitoring of cardiac gene therapy is critical to fully understand the biology of gene therapy in living subjects. We and others have monitored reporter gene expression in the myocardium of small (1) and large (2) animals (reviewed in reference 3). However, before these strategies are translated to the clinic, it is critical that they be tested using minimally invasive gene delivery approaches similar to those used clinically.
We tested the hypothesis that reporter genes can be delivered using a minimally invasive strategy to the myocardium of a swine, and expression can then be imaged using combined positron emission tomography-computed tomography (PET-CT).
Stanford’s Animal Care and Use Committee approved all procedures. Six domestic pigs (Pork Power Farms, Turlock, California) were used in the study. With sterile technique, 8-F vascular sheaths placed in the carotid arteries were used for vascular access. Percutaneous endomyocardial delivery systems (Biocardia, Inc., South San Francisco, California) (Fig. 1, left panel), placed into the sheaths, were used for fluoroscopy-guided delivery of genes (1010 plaque-forming units) or vehicle (phosphate-buffered saline [PBS]) (Fig. 1, right panel) in 3 doses of 0.2 cc each. Central mean arterial pressure (MAP) was measured. Forty-eight hours after gene delivery, animals were dynamically imaged after intravenous administration of 18F-labeled 9-[4-fluoro-3-(hydroxymethyl)butyl]guanine (18F-FHBG; tracer) (4) using a clinical combined PET-CT system (Discovery LS, GE Medical Systems, Milwaukee, Wisconsin) for a total scanning time of 180 min. Data are expressed as mean ± SEM.
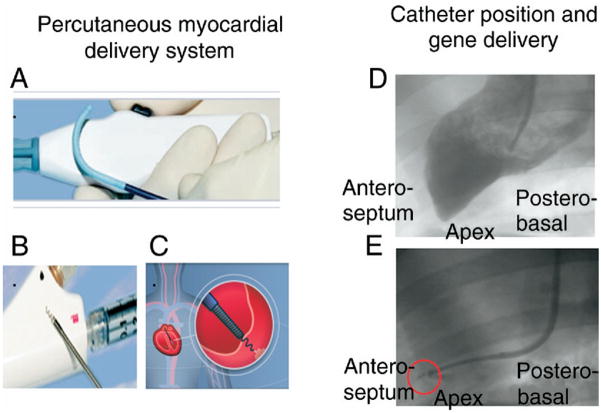
Figure 1. Fluoroscopy-Guided Delivery of Genes.
(Left) Percutaneous delivery system consisting of (A) steerable-guiding catheter and (B) helical needle infusion catheter. A steerable catheter provides maximum flexibility, allowing catheter positioning in virtually any area of the left ventricular cavity. The infusion catheter is used first to confirm intramyocardial positioning of the catheter and then for the delivery of therapeutic material. (C) The infusion catheter is “screwed’ inside the myocardium. (Right) Representative image of gene delivery in a swine model. A left ventricular angiogram is performed for delineation of the left ventricular endocardial contour (D). Intramyocardial positioning of the helical catheter is confirmed using contrast media (E), and gene therapy is then delivered.
There were no significant differences in weight (control, 37.4 ± 0.4 kg; gene therapy, 36.3 ± 0.7 kg; p = NS), MAP (control, 107 ± 11 mm Hg; gene therapy, 111 ± 14 mm Hg; p = NS), or heart rate (control, 90 ± 7 beats/min; gene therapy, 95 ± 9 beats/min; p = NS) between the 2 groups. There was no morbidity or mortality associated with the procedures. A total of 9.37 ± 1.31 mCi of 18F-FHBG (in 5 ml of PBS) was administered per animal.
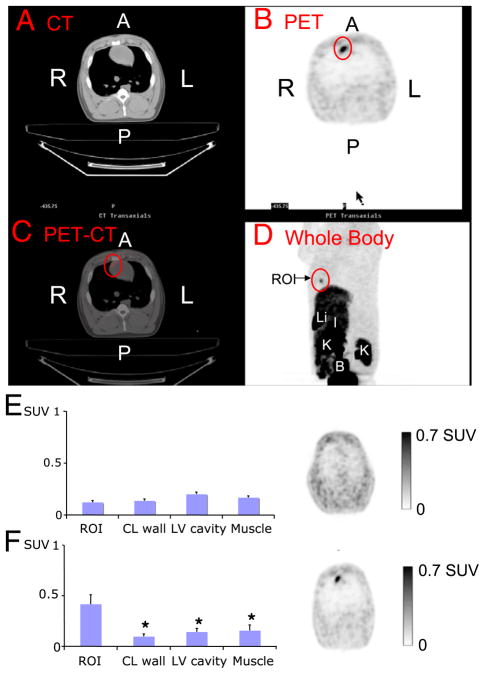
Figure 2 (top panel, A to D) shows a representative PET-CT scan of the gene therapy group. The CT images (Fig. 2A, top panel) were used for anatomic localization, and 18F-FHBG uptake (Fig. 2B, top panel) was located in the area into which gene therapy was delivered (anteroseptum) (Fig. 2C, top panel). Whole-body images (Fig. 2D, top panel) clearly showed the cardiac uptake in chest and abdominal structures.
Figure 2. Representative Positron Emission Tomography (PET) Computed Tomography (CT).
(Top) Image of gene therapy 48 h after percutaneous gene delivery (A to D). (Bottom) Uptake of 18F-labeled 9-[4-fluoro-3-(hydroxymethyl)butyl]guanine (standardized uptake value [SUV]) in (E) control (n = 2) and (F) gene therapy (n = 4) groups. *p < 0.05 compared to region of interest (ROI) (red circle). A = anterior; B = bladder; CL = contralateral; I = intestines; K = kidney; L = left; Li = liver; LV = left ventricular; P = posterior; R = right.
Animals from both groups had comparable 18F-FHBG uptake in paraspinal muscles and the nondelivered myocardial wall (Figs. 2E and 2F). Whereas control animals showed no distinct myocardial tracer uptake, experimental animals had significantly increased (p < 0.05) 18F-FHBG uptake (Figs. 2E and 2F, respectively). The best myocardial signal/background (left ventricular) ratio was obtained 3 h post-injection (180 min, 4.63 ± 1.4 vs. 90 min, 1.78 ± 0.6; p < 0.05). Autoradiography and microPET confirmed the increased 18F-FHBG uptake in the anteroseptum of the gene therapy animals.
Many different delivery methods have been developed for percutaneous cardiac delivery of gene therapy. The helical needle injection catheter system, used in this study, has the theoretic advantages of endocardial engagement and helical needle-track and has been shown to have good acute delivery success and retention (5). This delivery method has been designed to deliver material (e.g., genes, cells) to a specific and delimited area. Multiple injections or vascular-based delivery methods (e.g., intracoronary) may be more useful if the target area is a larger myocardial region or a specific coronary distribution.
Adenoviral infection results in strong, albeit relatively short-lived, transgene expression (6). For performing long-term longitudinal monitoring of therapy, other reporter gene strategies will be needed, such as adeno-associated or gutless adenovirus (7,8).
PET has nanomolar to picomolar (10−12 mol/l) sensitivity and tomographic capabilities, which makes PET the most suitable imaging modality for use in living subjects (compared with magnetic resonance and single photon emission-computed tomography) (9). Based on this study, 3 h after tracer administration appears to be a good time point for assessment of 18F-FHBG uptake in the myocardium.
These studies will play a critical role in the monitoring of gene therapy first in pre-clinical large animal models of cardiac disease and then in clinical therapeutic trials.
Acknowledgments
Please note: this study was supported by grants NCI SAIRP (to Dr. Gambhir), NHLBI R01 HL078632 (to Dr. Gambhir), NCI ICMIC CA114747 P50 (to Dr. Gambhir), NHLBI R21HL089027 (to Dr. Wu), and the Mayo Clinical Scholarship Program, Mayo Clinic College of Medicine, Rochester, Minnesota (to Dr. Rodriguez-Porcel). The authors thank Olin Palmer and Daniel Rosenman from BioCardia, Inc., for their technical assistance with the catheter delivery system and the Stanford cyclotron team (Dr. Fren Chin and Dr. David Dick) for 18F-FHBG production.
References
- 1.Wu JC, Chen IY, Wang Y, et al. Molecular imaging of the kinetics of vascular endothelial growth factor gene expression in ischemic myocardium. Circulation. 2004;110:685–91. doi: 10.1161/01.CIR.000013815302213.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bengel FM, Anton M, Richter T, et al. Noninvasive imaging of transgene expression by use of positron emission tomography in a pig model of myocardial gene transfer. Circulation. 2003;108:2127–33. doi: 10.1161/01.CIR.0000091401.26280.A0. [DOI] [PubMed] [Google Scholar]
- 3.Wu JC, Tseng JR, Gambhir SS. Molecular imaging of cardiovascular gene products. J Nucl Cardiol. 2004;11:491–505. doi: 10.1016/j.nuclcard.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Yaghoubi S, Barrio JR, Dahlbom M, et al. Human pharmacokinetic and dosimetry studies of [18F]FHBG: a reporter probe for imaging herpes simplex virus type-1 thymidine kinase reporter gene expression. J Nucl Med. 2001;42:1225–34. [PubMed] [Google Scholar]
- 5.Hou D, Youssef EA, Brinton TJ, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;12 (Suppl 9):I150–6. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 6.Min JJ, Iyer M, Gambhir SS. Comparison of [18F]FHBG and [14C]FIAU for imaging of HSV1-tk reporter gene expression: adenoviral infection vs stable transfection. Eur J Nucl Med Mol Imaging. 2003;30:1547–60. doi: 10.1007/s00259-003-1238-6. [DOI] [PubMed] [Google Scholar]
- 7.Yant SR, Ehrhardt A, Mikkelsen JG, Meuse L, Pham T, Kay MA. Transposition from a gutless adenotransposon vector stabilizes transgene expression in vivo. Nat Biotechnol. 2002;20:999–1005. doi: 10.1038/nbt738. [DOI] [PubMed] [Google Scholar]
- 8.Zou L, Zhou H, Pastore L, Yang K. Prolonged transgene expression mediated by a helper-dependent adenoviral vector (hdAd) in the central nervous system. Mol Ther. 2000;2:105–13. doi: 10.1006/mthe.2000.0104. [DOI] [PubMed] [Google Scholar]
- 9.Bengel FM, Schachinger V, Dimmeler S. Cell-based therapies and imaging in cardiology. Eur J Nucl Med Mol Imaging. 2005;32 (Suppl 2):S404–16. doi: 10.1007/s00259-005-1898-5. [DOI] [PubMed] [Google Scholar]