Abstract
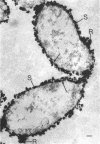
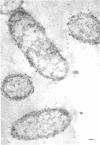
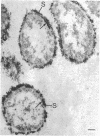
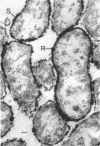
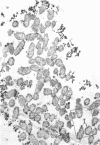
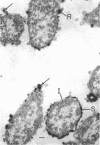
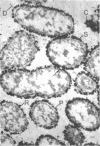
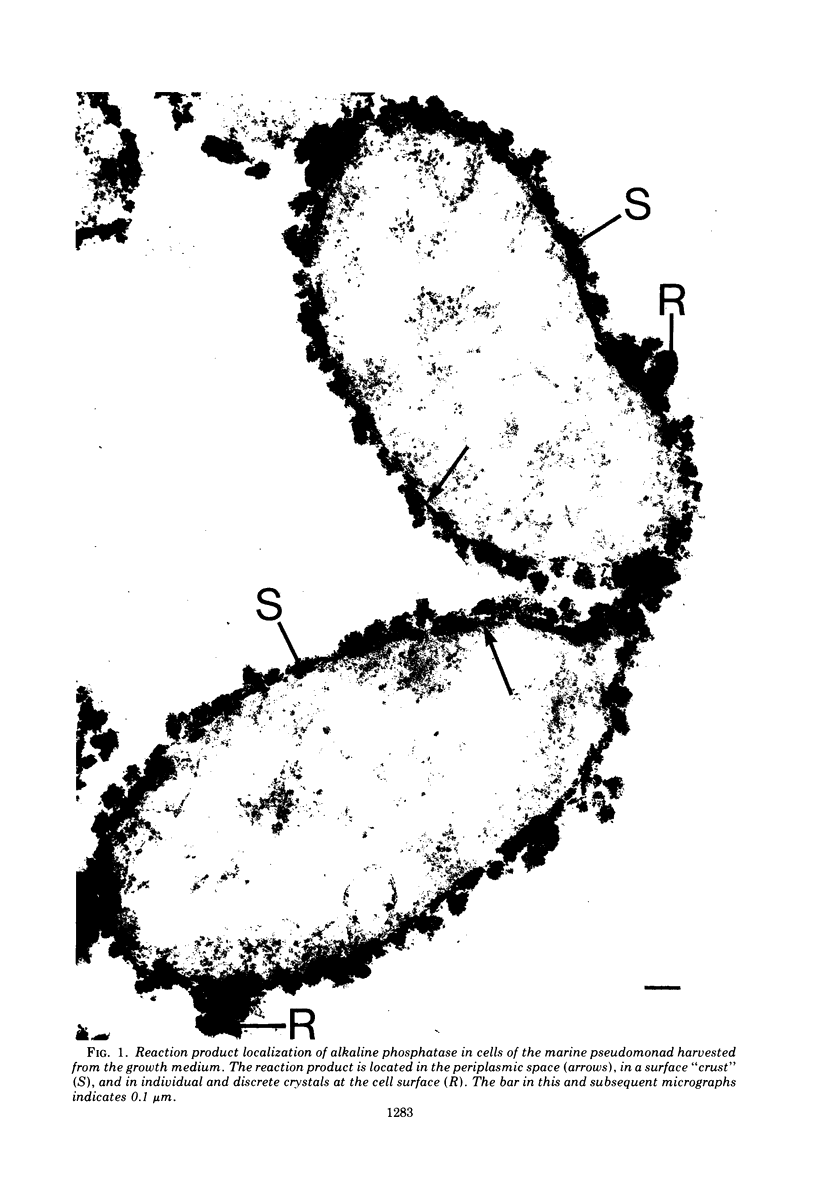
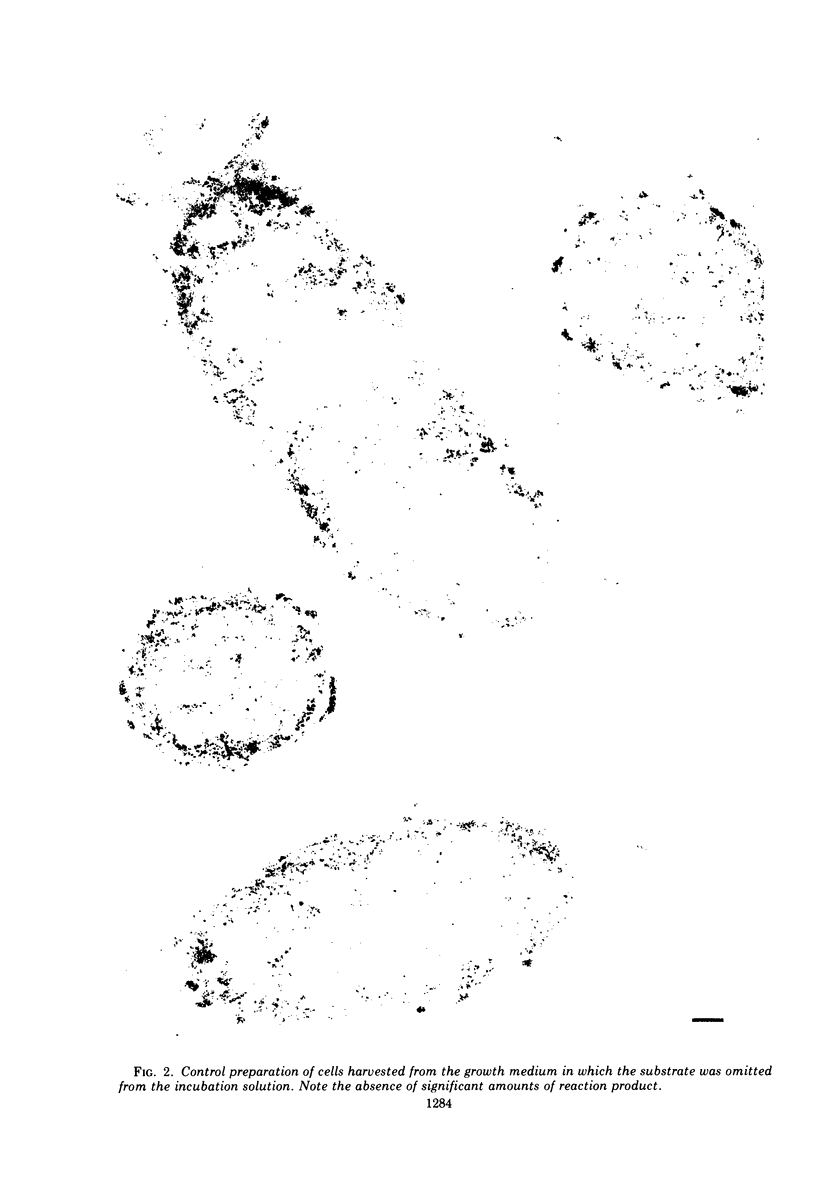
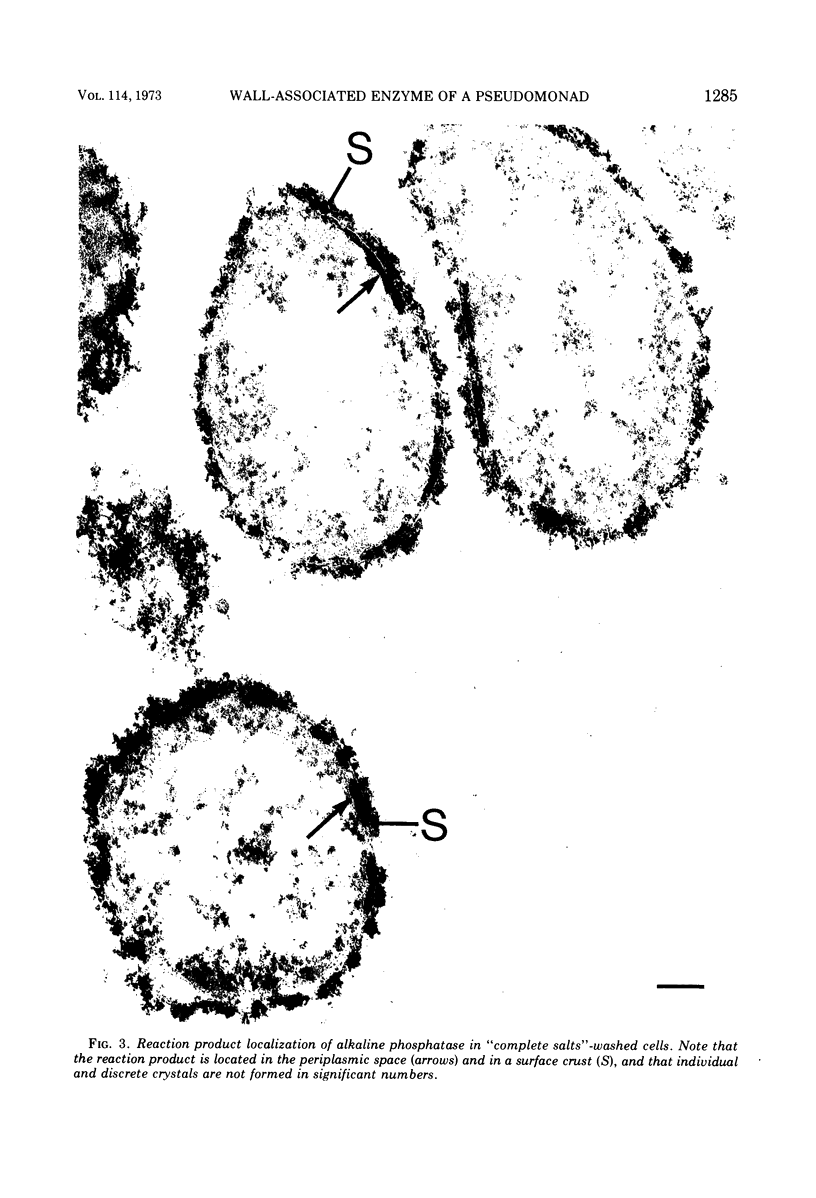
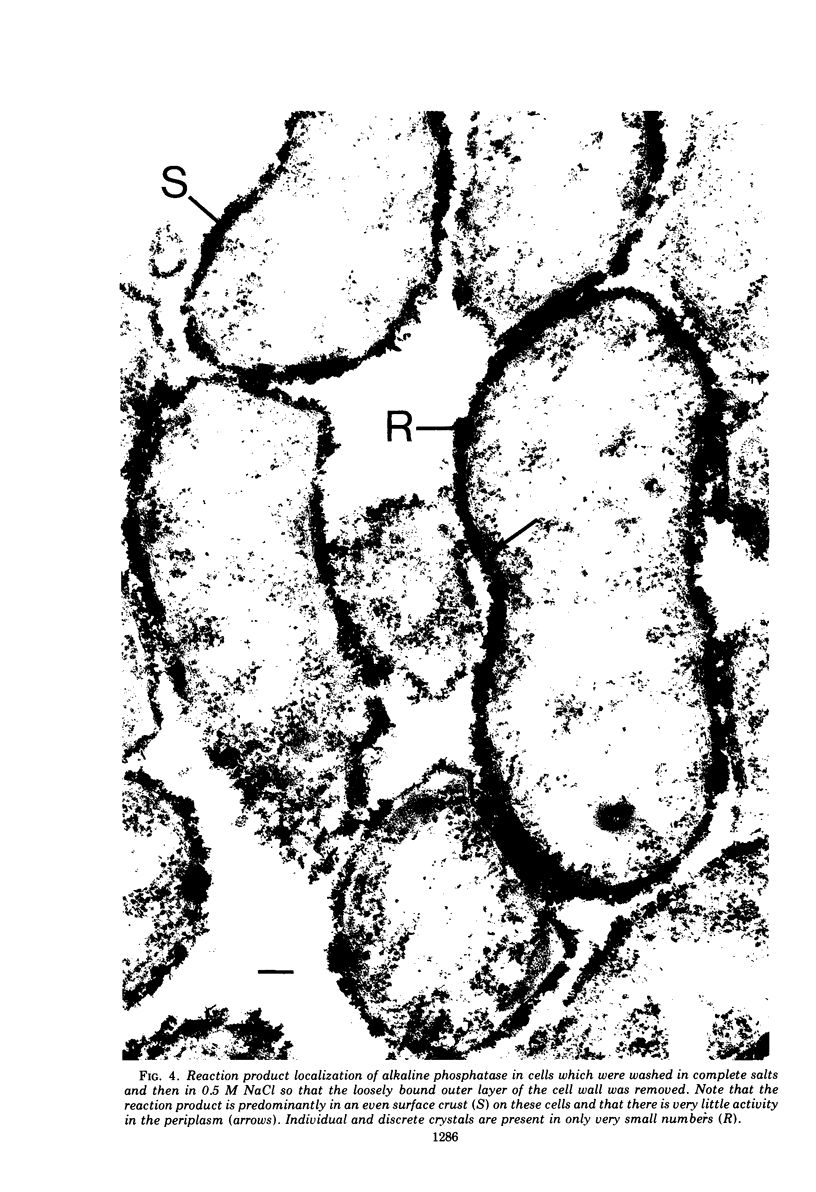
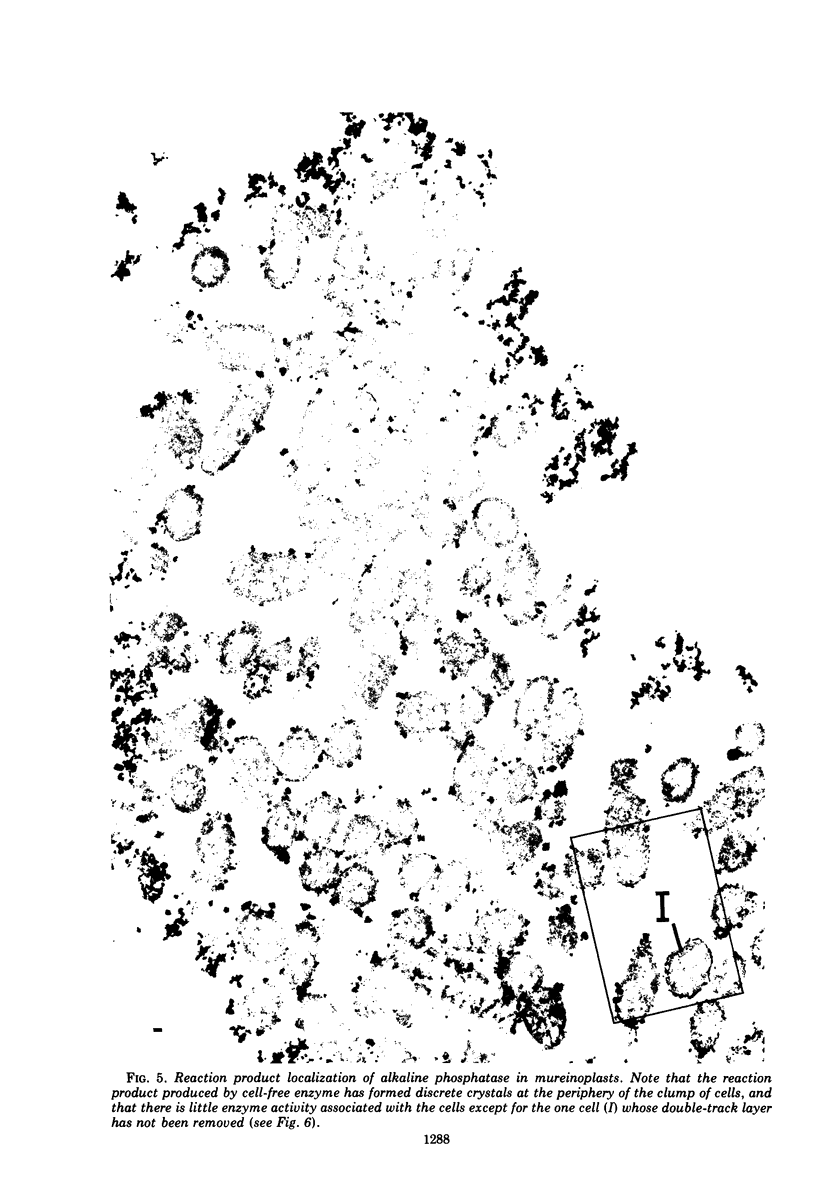
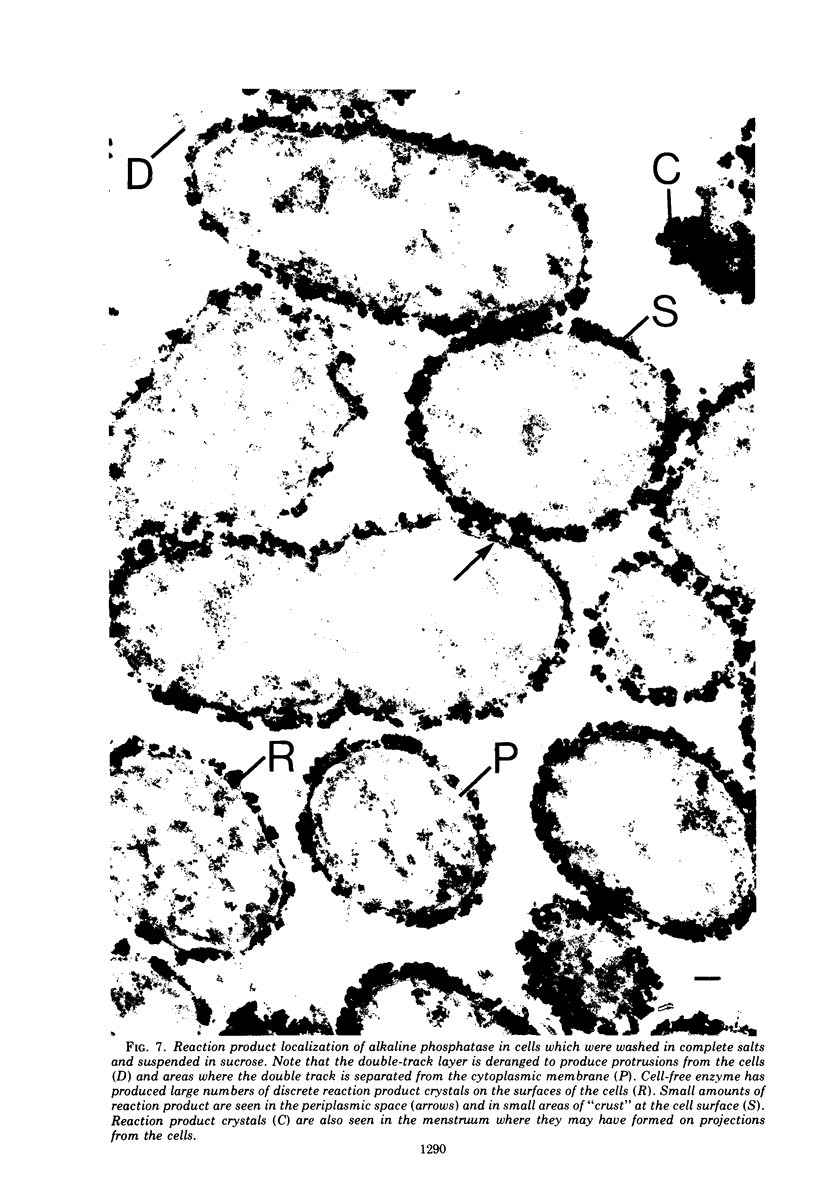
In untreated cells of the marine pseudomonad studied here, alkaline phosphatase was found to be located in the periplasmic space, at the cell surface, and in the medium into which it had been shed during growth. Washing in 0.5 M NaCl, which removed the loosely bound outer layer, caused a shift of periplasmic enzyme to the outer aspect of the double-track layer and released some of the cell surface-associated enzyme. When the double-track layer of the cell wall was partially deranged, large amounts of this cell wall-associated enzyme were released, and, when the double-track was removed from the cells to produce mureinoplasts, alkaline phosphatase was released into the menstruum. There was no significant association of the enzyme with the peptidoglycan layer of the cell wall, which is the outermost structure of the mureinoplast, and no association of the enzyme with the cytoplasmic membrane of these modified cells. This study has shown that alkaline phosphatase is specifically associated with the outer layers of the cell walls of cells of this organism and is retained within the cell wall by virtue of this association.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockman R. W., Heppel L. A. On the localization of alkaline phosphatase and cyclic phosphodiesterase in Escherichia coli. Biochemistry. 1968 Jul;7(7):2554–2562. doi: 10.1021/bi00847a016. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Day D. F., Costerton J. W., Ingram J. M. Alkaline phosphatase subunits in the culture filtrate of Pseudomonas aeruginosa. Can J Biochem. 1972 Mar;50(3):268–276. doi: 10.1139/o72-038. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Alkaline phosphatase localization and spheroplast formation of Pseudomonas aeruginosa. Can J Microbiol. 1970 Dec;16(12):1319–1324. doi: 10.1139/m70-218. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Interactions of alkaline phosphatase and the cell wall of Pseudomonas aeruginosa. J Bacteriol. 1971 Jul;107(1):325–336. doi: 10.1128/jb.107.1.325-336.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J Bacteriol. 1970 Nov;104(2):748–753. doi: 10.1128/jb.104.2.748-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Forsberg C., Matula T. I., Buckmire F. L., MacLeod R. A. Nutrition and metabolism of marine bacteria. XVI. Formation of protoplasts, spheroplasts, and related forms from a gram-negative marine bacterium. J Bacteriol. 1967 Nov;94(5):1764–1777. doi: 10.1128/jb.94.5.1764-1777.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W. The structure and function of the cell envelope of gram-negative bacteria. Rev Can Biol. 1970 Sep;29(3):299–316. [PubMed] [Google Scholar]
- Costerton J. W., Thompson J. Induced morphological changes in the stainable layers of the cell envelope of a gram-negative bacterium. Can J Microbiol. 1972 Jun;18(6):937–940. doi: 10.1139/m72-144. [DOI] [PubMed] [Google Scholar]
- De Voe I. W., Thompson J., Costerton J. W., MacLeod R. A. Stability and comparative transport capacity of cells, mureinoplasts, and true protoplasts of a gram-negative bacterium. J Bacteriol. 1970 Mar;101(3):1014–1026. doi: 10.1128/jb.101.3.1014-1026.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W., Costerton J. W., MacLeod R. A. Demonstration by freeze-etching of a single cleavage plane in the cell wall of a gram-negative bacterium. J Bacteriol. 1971 May;106(2):659–671. doi: 10.1128/jb.106.2.659-671.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Wetzel B. K., Heppel L. A. Biochemical and cytochemical evidence for the polar concentration of periplasmic enzymes in a "minicell" strain of Escherichia coli. J Bacteriol. 1970 Oct;104(1):543–548. doi: 10.1128/jb.104.1.543-548.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A., Costerton J. W., Kerr K. A. Freeze-etching and x-ray diffraction of the isolated double-track layer from the cell wall of a gram-negative marine pseudomonad. J Bacteriol. 1973 Jan;113(1):445–451. doi: 10.1128/jb.113.1.445-451.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Costerton J. W., Macleod R. A. Quantitation, chemical characteristics, and ultrastructure of the three outer cell wall layers of a gram-negative bacterium. J Bacteriol. 1970 Dec;104(3):1354–1368. doi: 10.1128/jb.104.3.1354-1368.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Costerton J. W., Macleod R. A. Separation and localization of cell wall layers of a gram-negative bacterium. J Bacteriol. 1970 Dec;104(3):1338–1353. doi: 10.1128/jb.104.3.1338-1353.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Rayman M. K., Costerton J. W., MacLeod R. A. Isolation, characterization, and ultrastructure of the peptidoglycan layer of a marine pseudomonad. J Bacteriol. 1972 Feb;109(2):895–905. doi: 10.1128/jb.109.2.895-905.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnarev V. M., Smirnova T. A. Electron microscopy of alkaline phosphatase of Escherichia coli. Can J Microbiol. 1966 Aug;12(4):605–607. doi: 10.1139/m66-086. [DOI] [PubMed] [Google Scholar]
- Lopes J., Gottfried S., Rothfield L. Leakage of periplasmic enzymes by mutants of Escherichia coli and Salmonella typhimurium: isolation of "periplasmic leaky" mutants. J Bacteriol. 1972 Feb;109(2):520–525. doi: 10.1128/jb.109.2.520-525.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlister T. J., Costerton J. W., Thompson L., Thompson J., Ingram J. M. Distribution of alkaline phosphatase within the periplasmic space of gram-negative bacteria. J Bacteriol. 1972 Sep;111(3):827–832. doi: 10.1128/jb.111.3.827-832.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisonson I., Tannenbaum M., Neu H. C. Surface localization of Escherichia coli 5'-nucleotidase by electron microscopy. J Bacteriol. 1969 Nov;100(2):1083–1090. doi: 10.1128/jb.100.2.1083-1090.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary G. P., Nelson J. D., Jr, MacLeod R. A. Requirement for salts for the isolation of lipopolysaccharide from a marine pseudomonad. Can J Microbiol. 1972 May;18(5):601–606. doi: 10.1139/m72-095. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E. L'inclusion au polyester pour l'ultramicrotomie. J Ultrastruct Res. 1958 Dec;2(2):200–214. doi: 10.1016/s0022-5320(58)90018-2. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. P., Cheng K. J., Costerton J. W., Idziak E. S., Ingram J. M. Sensitivity of normal and mutant strains of Escherichia coli to actinomycin-D. Can J Microbiol. 1972 Jun;18(6):909–915. doi: 10.1139/m72-139. [DOI] [PubMed] [Google Scholar]
- Thompson J., Costerton J. W., MacLeon R. A. K plus-dependent deplasmolysis of a marine pseudomonad plasmolyzed in a hypotonic solution. J Bacteriol. 1970 Jun;102(3):843–854. doi: 10.1128/jb.102.3.843-854.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel B. K., Spicer S. S., Dvorak H. F., Heppel L. A. Cytochemical localization of certain phosphatases in Escherichia coli. J Bacteriol. 1970 Oct;104(1):529–542. doi: 10.1128/jb.104.1.529-542.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]