Abstract
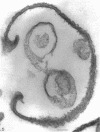
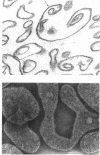
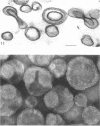
A method has been developed for the isolation of outer membranes from Acinetobacter sp. strain MJT/F5/199A. Washed cells were broken in a French press and, after deoxyribonuclease and ribonuclease treatment, removal of intact cells, and four washes in 20 mosmol phosphate buffer, pH 7.4, with centrifugation at 25,000 × g for 10 min, preparations of cell wall fragments from which almost all pieces of plasma membrane had been removed resulted. Treatment of the cell walls with lysozyme and further washing, in the presence of 20 mM MgCl2, yielded preparations of outer membranes. Electron microscopy of freeze-etched preparations shows that a regular pattern of subunits is present on the outer surfaces of intact cells. After negative staining, these subunits are visible on isolated walls and outer membranes; they can be removed by brief treatment with papain. In section, the cell wall structure is that typical of gram-negative bacteria, but the subunits are not detectable on the surface of the outer membrane. The outer membrane retains the appearance of a “unit membrane” in the cell wall, isolated outer membrane, and papain-treated outer membrane fractions. Both cell walls and outer membranes contain a high percentage of protein (76 and 84%, respectively) and not more than 5% carbohydrate, of which glucose and galactose are constitutents. The outer membranes of this Acinetobacter thus differ in structure and composition from those of bacteria in the Enterobacteriaceae.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. A., Quadling C., Yaguchi M., Tornabene T. G. The chemical composition of cell-wall lipopolysaccharides from Moraxella duplex and Micrococcus calco-aceticus. Can J Microbiol. 1970 Jan;16(1):1–8. doi: 10.1139/m70-001. [DOI] [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- Barker D. C., Thorne K. J. Spheroplasts of Lactobacillus casei and the cellular distribution of bactoprenol. J Cell Sci. 1970 Nov;7(3):755–785. doi: 10.1242/jcs.7.3.755. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. 1. Isolation and partial purification of the outermost cell wall layer. Can J Microbiol. 1970 Oct;16(10):1011–1022. doi: 10.1139/m70-171. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Ellar D. J., Muñoz E., Salton M. R. The effect of low concentrations of glutaraldehyde on Micrococcus lysodeikticus membranes: changes in the release of membrane-associated enzymes and membrane structure. Biochim Biophys Acta. 1971 Jan 5;225(1):140–150. doi: 10.1016/0005-2736(71)90292-6. [DOI] [PubMed] [Google Scholar]
- FISCHER F. G., DORFEL H. Die papierchromatographische Trennung und Bestimmung der Uronsäuren. Hoppe Seylers Z Physiol Chem. 1955 Sep 2;301(4-6):224–234. [PubMed] [Google Scholar]
- Garrard W. T. Selective release of proteins from Spirillum itersonii by tris (hydroxymethyl) aminomethane and ethylenediaminetetraacetate. J Bacteriol. 1971 Jan;105(1):93–100. doi: 10.1128/jb.105.1.93-100.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Gray G. W., Wilkinson S. G. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J Gen Microbiol. 1965 Jun;39(3):385–399. doi: 10.1099/00221287-39-3-385. [DOI] [PubMed] [Google Scholar]
- Henriksen S. D., Bovre K. The taxonomy of the genera Moraxella and Neisseria. J Gen Microbiol. 1968 May;51(3):387–392. doi: 10.1099/00221287-51-3-387. [DOI] [PubMed] [Google Scholar]
- Jermyn M. A., Isherwood F. A. Improved separation of sugars on the paper partition chromatogram. Biochem J. 1949;44(4):402–407. doi: 10.1042/bj0440402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Anderson R. S., Ordal E. J. Nucleic acid homologies among oxidase-negative Moraxella species. J Bacteriol. 1970 Feb;101(2):568–573. doi: 10.1128/jb.101.2.568-573.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOVACS N. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature. 1956 Sep 29;178(4535):703–703. doi: 10.1038/178703a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation and properties of outer and cytoplasmic membranes in Escherichia coli. Biochim Biophys Acta. 1969;193(2):268–276. doi: 10.1016/0005-2736(69)90188-6. [DOI] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation by density gradient centrifugation of two types of membranes from spheroplast membrane of Escherichia coli K12. Biochim Biophys Acta. 1968 Jan 3;150(1):159–161. doi: 10.1016/0005-2736(68)90020-5. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B. Fracture faces in intact cells and protoplasts of Bacillus stearothermophilus. A study by conventional freeze-etching and freeze-etching of corresponding fracture moieties. Protoplasma. 1970;71(3):295–312. doi: 10.1007/BF01279638. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Takacs B. J., Holt S. C. Thiocapsa floridana; a cytological, physical and chemical characterization. I. Cytology of whole cells and isolated chromatophore membranes. Biochim Biophys Acta. 1971 Apr 13;233(2):258–277. doi: 10.1016/0005-2736(71)90325-7. [DOI] [PubMed] [Google Scholar]
- Thornley M. J. A taxonomic study of Acinetobacter and related genera. J Gen Microbiol. 1967 Nov;49(2):211–257. doi: 10.1099/00221287-49-2-211. [DOI] [PubMed] [Google Scholar]
- Thornley M. J., Glauert A. M. Fine structure and radiation resistance in Acinetobacter: studies on a resistant strain. J Cell Sci. 1968 Jun;3(2):273–294. doi: 10.1242/jcs.3.2.273. [DOI] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Release of membrane components from viable Haemophilus parainfluenzae by ethylenediaminetetraacetic acid-tris(hydroxymethyl)-aminomethane. J Bacteriol. 1970 May;102(2):498–507. doi: 10.1128/jb.102.2.498-507.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Remsen C. C. Cell envelope of Nitrosocystis oceanus. J Ultrastruct Res. 1970 Oct;33(1):148–160. doi: 10.1016/s0022-5320(70)90122-x. [DOI] [PubMed] [Google Scholar]
- White D. A., Lennarz W. J., Schnaitman C. A. Distribution of lipids in the wall and cytoplasmic membrane subfractions of the cell envelope of Escherichia coli. J Bacteriol. 1972 Feb;109(2):686–690. doi: 10.1128/jb.109.2.686-690.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]