Abstract
Objective
We examined associations between vitamin C intake and serum uric acid in a population-based study.
Methods
We included 1,387 men without hypertension, and Body Mass Index <30 kg/m2 in the Health Professional Follow-up Study. Dietary intake was assessed with a semi-quantitative food frequency questionnaire validated for use in this population. Serum uric acid concentrations were measured.
Results
Greater intakes of total vitamin C were significantly associated with lower serum uric acid concentrations, after adjustment for smoking, BMI, ethnicity, blood pressure, presence of gout, use of aspirin, and intake of energy, alcohol, dairy protein, fructose, meat, seafood, and coffee. An inverse dose-response association was observed through vitamin C intake of 400–500 mg/d, and then reached a plateau. Adjusted mean uric acid concentrations across total vitamin C intake categories (<90, 90–249, 250–499, 500–999, or ≥ 1000 mg/d) were 6.4, 6.1, 6.0, 5.7, and 5.7 mg/dl, respectively (P for trend<0.001). Greater vitamin C intake was associated with lower prevalence of hyperuricemia (serum uric acid > 6 mg/dl). The multivariate ORs for hyperuricemia across total vitamin C intake categories were 1 (reference), 0.58, 0.57, 0.38, and 0.34 (95% CI: 0.20–0.58; P-trend< 0.001). When we used dietary data, which were assessed 4–8 years before blood collection, as predictors, we observed similar inverse associations between vitamin C intake and uric acid.
Conclusions
These population-based data indicate that vitamin C intake is inversely associated with serum uric acid concentrations. These findings support a potential role of vitamin C in the prevention of hyperuricemia and gout.
Keywords: gout, diet, epidemiology
Hyperuricemia is considered a precursor of gout, which is the most common inflammatory arthritis in adult men (1). Among the potentially useful protective factors against hyperuricemia and gout, vitamin C is an essential micronutrient for humans. Previous metabolic experiments have shown that high-dose vitamin C supplementation (3+ g/d) lowers serum uric acid via a uricosuric effect (2–4). This effect may be due to competition for renal reabsorption via an anion-exchange transport system in the proximal tubules (4, 5). Recently, a double-blinded placebo-controlled randomized trial (n=184) showed that supplementation with vitamin C as low as 500 mg daily for two months reduced serum uric acid by 0.5mg/dl, compared to no change in the placebo group (6). However, no population-based study has investigated whether vitamin C intake is associated with serum uric acid levels. Further, most of trials used a single large dose of vitamin C, therefore, it is unclear whether there is a dose-response relationship between vitamin C intake and uric acid concentrations. We therefore examined associations between vitamin C intake, assessed with a semi-quantitative food frequency questionnaire, and serum uric acid in a sub-sample of the Health Professional Follow-up Study (HPFS) 2, a large ongoing cohort of US men.
Materials and Methods
Study population
The HPFS is a large and well-characterized prospective cohort designed to study the association between diet and chronic diseases. The HPFS was established in 1986, when 51,529 male US health professionals (dentists, optometrists, osteopaths, podiatrists, pharmacists, and veterinarians) aged 40–75 years completed a mailed questionnaire about their medical history and lifestyle. Dietary intake data have been collected since 1986 and updated every four years. Follow-up questionnaires have been mailed to participants every 2 years to update information on potential risk factors and to ascertain newly diagnosed diseases. Blood samples were collected in 1993 and 1994, as previously described (7) and 18,025 men contributed blood samples that were stored in liquid nitrogen (−130°C). In the current study, we used data from a sub-sample of 1,387 HPFS participants previously selected for a prospective nested case-control study of serum uric acid and hypertension among men with available blood samples and without prevalent hypertension in 1994 (approximately 1yr after blood samples were collected) (8). Criteria for inclusion in the case-control study were: 1) blood sample drawn after fasting ≥ 8 hours; 2) body mass index (BMI) < 30 kg/m2 in 1994; and 3) no history of hypertension in 1994. The BMI restriction was imposed because obesity is a strong predictor of UA level and is a powerful predictor of hypertension and because the association between UA and hypertension may be modified by obesity (8).
Assessment of dietary and non-dietary exposures
Dietary intakes were assessed with a semi-quantitative food frequency questionnaire (FFQ) validated for use in this population (9, 10). In brief, participants were asked how often on average over the previous year they had consumed a specific amount of each food item with nine possible responses ranging from “never” to “six or more times per day”. Food composition values for nutrients were obtained from the Harvard University Food Composition Database derived from US Department of Agriculture sources (11). For supplemental vitamin C, respondents chose from the following categories: 0, 1 to 399, 400 to 700, 750 to 1250, and 1300 mg or more daily. The amount of vitamin C in multivitamin preparations was determined by the brand, type, and frequency of reported use. The correlations between the intakes measured by a food frequency questionnaire and diet records were 0.86 for total vitamin C and 0.68 for dietary vitamin C intake. (9, 10) Information on age, ethnicity, weight, height, smoking status, presence of gout, blood pressure, and use of aspirin was collected through questionnaires. BMI was calculated as weight (kg)/height (m) 2.
Assessment of serum uric acid
Uric acid concentrations were determined by oxidization with the specific enzyme uricase to form allantoin and H2O2 (Roche Diagnostics, Indianapolis, IN) at Boston Children’s Hospital Laboratory (Nader Rifai, director). The coefficient of variation using blind quality control specimens was 2.7%. Hyperuricemia was defined as > 6mg/dL (360 μmol/L) (12).
Statistical analyses
All statistical analyses were completed with SAS 9.1 (SAS Institute, Inc, Cary, NC). We categorized vitamin C intake into five groups as we did previously, for total vitamin C, i.e., vitamin C from both food and supplements: <90, 90–249, 250–499, 500–999, or ≥ 1000 mg/d; for dietary vitamin C, i.e., vitamin C from food alone: <50, 50–99, 100–199, 200–299, or ≥ 300 mg/d; and for vitamin C supplement: 0, 1–249, 250–499, 500–999, or ≥ 1000 mg/d. (13) We used the General Linear Models procedure to compare mean differences in levels of serum uric acid across vitamin C intake categories, with Duncan adjustment for multiple comparisons (the lowest category as reference) (14). Logistic regression was used to test differences in prevalence of hyperuricemia across vitamin C intake categories and to calculate odds ratios (ORs) and 95% confidence intervals (CIs). We adjusted for age (<60, 60–64, 65–69, 70–74, ≥ 75 y), smoking (never, past, current: 1–14, or ≥ 15 cigarettes/d), BMI (<23, 23–24.9, 25–26.9, 27–28.9, or ≥ 29 kg/m2, ethnicity (Caucasian vs. others), systolic blood pressure (<105, 105–114, 115–124, or ≥125 mm Hg), presence of gout (yes/no), use of aspirin (yes/no), as well as intake of total energy (Kcal/d), alcohol (0, <5, 5–9, 10–14, 15–29, 30–49, or ≥ 50 g/d), fructose (g/d), dairy protein (g/d), meat (servings/d), seafood (servings/d), and coffee (0, <1, 1–3, 4–5, or ≥ 6 cups/d). We also examined potential interactions of vitamin C intake with age (<60 versus ≥ 60 y in 1994), alcohol (none versus >0 g/d), smoking status (never versus ever), and BMI (<25 versus ≥ 25 kg/m2). To test significance for interaction, we included multiplicative terms in the linear regression models, with adjustment for other potential confounders. The continuous measure of vitamin C intake was used to fit a restricted cubic spline model and to obtain a smooth representation of the OR as a function of vitamin C intake with adjustment for the effects of potential confounders (15). We used 4 knots to divide continuous vitamin C intake into 5 intervals.
In primary analyses, we used dietary intakes collected in 1994 as exposures to examine the cross-sectional relationship between vitamin C intake and serum uric acid concentrations. In secondary analyses, we used average of diets collected in 1986 and 1990 as exposures to reflect long-term dietary intake patterns. All P-values are two sided.
Results
Participants with higher total vitamin C intake were more likely to had lower BMI and lower intake of total meat and coffee, had higher intake of fructose, alcohol, and seafood, and were more likely to use aspirin, and less likely to be current smokers, and Caucasian, relative to those in the lowest intake quartile (Table 1). There was no clear relationship observed between vitamin C intake and other characteristics.
Table 1.
Characteristics according to vitamin C intake in a sub-sample of the Health Professionals Follow-up Study in 1994 (n=1,387)
| Total vitamin C intake, mg/d | |||||
|---|---|---|---|---|---|
| <90 | 90–249 | 250–499 | 500–999 | ≥1000 | |
| N | 96 | 605 | 245 | 214 | 227 |
| Dietary vitamin C intake1, mg/d | 72.0 | 145 | 204 | 180 | 181 |
| Use of vitamin C supplement, % | 8.5 | 38.1 | 81.2 | 99.4 | 100 |
| Vitamin C supplement1, mg/d | 1.2 | 20.8 | 131 | 513 | 1263 |
| Age1, y | 59.3 | 61.2 | 61.7 | 61.6 | 60.7 |
| Current smokers, % | 12.5 | 5.1 | 3.7 | 4.2 | 5.8 |
| Past smokers, % | 50.0 | 45.7 | 40.5 | 47.8 | 51.6 |
| BMI1, kg/m2 | 25.4 | 25.0 | 24.9 | 25.0 | 24.8 |
| Caucasian, % | 95.1 | 90.9 | 91.0 | 92.0 | 90.9 |
| Systolic blood pressure1, mm Hg | 125 | 124 | 123 | 123 | 122 |
| Presence of gout, % | 4.1 | 2.8 | 4.3 | 2.7 | 3.6 |
| Use of aspirin, % | 24.1 | 35.2 | 40.9 | 55.9 | 46.6 |
| Total energy intake1, kcal/d | 1839 | 2053 | 2106 | 2104 | 1954 |
| Alcohol intake1, g/d | 11.3 | 13.0 | 11.0 | 12.1 | 12.0 |
| Fructose intake1, g/d | 17.7 | 24.5 | 28.6 | 27.0 | 28.2 |
| Dairy protein intake1, g/d | 15.2 | 15.4 | 14.6 | 15.7 | 13.5 |
| Total meat intake1, servings/d | 1.4 | 1.3 | 1.2 | 1.1 | 1.1 |
| Seafood intake1, servings/d | 0.26 | 0.30 | 0.31 | 0.33 | 0.36 |
| Coffee intake1, cups/d | 2.6 | 2.0 | 1.8 | 2.1 | 2.0 |
Means
A higher intake of total vitamin C was significantly associated with lower serum uric acid concentrations, after adjustment for smoking, BMI, intake of total energy, dairy protein, and alcohol, and other potential confounders (Table 2). We observed two plateaus for the inverse associations: the first was seen at 90–499 mg/d and then 500 mg/d and higher. Adjusted mean uric acid concentrations across total vitamin C intake categories were 6.4, 6.1, 6.0, 5.7, and 5.7 mg/dl (P for trend<0.001). Greater vitamin C supplement intake was significantly associated with a lower serum uric acid (P for trend < 0.001). Although higher dietary vitamin C intake categories tended to have lower serum uric acid levels than the lowest category, the linear trend was not significant (P for trend =0.10) (Table 2). Of note, the range of dietary vitamin C exposure was substantially smaller than that of total or supplemental vitamin C intake. After excluding subjects with vitamin C supplement intake, we observed a similar non-linear pattern with dietary vitamin C intake. Further adjustment for beer intake did not materially change the associations between vitamin C intake and uric acid concentrations.
Table 2.
Serum uric acid concentration according to total, dietary and supplement vitamin C intake (1994) in a sub-sample of the Health Professional Follow up Study (n=1,387)
| Serum Uric Acid concentration, mg/dL | Ptrend | |||||
|---|---|---|---|---|---|---|
| Total vitamin C intake, mg/d | <90 | 90–249 | 250–499 | 500–999 | ≥ 1000 | |
| n | 96 | 605 | 245 | 214 | 227 | |
| Age- and BMI - adjusted | 6.3±0.11 | 6.1±0.05 | 6.1±0.08 | 5.7±0.08*** | 5.8±0.08** | <0.001 |
| Multivariate2 | 6.4±0.1 | 6.1±0.05* | 6.0±0.08* | 5.7±0.08*** | 5.7±0.08*** | <0.001 |
| Vitamin C supplement, mg/d | 0 | 1–249 | 250–499 | 500–999 | ≥ 1000 | |
| n | 509 | 405 | 105 | 165 | 203 | |
| Age- and BMI - adjusted | 6.2±0.05 | 6.0±0.06 | 5.8±0.12* | 5.8±0.09** | 5.8±0.08*** | <0.001 |
| Multivariate2 | 6.2±0.05 | 6.0±0.06 | 5.8±0.12** | 5.8±0.09*** | 5.7±0.08*** | <0.001 |
| Dietary vitamin C, mg/d | <50 | 50–99 | 100–199 | 200–299 | ≥300 | |
| n | 26 | 241 | 791 | 271 | 58 | |
| Age- and BMI - adjusted | 6.6±0.24 | 6.0±0.07* | 6.0±0.04* | 6.0±0.07* | 6.1±0.16 | 0.49 |
| Multivariate2 | 6.6±0.24 | 6.1±0.08 | 6.0±0.04* | 6.0±0.08* | 5.9±0.17 | 0.10 |
| Excluding vitamin C supplement users2 | 6.9±0.36 | 6.3±0.13 | 6.2±0.07 | 6.0±0.13 | 6.2±0.29 | 0.12 |
Mean ± SE
Adjusted for age (y), smoking status (never smoker, past smoker, or current smoker: 1–14 or ≥ 15 cigarettes/d), BMI (<23, 23–24.9, 25–26.9, 27–28.9, or ≥29 kg/m2), ethnicity (Caucasian vs. others), systolic blood pressure (<105, 105–114, 115–124, or ≥125 mm Hg), presence of gout (yes/no), use of aspirin (yes/no), total energy (kcal/d), meat (servings/d), seafood (servings/d), dairy protein (g/d), fructose (g/d), alcohol (0, <5, 5–9, 10–14, 15–29, 30–49, or ≥ 50 g/d), and coffee (0, <1, 1–3, 4–5, or ≥ 6 cups/d).
P<0.05,
P<0.01, and
P<0.001
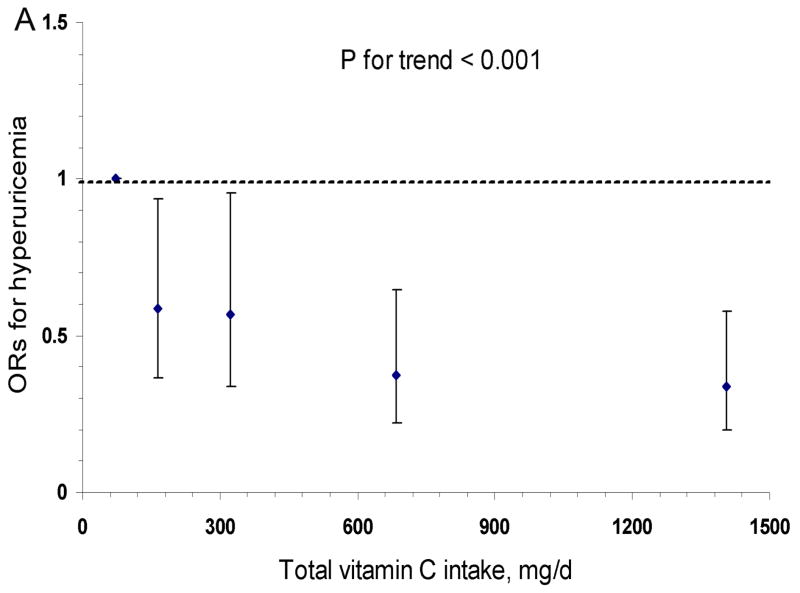
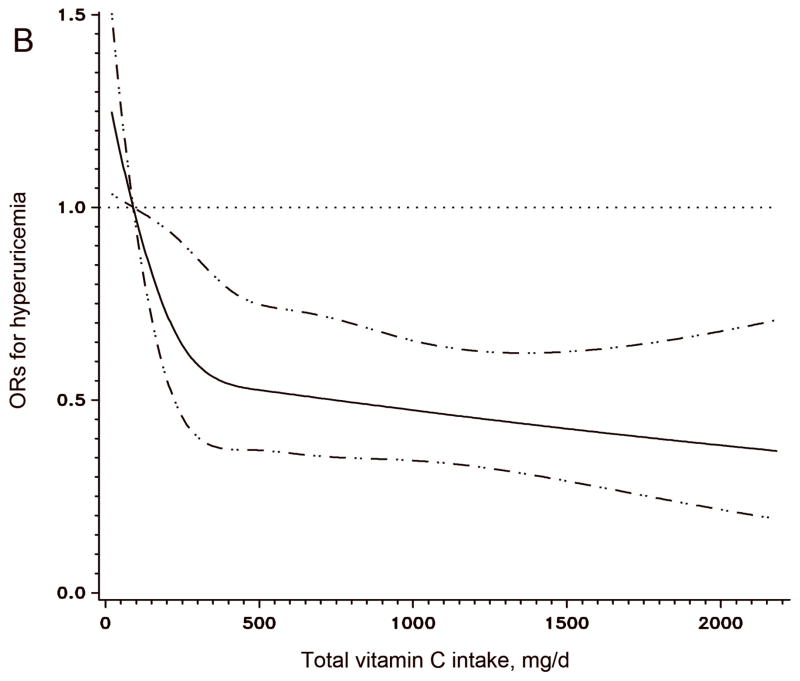
The results of logistic regression with hyperuricemia (serum uric acid > 6 mg/dl) as a dichotomous outcome were similar (Figure 1A). The multivariate ORs for hyperuricemia across total vitamin C intake categories were 1 (reference), 0.58, 0.57, 0.38, and 0.34 (95% CI: 0.20–0.58; P for trend < 0.001). The cubic Spline curve (Figure 1B) showed a similar pattern for the association between total vitamin C and hyperuricemia. A similar inverse association persisted with supplement vitamin C intake (P for trend < 0.001), but not with dietary vitamin C intake (P for trend = 0.15). An alternative definition of hyperuricemia (serum uric acid level > 7 mg/dl) (16) resulted in similar significant results. The multivariate OR for the highest versus lowest categories of total vitamin C intake was 0.31 (95% CI: 0.17–0.56, P for trend=0.009).
Figure 1. Odds Ratio and 95% confidence interval of hyperuricemia (serum uric acid > 360 μmol/L) according to total vitamin C intake category.
Panel A was calculated by a logistic regression model with the lowest intake category (<90 mg/d) as the reference group; Panel B was fitted by a cubic Spline logistic model with 90 mg/d as the reference group and the 95% confidence intervals are indicated by the dashed lines. Both models were adjusted for age (y), smoking status (never smoker, past smoker, or current smoker: 1–14 or ≥ 15 cigarettes/d), BMI (<23, 23–24.9, 25–26.9, 27–28.9, or ≥29 kg/m2), ethnicity (Caucasian vs. others), systolic blood pressure (<105, 105–114, 115–124, or ≥125 mm Hg), presence of gout (yes/no), use of aspirin (yes/no), total energy (kcal/d), dairy protein (g/d), fructose (g/d), alcohol (0, <5, 5–9, 10–14, 15–29, 30–49, or ≥ 50 g/d), and coffee (0, <1, 1–3, 4–5, or ≥ 6 cups/d).
Significant associations between vitamin C intake and uric acid concentrations did not materially change after excluding subjects with gout or those who are not Caucasian. When we used the average of diets in 1986 and 1990, we found similar results. Total vitamin C were inversely associated with serum uric acid concentrations (P for trend=0.002) and hyperuricemia (P for trend =0.02).
We did not find significant interactions between vitamin C and age, alcohol intake, smoking status and BMI (P for interaction > 0.1 for all). The inverse association between vitamin C intake and serum uric acid concentration persisted in subgroup analysis according to age, smoking, overweight, and alcohol intakes (Table 3).
Table 3.
Serum uric acid concentration according to total vitamin C intake (1994) in a sub-sample of the Health Professional Follow up Study (n=1,387), stratified by age, smoking status, BMI, and alcohol intake1
| Serum Uric Acid concentration, mg/dL | Ptrend | |||||
|---|---|---|---|---|---|---|
| Total vitamin C intake, mg/d | <90 | 90–249 | 250–499 | 500–999 | ≥1000 | |
| Age, y | ||||||
| < 60 | 6.3±0.17 | 6.1±0.07 | 6.1±0.12 | 5.8±0.13 | 5.8±0.12 | 0.02 |
| ≥ 60 | 6.5±0.18 | 6.2±0.07 | 6.0±0.10* | 5.7±0.11*** | 5.7±0.11*** | <0.001 |
| Smoking | ||||||
| Never | 6.2±0.20 | 6.0±0.07 | 6.0±0.10 | 5.6±0.12 | 5.8±0.12 | 0.05 |
| Ever | 6.6±0.16 | 6.2±0.07 | 6.1±0.12 | 5.8±0.11*** | 5.7±0.11*** | <0.001 |
| BMI, kg/m2 | ||||||
| <25 | 6.1±0.20 | 5.9±0.07 | 5.8±0.10 | 5.6±0.11* | 5.5±0.11* | 0.05 |
| ≥ 25 | 6.6±0.17 | 6.3±0.07 | 6.3±0.12 | 5.9±0.12** | 6.0±0.12** | 0.01 |
| Alcohol intake, g/d | ||||||
| none | 6.0±0.22 | 5.9±0.10 | 5.6±0.17 | 5.4±0.18 | 5.6±0.17 | 0.12 |
| > 0 | 6.5±0.16 | 6.2±0.06 | 6.2±0.09 | 5.8±0.09*** | 5.8±0.09*** | 0.002 |
Adjusted for age (y), smoking status (never smoker, past smoker, or current smoker: 1–14 or ≥ 15 cigarettes/d), BMI (<23, 23–24.9, 25–26.9, 27–28.9, or ≥29 kg/m2), ethnicity (Caucasian vs. others), systolic blood pressure (<105, 105–114, 115–124, or ≥125 mm Hg), presence of gout (yes/no), use of aspirin (yes/no), total energy (kcal/d), meat (servings/d), seafood (servings/d), dairy protein (g/d), fructose (g/d), alcohol (0, <5, 5–9, 10–14, 15–29, 30–49, or ≥ 50 g/d), and coffee (0, <1, 1–3, 4–5, or ≥ 6 cups/d).
P<0.05,
P<0.01, and
P<0.001, relative to the lowest intake category.
Discussion
In men without hypertension and BMI< 30 kg/m2, we found intakes of vitamin C to be inversely related to serum uric acid concentrations, independent of dietary and other risk factors for gout such as body mass index, age, and alcohol intake. The associations were largely derived by vitamin C supplement use. Furthermore, we observed similar significant associations when we used dietary exposure data, which were assessed 4–8 years before blood collection, as predictors.
Our results showed that total vitamin C intake of 500 mg/d or higher is associated with a ~0.6–0.7 mg/dL lower level of serum uric acid relative to those with intake < 90 mg/d. The magnitude of difference of serum uric acid associated with total vitamin C intake of 500mg/d was closely in line with that from a recent trial (6). This randomized trial showed that supplementation with vitamin C as low as 500 mg/d for two months reduced serum uric acid by 0.5 mg/dL, compared to no change in the placebo group (6). This level of population mean difference of serum uric acid levels (17, 18) can be translated into a clinically relevant difference in the risk for incident gout, as demonstrated in our previous studies (19, 20). For example, one daily serving increase in beer intake was associated with a mean serum uric acid level increase of 0.4 mg/dL in the cross-sectional analysis of National Health and Nutrition Examination Survey III (17) and with a 50% increased risk of incident gout in our prospective analysis of the HPFS (19). This potentially significant impact on the eventual risk of gout is also supported by our results, using hyperuricemia as a dichotomous outcome. Nevertheless, prospective studies with outcome of incident gout would be valuable.
Vitamin C likely modulates serum uric acid concentration via its uricosuric effect. Vitamin C and uric acid are reabsorbed through anion-exchange transport in the proximal tubule (6). Increased vitamin C concentration in the filtrate may competitively inhibit uric acid reabsorption (5). Recent advances in our understanding of the molecular mechanisms of renal uric acid transport suggest that the uricosuric effect may be through cis-inhibition of URAT1 (uric acid transporter 1, the key target of typical uricosurics) (21), Na+-dependent anion cotransporter (e.g. SLC5A8/A12) (22) or both in the proximal tubules(23). Furthermore, greater vitamin C intake may possibly improve renal function and increase the glomerular filtration rate (6, 24, 25), providing another potential mechanism for the uricosuric effect of vitamin C intake. Both human and animal studies have demonstrated that administration of vitamin C increases renal plasma flow and glomerular filtration rate and attenuates the increases in arterial pressure (24, 26). The antioxidant property of Vitamin C could reduce oxidative stress and inflammation, and could, therefore, be related to lower uric acid synthesis (16).
Strengths of the current study include use of a validated FFQ to assess dietary intake, and multiple measurements of dietary exposure. Besides use of dietary intake in 1994 as the predictor of serum uric acid, we conducted a sensitivity analysis using average dietary intake collected in 1986 and 1990. In this way we may reduce random errors introduced by a single dietary measurement, and, because of both dietary assessments prior to blood collection, minimize misclassification of exposure (vitamin C intake) due to change of diet related to high serum uric acid. We observed similar results with the main analyses, suggesting robustness of our findings. Our study population consisted of participants without hypertension and with BMI <30 kg/m2, limiting the generalizability of our findings. Our study is also limited by including only men; thus, the effect of gender could not be studied. Another limitation is that our cohort does not represent random samples of US men; therefore, the dietary patterns cannot be taken to reflect the general population. Nevertheless, the biological effects of diet in this cohort should be the same as those among men in general. Although validation studies suggested a high level of validity in Vitamin C intake measured by the FFQ used in the current study, (9, 10) measurement of plasma vitamin C concentration could provide a more accurate estimate of vitamin C status. Further, because of the observational design of the current study, we cannot exclude a possibility of residual confounding due to unmeasured confounders. For example, lack of information of use of gout-specific medicines may confound the association between vitamin C and uric acid concentration. However, we obtained similar results after excluding patients with gout.
In conclusion, we found that that intake of vitamin C is inversely associated with serum uric acid concentrations in a population-based study. These findings support a potential role for vitamin C in the prevention of hyperuricemia and gout. Our findings are most directly generalizable to men aged 50 years or older without hypertension and obesity. Corresponding studies of men with these conditions and of women would be valuable.
Footnotes
The study was supported by TAP Pharmaceuticals and NIH/NINDS grant R01 NS048517. The funding sources had no role in the design, conduct, or reporting of the study or in the decision to submit the manuscript for publication.
Abbreviations used: HPFS, Health Professional Fellow-up Study; BMI, body mass index; FFQ, food frequency questionnaire; OR, odds ratio; and CI, confidence interval
Publisher's Disclaimer: This is a pre-copy-editing, author-produced PDF of an article accepted for publication in The Journal of Rheumatology following peer review. The definitive publisher-authenticated version J Rheumatol. 2008 Sep;35(9):1853-8 is available online at: http://www.jrheum.org/content/35/9/1853.long
Contributor Information
Xiang Gao, Research associate in Department of Nutrition, Harvard University School of Public Health, Boston, MA.
Gary Curhan, Associate professor in Department of Epidemiology, Harvard University School of Public Health, and Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
John P. Forman, Instructor in Medicine, Harvard Medical School and an associate physician, Renal Division, Brigham and Women’s Hospital.
Alberto Ascherio, Associate professor in Department of Nutrition and Department of Epidemiology, Harvard University School of Public Health and in Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School.
Hyon K. Choi, Associate professor in Department of Medicine, Vancouver General Hospital, University of British Columbia, Vancouver, Canada.
References
- 1.Roubenoff R, Klag MJ, Mead LA, Liang KY, Seidler AJ, Hochberg MC. Incidence and risk factors for gout in white men. Jama. 1991 Dec 4;266:3004–7. [PubMed] [Google Scholar]
- 2.Mitch WE, Johnson MW, Kirshenbaum JM, Lopez RE. Effect of large oral doses of ascorbic acid on uric acid excretion by normal subjects. Clin Pharmacol Ther. 1981 Mar;29:318–21. doi: 10.1038/clpt.1981.42. [DOI] [PubMed] [Google Scholar]
- 3.Sutton JL, Basu TK, Dickerson JW. Effect of large doses of ascorbic acid in man on some nitrogenous components of urine. Hum Nutr Appl Nutr. 1983 Apr;37:136–40. [PubMed] [Google Scholar]
- 4.Stein HB, Hasan A, Fox IH. Ascorbic acid-induced uricosuria. A consequency of megavitamin therapy. Ann Intern Med. 1976 Apr;84:385–8. doi: 10.7326/0003-4819-84-4-385. [DOI] [PubMed] [Google Scholar]
- 5.Berger L, Gerson CD, Yu TF. The effect of ascorbic acid on uric acid excretion with a commentary on the renal handling of ascorbic acid. Am J Med. 1977 Jan;62:71–6. doi: 10.1016/0002-9343(77)90351-5. [DOI] [PubMed] [Google Scholar]
- 6.Huang HY, Appel LJ, Choi MJ, Gelber AC, Charleston J, Norkus EP, Miller ER., 3rd The effects of vitamin C supplementation on serum concentrations of uric acid: results of a randomized controlled trial. Arthritis Rheum. 2005 Jun;52:1843–7. doi: 10.1002/art.21105. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Pollak M, Liu Y, Platz EA, Majeed N, Rimm EB, Willett WC. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev. 2003 Feb;12:84–9. [PubMed] [Google Scholar]
- 8.Forman JP, Choi H, Curhan GC. Plasma uric acid level and risk for incident hypertension among men. J Am Soc Nephrol. 2007 Jan;18:287–92. doi: 10.1681/ASN.2006080865. [DOI] [PubMed] [Google Scholar]
- 9.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993 Jul;93:790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 10.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992 May 15;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 11.USDA. UDSA nutrient database for standard reference, release 10: Nutritient Dat Laboratory homepage. 1993 Available at: http://www.nal.usda.gov/fnic/foodcomp.
- 12.Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005 Dec 8;353:2450–61. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 13.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of the intake of vitamins C and B6, and the risk of kidney stones in men. J Urol. 1996 Jun;155:1847–51. [PubMed] [Google Scholar]
- 14.Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–12. [Google Scholar]
- 15.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989 May;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 16.Hayden MR, Tyagi SC. Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: The urate redox shuttle. Nutr Metab (Lond) 2004 Oct 19;1:10. doi: 10.1186/1743-7075-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi HK, Curhan G. Beer, liquor, and wine consumption and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2004 Dec 15;51:1023–9. doi: 10.1002/art.20821. [DOI] [PubMed] [Google Scholar]
- 18.Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005 Jan;52:283–9. doi: 10.1002/art.20761. [DOI] [PubMed] [Google Scholar]
- 19.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004 Apr 17;363:1277–81. doi: 10.1016/S0140-6736(04)16000-5. [DOI] [PubMed] [Google Scholar]
- 20.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004 Mar 11;350:1093–103. doi: 10.1056/NEJMoa035700. [DOI] [PubMed] [Google Scholar]
- 21.Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002 May 23;417:447–52. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 22.Thangaraju M, Ananth S, Martin PM, Roon P, Smith SB, Sterneck E, Prasad PD, Ganapathy V. c/ebpdelta Null mouse as a model for the double knock-out of slc5a8 and slc5a12 in kidney. J Biol Chem. 2006 Sep 15;281:26769–73. doi: 10.1074/jbc.C600189200. [DOI] [PubMed] [Google Scholar]
- 23.Choi HK, Mount DB, Reginato AM. Pathogenesis of Gout. Ann Intern Med. 2005;143:499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- 24.Tian N, Thrasher KD, Gundy PD, Hughson MD, Manning RD., Jr Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension. 2005 May;45:934–9. doi: 10.1161/01.HYP.0000160404.08866.5a. [DOI] [PubMed] [Google Scholar]
- 25.Chade AR, Rodriguez-Porcel M, Herrmann J, Krier JD, Zhu X, Lerman A, Lerman LO. Beneficial effects of antioxidant vitamins on the stenotic kidney. Hypertension. 2003 Oct;42:605–12. doi: 10.1161/01.HYP.0000089880.32275.7C. [DOI] [PubMed] [Google Scholar]
- 26.Schaufele TG, Schlaich MP, Delles C, Klingbeil AU, Fleischmann EH, Schmieder RE. Impaired basal NO activity in patients with glomerular disease and the influence of oxidative stress. Kidney Int. 2006 Sep;70:1177–81. doi: 10.1038/sj.ki.5001745. [DOI] [PubMed] [Google Scholar]