Abstract
Purpose
Men with PSA-only relapse of prostate cancer after primary therapy are generally fully functional and asymptomatic with a life expectancy of up to ten or more years. Androgen deprivation therapy (ADT) is a common treatment option. This study examined mood and cognitive changes in otherwise healthy men with prostate cancer prior to, during and after ADT.
Experimental Design
Twenty hormone naïve, eugonadal prostate cancer patients without evidence of metastases and with a rising PSA were treated with intermittent ADT consisting of nine months of complete androgen blockade achieved with combined leuprolide and flutamide followed by an “off treatment” period. Cognitive function tests and mood measures were administered at baseline, after three and nine months of ADT and after three months of no treatment. Twenty healthy control patients without prostate cancer range matched for age and education were tested at the same time intervals.
Results
ADT patients evidenced a significant decline in spatial reasoning, spatial abilities and working memory during treatment compared to baseline. No changes were noted for measures of verbal or spatial memory, selective attention or language. Significant changes in self-rated mood such as increased depression, tension, anxiety, fatigue and irritability were evident during treatment compared to baseline for ADT patients. No significant changes in either cognitive tests or mood measures were noted for the healthy control group.
Conclusions
These findings, suggest that nine months of combined androgen blockade may result in some adverse changes in cognition and mood. However, many but not all of these changes can return to baseline after cessation of ADT.
Keywords: Cancer, Oncology, Complete Androgen Blockade (CAB), Mood, Cognition, Memory, Prostate Cancer, Testosterone, Flutamide, Intermittent Androgen Ablation Therapy (ADT)
INTRODUCTION
Prostate cancer is the most common form of non-skin cancer diagnosed in men in the United States. Over 186,320 new cases will be diagnosed in 2008 [1]. Androgen deprivation therapy (ADT) used to be reserved for those with metastatic disease and resulted in a median survival of 2-5 years [2, 3]. ADT is now prescribed in the setting of localized disease (e.g. in combination with radiation therapy) or for those who have a rising PSA without evidence of metastases after primary therapy. In contrast to those with metastatic disease, such patients often have no disease related symptoms and may live for many years. Androgen deprivation is a common treatment option for many patients that have reoccurrence or biochemical relapse after primary therapy [4].
Intermittent androgen deprivation therapy (ADT) is a treatment strategy that cycles androgen withdrawal with an “off treatment” period allowing the testosterone levels to return to eugonadal levels. This approach has allowed for characterization of the biological, psychological, and quality of life effects of ADT and which effects are reversible or attenuated when the testosterone levels increase during the ‘off treatment’ period. In addition to these benefits, animal data suggests that intermittent exposure to androgen deprivation may prolong the interval to androgen independence [5, 6]. Physiological consequences of androgen deprivation include obesity, anemia, loss of bone mineral density, muscle atrophy, gynecomastia and mood changes [2, 7, 8]. A recent population based study showed that ADT was associated with an increased risk of diabetes, myocardial infarction, and hypertension [9].
Changes in quality of life in men undergoing ADT have been previously reported including symptoms of cognitive difficulties [10-15] with one study reporting approximately 25% of men reporting cognitive difficulties [16]. However, despite these changes, the treatment is generally well accepted [17].
Animal studies indicate that androgens effect brain regions such as the hippocampus and frontal lobes with observable changes in spatial memory and executive function tasks [18-21]. Human studies also indicate a positive relationship between testosterone levels and cognitive function including spatial abilities, spatial memory and executive functions [22-25].
Previous studies examining cognition in men undergoing androgen ablation have found impairments in verbal memory [26, 27], spatial abilities [28] and attention [29]. However, other studies have found no appreciable change in cognition from androgen ablation [30], or improvement in verbal memory [15], or decline for only a subset of participants [16]. Variability in findings may be due to differences in study methods, study samples, disease factors and medications.
Epidemiological studies of healthy older men without prostate cancer have suggested a positive relationship between testosterone and cognition. In our local clinic, patients sometimes complain of cognitive difficulties that they attribute to hormone deprivation therapy although this information is anecdotal. The aim of this study was to examine whether any objective evidence of cognitive decline could be found to support the anecdotal reports of cognitive decline from patients. In addition we attempted to replicate and expand on our earlier study that examined cognitive changes in patients with prostate cancer by including measures of mood which might also impact daily functioning. We examined patients prospectively, taking advantage of the intermittent aspect of androgen deprivation therapy to include off –on-off assessments. Patients were assessed before, during and after treatment with complete androgen blockage (CAB) (combined GnRH agonist and antiandrogen) including multiple assessments at baseline and during treatment. Few studies have included follow up assessment during the off treatment period. We hypothesized that complete androgen blockade would alter and possibly impair memory and executive functions and would have some detrimental impact on mood.
MATERIALS AND METHODS
Participants
Included both the treatment group and a control or no treatment comparison group. Patients were recruited from the Genito-urinary clinic at the Seattle Cancer Care Alliance and the Urology clinics at the University of Washington Medical Center. Physicians and nurses informed patients of the opportunity to participate and if they demonstrated some interest, they contacted the study coordinator who conducted a brief phone screen to determine eligibility. Although a formal assessment of reasons for refusal was not undertaken, Physicians and nurses reported that nearly all patients were very interested in participating, but the majority of patients who refused stated time constraints as the reason. Control participants were recruited from print advertisements and flyers in the medical center and in the local newspapers. In order to be eligible for treatment with androgen deprivation, patients had to have a rising PSA following primary therapy (prostatectomy, external beam radiation, or brachytherapy) without evidence of metastatic disease by imaging on bone scan and CT scan of the chest, abdomen and pelvis. Patients were required to have a WHO performance status of 0 or 1, normal baseline serum testosterone, and no prior ADT. Patients who had pain due to prostate cancer, prior psychiatric illness involving hospitalization, dementia, central nervous system metastasis, history of systemic chemotherapy, and current renal dysfunction or hepatic dysfunction were not eligible. The control or no-treatment group included community dwelling, eugonadal men without prostate cancer who were range matched for age and education. All participants signed written informed consent approved by the University of Washington Institutional Review Board prior to study procedures.
Study Design
The treatment and testing schema for ADT patients is summarized in Figure 1. The treatment group received combined androgen blockade (CAB) with an anti-androgen and GnRH analog for a total of nine months duration whereupon ADT was stopped for a variable period of time. Both the treatment and no treatment groups were tested at baseline, 3, 9 and 12 months with a battery of cognitive function and mood measures. The pre-baseline and baseline assessments were completed before the start of any treatment medication. Blood samples for total testosterone (T), and estradiol (E) were also obtained at the same intervals.
Figure 1.
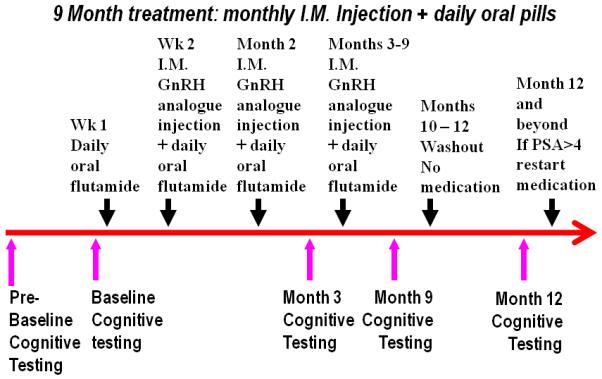
Study procedure outline.
For all the following figures: Red solid line is the ADT group and blue dashed line is the controls at baseline before treatment, during treatment and off treatment at the 12 month assessment. Error bars represent standard deviation.
Outcome Measures
The primary outcome measure was a battery of cognitive tests assessing memory, attention and visuospatial abilities and mood measures. Several precautions were implemented to control for possible practice effects. First, both treatment and control participants were administered the battery of cognitive tests twice prior to onset of hormone treatment. The first cognitive testing session was considered the pre-baseline testing session and the second testing session was used as the true baseline and these were administered from three to 12 days apart. The largest observed practice effect occurs between the first and second testing sessions, as participants become familiar with the cognitive tasks at the first testing [31]. Thus by using the second testing session as the true baseline, practice effects are minimized. Second, we used comparable alternate versions of each test that were randomly ordered and counterbalanced among participants. The following test descriptions are grouped according to cognitive domains.
Spatial Memory Measure
1) Puget Sound Route Learning Test
This test measured the ability to navigate a short route within a room [32] and has been shown to be sensitive to changes in testosterone levels [33, 34] and reliable and valid in an older population [32]. Three trials were administered followed by three trials of a new route using pictures placed on the floor as landmarks. A delayed recall is administered after twenty minutes. Performance was assessed by calculating the number of correct sequential units recalled for the delayed recall and this was used in the analysis.
Spatial Ability Measure
Complex Design Construction
This test is based on the Wechsler Adult Intelligence Scale-Revised, Block Design subtest and measures participants’ ability to analyze and construct abstract figures from their component parts [35]. This test was selected as spatial abilities, such as constructional abilities have been shown to be effected by changes in testosterone in men [25, 36] and the task has good reliability and validity [35, 37]. Time to completion is recorded for each design with a time limit of 3 minutes per design, for a total of nine designs. Total designs completed and average time per design were analyzed.
2) Mental Rotation
This test is based on the Vandenberg & Kuse (1978) Mental Rotation Test [38]. Several studies have shown an association between mental rotation and endogenous testosterone levels [39-43] and the task has been shown to be reliable and valid for measuring spatial abilities [44, 45]. Subjects are presented with line drawings of complex, three dimensional cubes on a computer screen. The subject must compare the two drawings and decide if they match. Reaction time and number correct are recorded.
Verbal Memory Measures
1)Proactive Interference (PI)
As adapted from Moscovitch (1994) [46]. The PI task is sensitive to damage to the frontal lobes and may be a more sensitive indicator of early memory impairments in older adults than declarative memory tasks [47-51]. It has been shown to be reliable with regard to repeated measures and valid in detecting brain damage or early dementia [47-52]. Participants listened to a list of 10 words from the same semantic category (e.g., articles of clothing), and then recalled as many of these words as possible. The procedure was repeated for a total of 4 trials followed by a trial of words from a new category (e.g. fruits). Total words recalled from the first four lists and the fifth trial were analyzed.
2)Story Recall
This task is based on the well known Wechsler Memory Scale –Revised Logical Memory task [53] but modified with new stories to reduce practice effects and to accommodate a repeated measure design. The modified version has been shown to have good test, re-test reliability and validity with regard to healthy and memory impaired populations [54-57]. Participants listened to two brief narratives (stories) and were asked to recall as much as possible immediately after hearing each story and following a 20-minute delay. Total words recalled for both stories at the delay were analyzed.
Verbal Ability Measure
Verbal Fluency
Verbal fluency has been shown to change with manipulation of testosterone levels [36, 58]. Participants were asked to verbally generate as many words beginning with a particular letter (e.g. P) within a 60 second period [59]. The task is very reliable and has been shown to be valid at detecting brain dysfunction, impairment, damage and dementia (See Lezak (1995) and Spreen and Strauss (1991) for an extensive review [59, 60]). Two trials were administered with two different letters. The total number of words generated was recorded for each letter and summed and analyzed.
Executive Functions
Stroop Color Word Interference Task
The Stroop task is very reliable and has been shown to be valid at detecting brain dysfunction and frontal lobe impairment (See Lezak (1995) and Spreen and Strauss (1991) for an extensive review [59, 60]) and has been shown to change with testosterone manipulation [58, 61]. Participants read 100 color words (red, green, blue), followed by identification of color blocks followed by reading the color of the ink and ignoring the word (e.g., the word ‘blue’ printed in green letters). Time to complete the task was recorded as well as errors and time was analyzed.
2)Visual Working Memory
This task is based on the Subject Ordered Pointing Task (SOPT) by Petrides (1982)[62] and measures working memory. The task has been shown to be reliable and valid with older adults [63, 64] and sensitive to changes in testosterone levels in men [25]. The participant is shown a grid array of 10, 12 or 16 abstract designs and they must choose a new design with each refresh of the screen. Total correct designs, and errors across all trials were recorded and total errors were analyzed.
Mood Measures
Profile of Mood States (POMS)
The POMS is a widely used and well validated mood instrument that assesses current mood state within weeks [65]. Participants responses were recorded and scored according to the manual directions and T scores were generated with comparisons to the manual sample of healthy outpatients. T scores were used in the analysis.
Visual Analogue Scale (VAS)
Participants were given a visual analogue scale of mood items that included irritability, tension, depression, and moodiness. This collection of mood descriptors has been shown to be sensitive to mood changes in women who suffer from premenstrual dysphoric disorder [66, 67] and VAS scales in general have been shown to be a reliable and valid measure of mood state [68, 69]. The distance between none and the marker is measured in millimeters and this number was analyzed.
Statistical Analyses
Neuropsychological measures
The primary study question was to assess whether significant decline in circulating hormone levels resulted in subsequent changes in cognitive functioning and mood as assessed by change from baseline. The primary outcome measure was change over time on a battery of neuropsychological tests measuring verbal and spatial memory, executive functions, attention and mood measures. A p value of 0.05 or less was accepted as significant. A mixed model, repeated measures, Multivariate Analysis of Variance (MANOVA) was used with group (ADT, no treatment) as the independent factor and time (baseline, 3 and 9 months treatment, 3 months off treatment) as the repeated factor and neuropsychological tests: spatial memory (Route Test), verbal memory (proactive interference and story recall) spatial ability (mental rotations) executive functions (SOPT, Stroop) and language (verbal fluency) as dependent measures. Use of a MANOVA with all cognitive tests together helps to control family wise error rate and reduces the risk of type 1 errors due to multiple comparisons. Findings from the overall MANOVA revealed significant interaction effects that were further analyzed using ANOVAs for each individual test as a dependent measure to ensure maximal sample size. Planned comparisons were performed for treatment and off treatment (12-month assessment) compared to baseline and post hoc comparisons were subjected to Bonferroni correction. A similar MANOVA was performed with all of the POMS subtests as dependent measures (tension-anxiety (TA), depression-dejection (Dep), anger-hostility (AH), vigor-activity (VA), fatigue-inertia (FI), confusion-bewilderment (CON)) and a MANOVA with the four VAS subscales as dependent measures (irritability (IR), tension (TEN), depression (DEP), moodiness (MO)). To examine potential interactions between changes in mood, additional MANOVAs with the Neuropsychological test results as dependent measures were performed with change in POMS subtest scores (baseline subtracted from month 9) as co-variates .
Hormone Analyses
A secondary analysis was performed to assess changes in circulating hormone levels testosterone (T) and estradiol in response to treatment. Serum samples were taken during clinic visits at baseline, and months, 3 and 9 of treatment and after 3 months off treatment and were sent to the UWMC clinical laboratory. Similar to neuropsychological measures a MANOVA was performed with total testosterone (T) and estradiol as dependent measures. We did not perform a correlation analysis between cognitive or mood tests and hormone levels during treatment as the hormones are at castrate levels which are at or below the detect ability level of the assay. Therefore the hormone values are both unstable and do not represent a normal distribution which is necessary for correlation analysis.
RESULTS
ADT study participants were recruited from the University of Washington Medical Center (UWMC) and the Seattle Cancer Care Alliance (SCCA) between June, 2003 and August 2006. Twenty-one ADT patients completed the screening visit and nine-teen ADT patients completed all study visits. Two ADT patients dropped out, one patient after the screening visit due to scheduling conflicts and one patient after the month 3 visit due to medical complications un-related to androgen deprivation treatment. Twenty-four control subjects completed the screening visit and nine-teen control participants completed all study visits. Three control participants dropped out after the screening visit due to scheduling conflicts and one participant dropped out after completing the 3 month visit due to a family emergency and one participant was excluded at screening due to low testosterone levels. Only participants who completed all visits were included in the analyses below. Both ADT patients and controls ranged in age from 54 to 78 years with a mean age of 64 years for both groups and education ranged from 12 to 22 years with a mean education level of 17 years. Groups were range matched for education within two years and age within four years. Because the groups were range matched they were not significantly different on these demographic variables. Demographic variables are included in Table 1.
Table 1.
Demographics, Serum Hormone Values and Neuropsychological Test Results
Testosterone ng/ml, Esradiol pg/ml, Route Test- number of correctly recalled sequences after a delay; Block Design- number of correctly completed designs, *ADT group demonstrated a significant decline over time and month 3 compared to baseline (p<.05) and there was group by time interaction effect (p<.05); Mental Rotation – number of correctly identified figures, *ADT group performed worse than the control group on treatment (p<.05) and there was group by time interaction effect (p<.05); Proactive Interference- Number of correctly recalled words summed across four trials; Story Recall- Number of correctly recalled bits of information after a delay; Verbal Fluency- Number of words generated for two letters; Stroop- time to complete the interference trial; SOPT- number of errors summed across all trials, *and there was group by time interaction effect (p<.05).
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
|---|---|---|---|---|
| Demographics | Age | Education | ||
| ADT | 62.05 (7.19) | 16.30 (3.01) | ||
| Control | 65.47 (7.99) | 17.38 (2.55) |
| Baseline | 3 Month | 9 Month | 12 Month | |
|---|---|---|---|---|
| Testosterone | ||||
| ADT | 406 (1.6) | 0.28 (0.24) | 0.20 (0.07) | 2.45 (1.35) |
| Control | 3.97 (1.4) | 4.39 (2.14) | 3.93 (1.66) | 3.79 (1.61) |
| Estradiol | ||||
| ADT | 33.60 (17.29) | 23.12 (6.56) | 22.76 (5.75) | 24.35 (6.46) |
| Control | 26.61 (10.18) | 33.65 (10.49) | 28.65 (10.47) | 27.06 (7.30) |
| Spatial Memory (Route Test) | ||||
| ADT | 19.50 (10.05) | 22.00 (9.39) | 23.76 (8.60) | 24.12 (8.86) |
| Control | 17.27 (7.72) | 18.00 (6.50) | 19.47 (7.21) | 19.88 (5.75) |
| Spatial Ability (Block Design) | ||||
| ADT | 8.65 (0.81) | 7.20 (1.21)* | 8.82 (0.53) | 8.47 (1.06) |
| Control | 8.94 (0.23) | 8.94 (0.24) | 8.82 (0.39) | 8.94 (0.24) |
| Spatial ability (Mental Rotation) | ||||
| ADT | 16.12 (3.39) | 13.00 (3.01)8 | 16.52 (2.42) | 15.53 (3.08) |
| Control | 16.27 (2.67) | 16.64 (3.21) | 16.35 (2.82) | 16.23 (2.65) |
| Verbal Memory (Proactive Interference) | ||||
| ADT | 21.31 (5.50) | 22.90 (5.03) | 22.70 (5.33) | 22.70 (4.33) |
| Control | 23.55 (4.84) | 24.38 (4.73) | 24.26 (5.18) | 23.85 (5.22) |
| Verbal Memory (Story Recall) | ||||
| ADT | 35.06 (11.44) | 37.86 (12.11) | 39.38 (10.90) | 41.16 (8.08) |
| Control | 40.84 (13.30) | 37.95 (10.88) | 43.25 (9.70) | 46.39 (11.94) |
| Verbal ability (Verbal Fluency) | ||||
| ADT | 24.65 (7.11) | 26.15 (9.14) | 26.29 (7.44) | 25.70 (8.29) |
| Control | 25.39 (6.04) | 26.58 (8.14) | 25.00 (7.32) | 26.76 (6.74) |
| Executive function (Stroop) | ||||
| ADT | 51.00 (18.15) | 55.52 (22.11) | 47.58 (11.64) | 48.82 (13.31) |
| Control | 47.61 (9.03) | 47.82 (8.06) | 46.11 (9.15) | 44.88 (11.11) |
| Executive function (SOPT) | ||||
| ADT | 13.22 (9.70) | 15.66 (8.50)* | 12.35 (10.16) | 14.00 (11.04) |
| Control | 13.66 (8.61) | 9.70 (5.71) | 10.53 (5.84) | 11.88 (6.52) |
Hormone Analyses
A repeated measures ANOVA revealed a significant change over time in the treatment group only for total testosterone F (3,26) = 65.9, p<.01 with planned comparisons revealing a significant difference between months 3 and 9 compared to baseline p<0.05. There was a group by time interaction for month 3 compared to baseline F (1,28) = 95.1, p<.01 and for month 9 compared to baseline F (1, 28) = 91.5, p<.01 (See Figure 2). Mean serum testosterone (ng/mL) levels and standard deviations are included in Table 1. Estradiol decreased significantly over time in the ADT group F (3,26) = 8.6, p<.01 with pairwise comparisons revealing a significant difference between months 3 and 9 and baseline p<0.05. The control group demonstrated an increase over time F (3,26) = 4.9 p<.01 due to a slight spike at month 3 compared to baseline p<0.05. There was a group by time interaction for month 3 compared to baseline F (1,28) = 34.3, p<.01 and month 9 compared to baseline F (1,28) = 11.8, p<.01 (See Figure 2). Mean serum testosterone and estradiol levels are included in Table 1. Accuracy of serum hormone levels are not reliable when they are near the detectable level of the assay. Thus the fluctuations in both the ADT and control group may be related to the very low levels of estradiol in men which are near the detectable level of the assay and therefore are not reliable.
Figure 2.
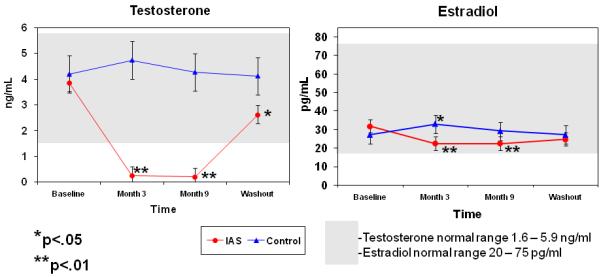
Mean serum Testosterone (left) and Estradiol (right) levels. ADT group demonstrated a significant decline in testosterone and estradiol levels during treatment. Controls demonstrated a significant increase in estradiol levels at month 3 compared to baseline. There was a group by time interaction effect (p<.05) for both Testosterone and estradiol.
Neuropsychological measures
Overall, compared to baseline in the ADT group, results indicate increased difficulties with executive functions as demonstrated by an increase in errors on the SOPT. Difficulties with spatial reasoning were evident from a decrease in the number of completed designs for complex design construction and increased errors on the mental rotation task. Other cognitive abilities appear to have remained stable or improved slightly such as the performance on story recall although this change was not significant. Details of significant findings between groups and across time follow and unless otherwise noted are non-significant. Omnibus MANOVA with all neuropsychological measures revealed a significant group by time interaction F(8,39) = 2.51 p<0.05. Subsequent ANOVAs for each test revealed a significant interaction effect for SOPT errors for month 3 compared to baseline F (1, 31) = 4.26, p<.05, number of completed block designs for month 3 compared to baseline F (1, 27) = 3.89, p<.05, and number of correct responses on mental rotation for month three compared to baseline F (1, 29) = 5.87, p<.05. Changes over time for mental rotation errors was significant in the ADT group alone F (1, 27) = 4.31, p<.05 and not the control group and the between group comparison was significant at month 3 F(1, 29) = 4.22, p<.05. Months 9 and 12 compared to baseline were non- significant as well as between group comparisons at baseline, months 9 or 12 for mental rotation. The ADT group evidenced a significant drop in the number of completed designs on the complex design construction task over time F (3, 25) = 3.21, p<.05 which was not observed in the control group. This was due to a drop in completed designs at month 3 compared to baseline (p<0.05) but not for months 9 and 12 compared to baseline or between groups at those timepoints. An interaction effect was observed for the SOPT with an increase in errors for the ADT group. However, due to a drop in errors at month three for the control group the interaction effect may be secondary to this pattern observed in both groups rather than due to change in the ADT group alone.
Mood measures
The ADT group evidenced an increase in self-rated depression, irritability, moodiness, tension, and fatigue. Details of significant findings between groups and across time follow and unless otherwise noted are non-significant. Omnibus MANOVA with all mood measures (POMS and VAS subscales) revealed a significant group by time interaction F(10,86) = 2.36 p<0.05. Subsequent ANOVAs for each mood measure revealed a significant increase in POMS fatigue in the ADT group at month 9 compared to baseline F(1,30)=11.7,(P<.05) and a trend for month three compared to baseline (p<0.08). There was a significant difference between the ADT and control group at month 3 F(1, 30) = 5.74, p<.05 with the ADT group endorsing a significantly higher level of depression symptoms on the POMS, although comparisons to baseline were not significant. For VAS items the ADT group evidenced a significant change over time F(12,19)=3.25, p<.01 which was not evident in the control group. An examination of the VAS items revealed a significant increase in self-endorsed irritability F(1,30) 4.81, p<.05 at month three compared to baseline p<0.05. To examine interactions between changes in mood and cognitive measures, MANOVAs as described above were repeated with cognitive measures as dependent variables, time and diagnosis (patient, control) as between factors and change (month nine minus baseline) in POMS subtests (tension-anxiety (TA), depression-dejection (Dep), anger-hostility (AH), vigor-activity (VA), fatigue-inertia (FI), confusion-bewilderment (CON)) as co-variates were performed. However, mood factors were not significant as co-variates and therefore did not appreciably add to the model beyond time and group factors.
DISCUSSION
Results indicate healthy men with non-metastatic prostate cancer, who are treated with ADT, may experience a decline in spatial ability, working memory and mood changes such as increased irritability, depressed mood, decreased energy and vigor. These changes were most pronounced after three months of combined androgen blockade with some evidence of a return to function toward the end of treatment at month 9. Most but not all of these changes appeared to return to baseline after stopping ADT. Although the sample size from this study is limited, it is comparable to other published studies of ADT, and current findings replicate our previous report of a decline in spatial reasoning ability [28]. Other cognitive domains appear to be unchanged including verbal memory, verbal fluency and selective attention. The pattern of some cognitive abilities remaining stable in contrast to other areas of cognition changing suggests selective effects of androgens and/or estrogen on cognition and mood. Previous studies examining endogenous levels of androgens generally support a relationship between androgens and spatial reasoning [41, 70]. However, not all studies are supportive [71] and some suggest that this relationship is modulated by hormone levels indicating there may be an ideal or optimal level [72, 73]. Animal models have shown that androgen ablation alters frontal dopaminergic axon density which affects selective aspects of executive functions such as working memory (e.g. mental scratch pad) but not set shifting (e.g. switching from one task to another) [18]. Our results also suggest that working memory as measured by the SOPT a task of working memory was adversely affected whereas performance on the Stroop test a measure of selective attention was unchanged. These cognitive changes are within the context of a change from baseline, and therefore do not necessarily represent impairment in comparison to age matched normative data. Further, due to a change in the control group, we cannot be confident of the findings for the SOPT task.
Previous studies examining cognitive abilities in men with prostate cancer undergoing androgen ablation have found mixed results. In one study by Green and colleagues (2002), men with advanced prostate cancer were randomized to one of four palliative care treatment regimens including “no treatment” with close monitoring [74]. Decreases in performance on a verbal memory test were found for patients assigned to GnRH analog (goserelin) but improved performance was noted for those assigned to single agent steroidal anti-androgen (cyproterone acetate -CPA). The authors suggest that the differences in findings amongst groups may be due to the various medications and their mechanisms of action. A particular strength of the Green et al. (2002) study is the use of a randomized design. However, the present study, like others utilized a healthy, non-metastatic sample with rising PSA and therefore a randomization scheme that includes no treatment is not feasible. Salminen and colleagues (2003) performed serial testing at baseline, 6 and 23 months with a computerized battery to examine men with localized prostate cancer who were receiving flutamide plus LHRH analogue. Patients were impaired compared to healthy controls at baseline for attention (digit span and digit symbol) and for verbal fluency. After 12 months of androgen deprivation, the patients improved on two memory tests compared to baseline and there were no changes on a depression measure [15]. A subsequent study by Salminen and colleagues (2004) with another sample of men undergoing their first cycle of 12 months of androgen deprivation revealed impairments on visuomotor tasks and a correlation between the magnitude of testosterone loss and cognitive performance [29]. However, improvement on the object recall memory test was replicated. Almeida et al. (2004) reported no significant cognitive changes in a group of men receiving ADT (one week of antiandrogen followed by nine months of GnRH analog) but an increase in depression and anxiety symptoms [30]. More recently, Jenkins and colleagues (2005) reported on a group of men with solid tumors who underwent three weeks of ADT (cyproterone acetate (CPA) followed by 3-5 months of goserelin) and radiotherapy [16]. Cognitive testing was done at baseline and after 3 – 5 months of ADT. Although there were no between group differences at any time point using parametric statistical analyses, a reliable change index approach revealed 47% of patients and 17% of controls had a decline on at least one task.
Our findings are consistent with other studies with regard to findings of spatial reasoning and executive function deficits [16, 29]. Also, similar to other studies we observed improvement in verbal memory although this finding was not statistically significant. However, current results are distinctive with regard to a strong off-on-off study design that included assessment of patients prior to, during and following cessation of androgen deprivation treatment and a battery of neurocognitive tests assessing multiple cognitive domains along with mood. Our findings suggest that ADT may result in cognitive impairments in some but not all men. This is certainly consistent with findings of Jenkins et al. (2005) and Green et al. (2002) in which a subset of men demonstrated cognitive impairments. It is possible that some patients may experience a significant decline whereas other patients may remain stable or experience a slight improvement due to practice effects. However, statistical analyses that use the mean or average over time in this situation would likely result in essentially ‘no’ findings or no change. This phenomenon of intra-individual response has been suggested in studies examining cognitive changes in women with breast cancer undergoing chemotherapy, hormonal therapy or both [75].
In addition to cognitive changes, we observed significant changes in mood. In particular, men undergoing ADT evidenced an increase in self endorsed fatigue, depression, moodiness, irritability, tension, anxiety and loss of vigor. While our findings are consistent with increases in depression and anxiety reported by other studies of men undergoing ADT, [26, 30] several other studies have failed to find significant changes in mood from androgen deprivation [16, 29, 74, 76]. POMS scores changed from baseline during androgen deprivation, although the changes were not in the clinically significant range (e.g. T score greater than 60). Therefore, it is possible that while patients may report noticing a change it is more reflective of a change from baseline rather than a mood state that might receive a clinical diagnosis. However, there is some indication that when ADT patients are given a diagnostic depression instrument, approximately 12.8% meet clinical criteria for depression [77]. Our results using the POMS, VAS and the study that utilized the diagnostic instrument may be different due to potential response bias with self report versus interviewer administered measures. Response biases can include social desirability and a tendency for men to have difficulty with or reluctance to report mood problems. We also included a visual analogue scale (VAS) for mood items shown to be sensitive to pre-menstrual dysphoric disorder (PDD) such as irritability and moodiness. Both of these mood items increased compared to baseline for ADT group. It is possible that due to the fluctuation in hormones, the mood changes that occur in men undergoing ADT may be best characterized as an atypical depression with prominent components of irritability, moodiness and emotional lability similar to PDD.
In the present study, men in the treatment group received both a GnRH analog as well as a non-steroidal anti-androgen (flutamide or bicalutimide). Animal studies indicate that flutamide administered directly into the brain of rats has adverse effects on cognitive performance [21, 78]. Many other studies have used an anti-androgen initially for a few weeks followed by a GnRH analog alone for the remainder of treatment. Thus, our findings have the most relevance for men undergoing combined androgen blockade (CAB) treatment (anti-androgen plus GnRH agonist). The anti-androgens used in this study were non-steroidal anti-androgens as compared to cyproterone acetate (CPA) a steroidal anti-androgen that lowers T, often to castrate levels. Non-steroidal antiandrogens alone do not usually lower T levels and can actually result in increasing T levels above normal over time. A recent review suggests that non-steroidal anti-androgens may be more tolerable to patients and have fewer quality of life side effects compared with agents like CPA [79]. Thus, the treatment agents used and duration of treatment may contribute to the mixture of findings regarding androgen deprivation treatment and cognition and mood reported in the literature. This is suggested by the findings of Green et al. (2002) in which participants randomized to one of four treatments had different outcomes with regard to cognitive and mood changes compared to the other treatment groups.
The present study has several limitations. The sample size is relatively small and findings should be replicated with a larger sample. However, these findings duplicate and expand our prior pilot experience in the same population of patients treated in the same manner with the addition of a healthy non-treated group in this study. ADT patients were referred from a specialized oncology clinic and it was not possible to compare demographics or other characteristics of participants who agreed to enter the study to those who refused. Several studies have reported significant differences between men with prostate cancer and healthy controls at baseline prior to the initiation of androgen deprivation [15, 26] suggesting that a non-cancer group may not be the optimal choice for a control group.
In summary, this study prospectively examined cognition and mood changes in otherwise healthy, men with non-metastatic prostate cancer undergoing to intermittent androgen deprivation therapy (ADT). Our findings indicate that men undergoing ADT evidence a decline in spatial reasoning and working memory abilities although the functional significance of these changes remains unclear. Individual patients may notice such changes more acutely based on pre-treatment abilities, activities or co-morbidities. In addition, men undergoing ADT endorse increases in fatigue, depression, moodiness, irritability, tension and anxiety and loss of vigor which returns to near baseline 3 months after stopping ADT. Future studies should address the functional significance of these changes and what potential biological or individual differences may pre-dispose some individuals to be more vulnerable to changes.
Acknowledgements
The authors wish to thank Marisa Johnson, Teresa Gambol, and Jennifer Jenkins for their excellent technical assistance. This study was supported in part by NIA K01AG00858, M01-RR-00037 and Department of Defense, Prostate Cancer Research Program (DAMD17-03-1-0045) and VAMC. Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
References
- 1.Society AC. Overview: Prostate Cancer. Vol. 2008 2008. [Google Scholar]
- 2.Higano C. Androgen deprivation therapy: monitoring and managing the complications. Hematol Oncol Clin North Am. 2006;20:909–23. doi: 10.1016/j.hoc.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Holmes L, Jr., Chan W, Jiang Z, Du XL. Effectiveness of androgen deprivation therapy in prolonging survival of older men treated for locoregional prostate cancer. Prostate Cancer Prostatic Dis. 2007 doi: 10.1038/sj.pcan.4500973. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez ML, Keane TE, Evans CP. Managing prostate cancer: the role of hormone therapy. Can J Urol. 2007;14(Suppl 1):10–8. [PubMed] [Google Scholar]
- 5.Akakura K, Bruchovsky N, Goldenberg SL, Rennie PS, Buckley AR, Sullivan LD. Effects of intermittent androgen suppression on androgen-dependent tumors. Apoptosis and serum prostate-specific antigen. Cancer. 1993;71:2782–90. doi: 10.1002/1097-0142(19930501)71:9<2782::aid-cncr2820710916>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Sato N, Gleave ME, Bruchovsky N, Rennie PS, Goldenberg SL, Lange PH, Sullivan LD. Intermittent androgen suppression delays progression to androgen- independent regulation of prostate-specific antigen gene in the LNCaP prostate tumour model. J Steroid Biochem Mol Biol. 1996;58:139–46. doi: 10.1016/0960-0760(96)00018-0. [DOI] [PubMed] [Google Scholar]
- 7.Stoch SA, Parker RA, Chen L, Bubley G, Ko YJ, Vincelette A, Greenspan SL. Bone loss in men with prostate cancer treated with gonadotropin- releasing hormone agonists. J Clin Endocrinol Metab. 2001;86:2787–91. doi: 10.1210/jcem.86.6.7558. [DOI] [PubMed] [Google Scholar]
- 8.Higano C, Shields A, Wood N, Brown J, Tangen C. Bone mineral density in patients with prostate cancer without bone metastases treated with intermittent androgen suppression. Urology. 2004;64:1182–6. doi: 10.1016/j.urology.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 10.Joly F, Alibhai SM, Galica J, Park A, Yi QL, Wagner L, Tannock IF. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J Urol. 2006;176:2443–7. doi: 10.1016/j.juro.2006.07.151. [DOI] [PubMed] [Google Scholar]
- 11.Kato T, Komiya A, Suzuki H, Imamoto T, Ueda T, Ichikawa T. Effect of androgen deprivation therapy on quality of life in Japanese men with prostate cancer. Int J Urol. 2007;14:416–21. doi: 10.1111/j.1442-2042.2007.01748.x. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro PJ, Coyne JC, Kruus LK, Palmer SC, Vaughn DJ, Malkowicz SB. Interest in services among prostate cancer patients receiving androgen deprivation therapy. Psychooncology. 2004;13:512–25. doi: 10.1002/pon.769. [DOI] [PubMed] [Google Scholar]
- 13.Herr HW, Kornblith AB, Ofman U. A comparison of the quality of life of patients with metastatic prostate cancer who received or did not receive hormonal therapy. Cancer. 1993;71:1143–50. doi: 10.1002/1097-0142(19930201)71:3+<1143::aid-cncr2820711437>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Herr HW, O’Sullivan M. Quality of life of asymptomatic men with nonmetastatic prostate cancer on androgen deprivation therapy. J Urol. 2000;163:1743–6. [PubMed] [Google Scholar]
- 15.Salminen E, Portin R, Korpela J, Backman H, Parvinen LM, Helenius H, Nurmi M. Androgen deprivation and cognition in prostate cancer. Br J Cancer. 2003;89:971–6. doi: 10.1038/sj.bjc.6601235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins VA, Bloomfield DJ, Shilling VM, Edginton TL. Does neoadjuvant hormone therapy for early prostate cancer affect cognition? Results from a pilot study. BJU Int. 2005;96:48–53. doi: 10.1111/j.1464-410X.2005.05565.x. [DOI] [PubMed] [Google Scholar]
- 17.Lamb DS, Denham JW, Mameghan H, Joseph D, Turner S, Matthews J, Franklin I, Atkinson C, North J, Poulsen M, Kovacev O, Robertson R, Francis L, Christie D, Spry NA, Tai KH, Wynne C, Duchesne G. Acceptability of short term neo-adjuvant androgen deprivation in patients with locally advanced prostate cancer. Radiother Oncol. 2003;68:255–67. doi: 10.1016/s0167-8140(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 18.Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav. 2007;51:183–94. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Hajszan T, MacLusky NJ, Johansen JA, Jordan CL, Leranth C. Effects of androgens and estradiol on spine synapse formation in the prefrontal cortex of normal and testicular feminization mutant male rats. Endocrinology. 2007;148:1963–7. doi: 10.1210/en.2006-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci. 2001;1:371–81. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- 21.Naghdi N, Oryan S, Etemadi R. The study of spatial memory in adult male rats with injection of testosterone enanthate and flutamide into the basolateral nucleus of the amygdala in Morris water maze. Brain Res. 2003;972:1–8. doi: 10.1016/s0006-8993(03)02227-3. [DOI] [PubMed] [Google Scholar]
- 22.Cherrier MM. Androgens and cognitive function. J Endocrinol Invest. 2005;28:65–75. [PubMed] [Google Scholar]
- 23.Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999;84:3681–5. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- 24.Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87:5001–7. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- 25.Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behavioral Neuroscience. 1994;108:325–32. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- 26.Beer TM, Bland LB, Bussiere JR, Neiss MB, Wersinger EM, Garzotto M, Ryan CW, Janowsky JS. Testosterone loss and estradiol administration modify memory in men. J Urol. 2006;175:130–5. doi: 10.1016/S0022-5347(05)00049-2. [DOI] [PubMed] [Google Scholar]
- 27.Green HJ, Pakenham KI, Gardiner RA. Effects of lutenizing hormone releasing hormone analogs on cognition in women and men: A review. Psychology, Health and Medicine. 2000;5:407–18. [Google Scholar]
- 28.Cherrier MM, Rose AL, Higano C. The effects of combined androgen blockade on cognitive function during the first cycle of intermittent androgen suppression in patients with prostate cancer. J Urol. 2003;170:1808–11. doi: 10.1097/01.ju.0000091640.59812.83. [DOI] [PubMed] [Google Scholar]
- 29.Salminen EK, Portin RI, Koskinen A, Helenius H, Nurmi M. Associations between serum testosterone fall and cognitive function in prostate cancer patients. Clin Cancer Res. 2004;10:7575–82. doi: 10.1158/1078-0432.CCR-04-0750. [DOI] [PubMed] [Google Scholar]
- 30.Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology. 2004;29:1071–81. doi: 10.1016/j.psyneuen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Falleti MG, Maruff P, Collie A, Darby DG. Practice effects associated with the repeated assessment of cognitive function using the CogState battery at 10-minute, one week and one month test-retest intervals. J Clin Exp Neuropsychol. 2006;28:1095–112. doi: 10.1080/13803390500205718. [DOI] [PubMed] [Google Scholar]
- 32.Tiernan K, Schenk K, Shimonova M, Schollaert D, Swadberg D, Boorkman P, Cherrier MM. An Examination of the Validity and Reliability of a Novel Route Test in Healthy Older Adults and AD Patients. The Clinical Neuropsychologist. 2004;8:39–42. [Google Scholar]
- 33.Cherrier MM, Asthana S, Baker LD, Plymate S, Matsumoto A, Peskind E, Raskind MA, Brodkin K, Bremner W, Petrova A, Latendresse S, Craft S. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57:80–8. doi: 10.1212/wnl.57.1.80. [DOI] [PubMed] [Google Scholar]
- 34.Cherrier MM, Matsumoto AM, Amory JK, Johnson M, Craft S, Peskind ER, Raskind MA. Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology. 2007;32:72–9. doi: 10.1016/j.psyneuen.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wechsler D. Weschler Adult Intelligence Scale -Revised. The Psychological Corporation; San Antonio, Texas: 1981. [Google Scholar]
- 36.O’Connor DB, Archer J, Hair WM, Wu FC. Activational effects of testosterone on cognitive function in men. Neuropsychologia. 2001;39:1385–94. doi: 10.1016/s0028-3932(01)00067-7. [DOI] [PubMed] [Google Scholar]
- 37.Wechsler D. WAIS-III administration and scoring manual. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 38.Vandenberg SG, Kuse AR. Mental rotations, a group test of three-dimensional spatial visualization. Perceptual and Motor Skills. 1978;47:599–601. doi: 10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- 39.Kimura D. Sex and Cognition. The MIT Press; Cambridge, MA: 1999. [Google Scholar]
- 40.Silverman I, Kastuk D, Choi J, Phillips K. Testosterone levels and spatial ability in men. Psychoneuroendocrinology. 1999;24:813–22. doi: 10.1016/s0306-4530(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 41.Hooven CK, Chabris CF, Ellison PT, Kosslyn SM. The relationship of male testosterone to components of mental rotation. Neuropsychologia. 2004;42:782–90. doi: 10.1016/j.neuropsychologia.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 42.Schoning S, Engelien A, Kugel H, Schafer S, Schiffbauer H, Zwitserlood P, Pletziger E, Beizai P, Kersting A, Ohrmann P, Greb RR, Lehmann W, Heindel W, Arolt V, Konrad C. Functional anatomy of visuo-spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia. 2007 doi: 10.1016/j.neuropsychologia.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Sommer IE, Cohen-Kettenis PT, van Raalten T, Vd Veer AJ, Ramsey LE, Gooren LJ, Kahn RS, Ramsey NF. Effects of cross-sex hormones on cerebral activation during language and mental rotation: An fMRI study in transsexuals. Eur Neuropsychopharmacol. 2008;18:215–21. doi: 10.1016/j.euroneuro.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science. 1971;171:701–3. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- 45.Shepard S, Metzler D. Mental rotation: effects of dimensionality of objects and type of task. J Exp Psychol Hum Percept Perform. 1988;14:3–11. [PubMed] [Google Scholar]
- 46.Moscovitch M. Cognitive resources and dual-task interference effects on retrieval in normal people: The role of the frontal lobes and medial temporal cortex. Neuropsychology. 1994;8:524–34. [Google Scholar]
- 47.Shimamura AP, Jurica PJ, Mangels JA, Gershberg FB. Susceptibility to memory interference effects following frontal lobe damage: Findings from tests of paired-associate learning. Journal of Cognitive Neuroscience. 1995;7:144–52. doi: 10.1162/jocn.1995.7.2.144. [DOI] [PubMed] [Google Scholar]
- 48.Loewenstein DA, Acevedo A, Agron J, Duara R. Vulnerability to proactive semantic interference and progression to dementia among older adults with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;24:363–8. doi: 10.1159/000109151. [DOI] [PubMed] [Google Scholar]
- 49.Postle BR, Brush LN, Nick AM. Prefrontal cortex and the mediation of proactive interference in working memory. Cogn Affect Behav Neurosci. 2004;4:600–8. doi: 10.3758/cabn.4.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–93. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 51.McDonald CR, Bauer RM, Grande L, Gilmore R, Roper S. The role of the frontal lobes in memory: evidence from unilateral frontal resections for relief of intractable epilepsy. Arch Clin Neuropsychol. 2001;16:571–85. [PubMed] [Google Scholar]
- 52.Jacoby LL, Debner JA, Hay JF. Proactive interference, accessibility bias, and process dissociations: valid subjective reports of memory. J Exp Psychol Learn Mem Cogn. 2001;27:686–700. [PubMed] [Google Scholar]
- 53.Wechsler D. Wechsler Memory Scale -Revised. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- 54.Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999;56:1135–40. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- 55.Craft S, Dagogo-Jack E, Wiethop CM, Nevins R, Fleischman S, Rice V, Newcomer J, Cryer PE. The effects of hyperglycemia on memory and hormone levels in Dementia of the Alzheimer’s Type: A longitudinal study. Behavioral Neuroscience. 1993;107:926–41. doi: 10.1037//0735-7044.107.6.926. [DOI] [PubMed] [Google Scholar]
- 56.Craft S, Murphy C, Wemstrom J. Glucose effects on memory and complex non-memory measures: The influence of age, sex, and glucoregulatory response. Psychobiology. 1994;22:95–105. [Google Scholar]
- 57.Craft S, Zallen G, Baker LD. Glucose and memory in mild senile dementia of the Alzheimer type. J Clin Exp Neuropsychol. 1992;14:253–67. doi: 10.1080/01688639208402827. [DOI] [PubMed] [Google Scholar]
- 58.Wolf OT, Kirschbaum C. Endogenous estradiol and testosterone levels are associated with cognitive performance in older women and men. Horm Behav. 2002;41:259–66. doi: 10.1006/hbeh.2002.1770. [DOI] [PubMed] [Google Scholar]
- 59.Spreen O, Strauss E. A compendium of neuropsychological tests. Oxford University Press; New York: 1991. [Google Scholar]
- 60.Lezak MD. Neuropsychologcial Assessment. Third ed. Oxford University Press; New York, NY: 1995. [Google Scholar]
- 61.Glueck CJ, Glueck HI, Stroop D, Speirs J, Hamer T, Tracy T. Endogenous testosterone, fibrinolysis, and coronary heart disease risk in hyperlipidemic men. The Journal of laboratory and clinical medicine. 1993;122:412–20. [PubMed] [Google Scholar]
- 62.Petrides M, Milner B. Deficits on subject-ordered tasks after frontal and temporal lobe lesions in man. Neuropsychologia. 1982;20:249–62. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 63.Bryan J, Luszcz MA. Adult age differences in Self-Ordered Pointing Task performance: Contributions from working memory, executive function and speed of information processing. J Clin Exp Neuropsychol. 2001;23:608–19. doi: 10.1076/jcen.23.5.608.1250. [DOI] [PubMed] [Google Scholar]
- 64.Daigneault S, Braun CM. Working memory and the Self-Ordered Pointing Task: Further evidence of early prefrontal decline in normal aging. J Clin Exp Neuropsychol. 1993;15:881–95. doi: 10.1080/01688639308402605. [DOI] [PubMed] [Google Scholar]
- 65.McNair DM, Lorr M, Droppleman LF. Profile of Mood States. Educational and Industrial Testing Service; San Diego, CA: 1981. [Google Scholar]
- 66.Steiner M, Streiner DL. Validation of a revised visual analog scale for premenstrual mood symptoms: results from prospective and retrospective trials. Canadian journal of psychiatry. 2005;50:327–32. doi: 10.1177/070674370505000607. [DOI] [PubMed] [Google Scholar]
- 67.Pearlstein TB, Halbreich U, Batzar ED, Brown CS, Endicott J, Frank E, Freeman EW, Harrison WM, Haskett RF, Stout AL, Yonkers KA. Psychosocial functioning in women with premenstrual dysphoric disorder before and after treatment with sertraline or placebo. J Clin Psychiatry. 2000;61:101–9. doi: 10.4088/jcp.v61n0205. [DOI] [PubMed] [Google Scholar]
- 68.Huber A, Suman AL, Rendo CA, Biasi G, Marcolongo R, Carli G. Dimensions of “unidimensional” ratings of pain and emotions in patients with chronic musculoskeletal pain. Pain. 2007;130:216–24. doi: 10.1016/j.pain.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 69.Di Benedetto M, Lindner H, Hare DL, Kent S. A Cardiac Depression Visual Analogue Scale for the brief and rapid assessment of depression following acute coronary syndromes. J Psychosom Res. 2005;59:223–9. doi: 10.1016/j.jpsychores.2005.06.070. [DOI] [PubMed] [Google Scholar]
- 70.Driscoll I, Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. Virtual navigation in humans: the impact of age, sex, and hormones on place learning. Horm Behav. 2005;47:326–35. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 71.McKeever WF, Rich DA, Deyo RA, Conner RL. Androgens and spatial ability: Failure to find a relationship between testosterone and ability measures. Bulletin of the Psychonomic Society. 1987;25:438–40. [Google Scholar]
- 72.Moffat SD, Hampson E. A curvilinear relationship between testosterone and spatial cognition in humans: possible influence of hand preference. Psychoneuroendocrinology. 1996;21:323–37. doi: 10.1016/0306-4530(95)00051-8. [DOI] [PubMed] [Google Scholar]
- 73.Hampson E, Moffat SD. Is Testosterone Related to Spatial Cognition and Hand Preference in Humans? Brain and Cognition. 1994;26:255–66. doi: 10.1006/brcg.1994.1060. [DOI] [PubMed] [Google Scholar]
- 74.Green HJ, Pakenham KI, Headley BC, Yaxley J, Nicol DL, Mactaggart PN, Swanson C, Watson RB, Gardiner RA. Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing hormone analogues and cyproterone acetate: a randomized controlled trial. BJU Int. 2002;90:427–32. doi: 10.1046/j.1464-410x.2002.02917.x. [DOI] [PubMed] [Google Scholar]
- 75.Falleti MG, Sanfilippo A, Maruff P, Weih L, Phillips KA. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: a meta-analysis of the current literature. Brain Cogn. 2005;59:60–70. doi: 10.1016/j.bandc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 76.Taxel P, Stevens MC, Trahiotis M, Zimmerman J, Kaplan RF. The effect of short-term estradiol therapy on cognitive function in older men receiving hormonal suppression therapy for prostate cancer. J Am Geriatr Soc. 2004;52:269–73. doi: 10.1111/j.1532-5415.2004.52067.x. [DOI] [PubMed] [Google Scholar]
- 77.Pirl WF, Siegel GI, Goode MJ, Smith MR. Depression in men receiving androgen deprivation therapy for prostate cancer: a pilot study. Psychooncology. 2002;11:518–23. doi: 10.1002/pon.592. [DOI] [PubMed] [Google Scholar]
- 78.Edinger KL, Frye CA. Androgens’ performance-enhancing effects in the inhibitory avoidance and water maze tasks may involve actions at intracellular androgen receptors in the dorsal hippocampus. Neurobiol Learn Mem. 2007;87:201–8. doi: 10.1016/j.nlm.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 79.Gillatt D. Antiandrogen treatments in locally advanced prostate cancer: are they all the same? Journal of cancer research and clinical oncology. 2006;132(Suppl 1):S17–26. doi: 10.1007/s00432-006-0133-5. [DOI] [PubMed] [Google Scholar]


