Abstract
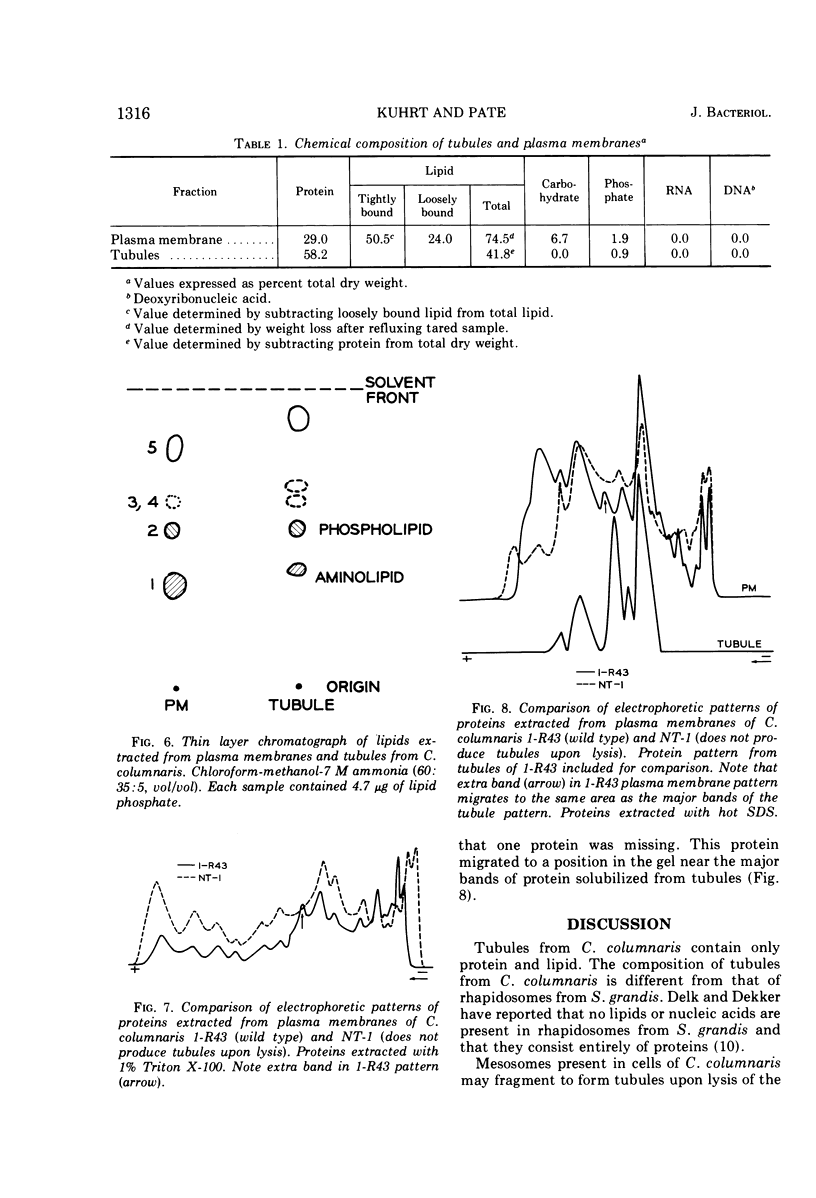
Tubular structures are released from cells of Cytophaga columnaris after lysis of the cells. To determine the nature of these tubules, they were purified and their composition was determined. Tubules were isolated after treating cell lysates with 1.0% sodium dodecyl sulfate at pH 8.1, which solubilizes all structural components except tubules. Plasma membranes from the same organism were isolated by discontinuous sucrose gradient centrifugation of lysed cells. Both tubules and membranes are composed of lipids and proteins. Lipids extracted from tubules and plasma membranes produced similar patterns when examined by thin-layer chromatography. Proteins solubilized from membranes were separated into 14 bands by polyacrylamide gel electrophoresis, whereas those solubilized from tubules separated into only 5 bands. The presence of lipids in tubules from C. columnaris supports the idea that they are derived from membranes of intact cells. In this respect they are similar to tubules produced by cells of Clostridium botulinum and different from other tubular structures (“rhapidosomes”) found in cells of Saprospira grandis.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baechler C. A., Taylor A. R., Berk R. S. Electron microscopic examination and quantitation of rhapidosomes, viruses and bacteria concentrated at an aqueous-air interface. Prep Biochem. 1972;2(3):287–296. doi: 10.1080/00327487208061478. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. The isolation and morphology of some new bacteriophages specific for Bacillus and Acetobacter species. J Gen Microbiol. 1965 Nov;41(2):233–241. doi: 10.1099/00221287-41-2-233. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D. Association of microcyst formation in Spirillum itersonii with the spontaneous induction of a defective bacteriophage. J Bacteriol. 1969 Feb;97(2):885–892. doi: 10.1128/jb.97.2.885-892.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S. Disruption of T-even bacteriophages by dimethyl sulfoxide. J Virol. 1968 Jun;2(6):610–620. doi: 10.1128/jvi.2.6.610-620.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Delk A. S., Dekker C. A. Characterization of rhapidosomes of Saprospira grandis. J Mol Biol. 1972 Feb 28;64(1):287–295. doi: 10.1016/0022-2836(72)90336-1. [DOI] [PubMed] [Google Scholar]
- Dulaney J. T., Touster O. The solubilization and gel electrophoresis of membrane enzymes by use of detergents. Biochim Biophys Acta. 1970 Jan 6;196(1):29–34. doi: 10.1016/0005-2736(70)90162-8. [DOI] [PubMed] [Google Scholar]
- Dworkin M. Biology of the myxobacteria. Annu Rev Microbiol. 1966;20:75–106. doi: 10.1146/annurev.mi.20.100166.000451. [DOI] [PubMed] [Google Scholar]
- Garnjobst L. Cytophaga columnaris (Davis) in Pure Culture: A Myxobacterium Pathogenic to Fish. J Bacteriol. 1945 Feb;49(2):113–128. doi: 10.1128/jb.49.2.113-128.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräf W. Bewegungsorganellen bei Myxobakterien. Arch Hyg Bakteriol. 1965 Jul;149(5):518–526. [PubMed] [Google Scholar]
- HOENIGER J. F. CELLULAR CHANGES ACCOMPANYING THE SWARMING OF PROTEUS MIRABILIS. I. OBSERVATIONS OF LIVING CULTURES. Can J Microbiol. 1964 Feb;10:1–9. doi: 10.1139/m64-001. [DOI] [PubMed] [Google Scholar]
- Horzinek M., Mussgay M. Studies on the substructure of togaviruses. I. Effect of urea, deoxycholate, and saponin on the Sindbis virion. Arch Gesamte Virusforsch. 1971;33(3):296–305. doi: 10.1007/BF01254686. [DOI] [PubMed] [Google Scholar]
- Hougen K. H., Birch-Andersen A. Electron microscopy of endoflagella and microtubules in Treponema reiter. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(1):37–50. doi: 10.1111/j.1699-0463.1971.tb00031.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Steers E., Jr Selective solubilization of a protein component of the red cell membrane. Science. 1968 Jan 12;159(3811):203–204. doi: 10.1126/science.159.3811.203. [DOI] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation by density gradient centrifugation of two types of membranes from spheroplast membrane of Escherichia coli K12. Biochim Biophys Acta. 1968 Jan 3;150(1):159–161. doi: 10.1016/0005-2736(68)90020-5. [DOI] [PubMed] [Google Scholar]
- Pate J. L., Johnson J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. II. Structure and formation of rhapidosomes. J Cell Biol. 1967 Oct;35(1):15–35. doi: 10.1083/jcb.35.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. I. Structure and formation of mesosomes. J Cell Biol. 1967 Oct;35(1):1–13. doi: 10.1083/jcb.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot J. Study of human red blood cell membrane using sodium deoxycholate. I. Mechanism of the solubilization. Biochim Biophys Acta. 1971 Feb 2;225(2):201–213. doi: 10.1016/0005-2736(71)90213-6. [DOI] [PubMed] [Google Scholar]
- Pope L. M., Jurtshuk P. Microtubule in Azotobacter vinelandii strain O. J Bacteriol. 1967 Dec;94(6):2062–2064. doi: 10.1128/jb.94.6.2062-2064.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach H. Die wahre Natur der Myxobakterien- "Rhapidosomen". Arch Mikrobiol. 1967 Apr 17;56(4):371–383. [PubMed] [Google Scholar]
- SHAFA F., SALTON M. R. Disaggregation of bacterial cell walls by anionic detergents. J Gen Microbiol. 1960 Aug;23:137–141. doi: 10.1099/00221287-23-1-137. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H. Composition of the membranes isolated from several Gram-positive bacteria. Biochim Biophys Acta. 1965 Oct 18;107(3):531–538. doi: 10.1016/0304-4165(65)90197-2. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. J Bacteriol. 1970 Nov;104(2):882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. Presence of rhapidosomes in various species of bacteria and their morphological characteristics. J Bacteriol. 1967 Nov;94(5):1746–1756. doi: 10.1128/jb.94.5.1746-1756.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iterson W., Hoeniger J. F., Nijman van Zanten E. A "microtubule" in a bacterium. J Cell Biol. 1967 Jan;32(1):1–10. doi: 10.1083/jcb.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]





