Abstract
γ-Secretase is a multiprotein, intramembrane-cleaving protease with a growing list of protein substrates, including the Notch receptors and the amyloid precursor protein. The four components of γ-secretase complex—presenilin (PS), nicastrin (NCT), Pen2, and Aph1—are all thought to be essential for activity. The catalytic domain resides within PS proteins, NCT has been suggested to be critical for substrate recognition, and the contributions of Pen2 and Aph1 remain unclear. The role of NCT has been challenged recently by the observation that a critical residue (E332) in NCT, which had been thought to be essential for γ-secretase activity, is instead involved in complex maturation. Here, we report that NCT is dispensable for γ-secretase activity. NCT-independent γ-secretase activity can be detected in two independent NCT-deficient mouse embryonic fibroblast lines and blocked by the γ-secretase inhibitors N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester and L-685,458. This catalytic activity requires prior ectodomain shedding of the substrate and can cleave ligand-activated endogenous Notch receptors, indicating presence of this activity at the plasma membrane. Small interfering RNA knockdown experiments demonstrated that NCT-independent γ-secretase activity requires the presence of PS1, Pen2, and Aph1a but can tolerate knockdown of PS2 or Aph1b. We conclude that a PS1/Pen2/Aph1a trimeric complex is an active enzyme, displaying biochemical properties similar to those of γ-secretase and roughly 50% of its activity when normalized to PS1 N-terminal fragment levels. This PS1/Pen2/Aph1a complex, however, is highly unstable. Thus, NCT acts to stabilize γ-secretase but is not required for substrate recognition.
Introduction
γ-Secretase is a multiprotein, intramembrane-cleaving protease that cleaves its substrates within their transmembrane domains (TMDs) (Selkoe and Kopan, 2003). Biologically and clinically important γ-secretase substrates (Fagan et al., 2007; Selkoe and Wolfe, 2007; Dejaegere et al., 2008) include amyloid precursor protein (APP) (Haass, 2004; Thinakaran and Koo, 2008; Wolfe, 2008), ErbB4 (Ni et al., 2001), neuregulin (Bao et al., 2003), and the Notch receptors (De Strooper et al., 1999). The physiological and pathological roles of γ-secretase, its potential as a drug target in cancer and Alzheimer's disease (Miele et al., 2006; Roy et al., 2007; Aster et al., 2008; Tomita, 2009), and its novel function as an intramembrane-cleaving protease have generated intense commercial and academic interest in this mysterious enzyme. A better understanding of its structure, substrate recognition, and regulation will improve therapeutic exploitation of this complex protease (Kukar et al., 2008; Wolfe, 2009).
Genetic and biochemical studies have provided strong evidence that γ-secretase is a multiprotein complex comprised of presenilin (PS; either PS1 or PS2), which contains the catalytic site (De Strooper et al., 1998; Wolfe et al., 1999; Li et al., 2000); nicastrin (NCT), which is thought to contain a substrate binding site (Shah et al., 2005); and Pen2 and Aph1 (1a, 1b, or 1c in rodents), which are of unclear contribution (De Strooper, 2003; Iwatsubo, 2004). Loss of any of these four proteins seemed to abolish γ-secretase activity (Francis et al., 2002; Takasugi et al., 2003), and only coexpression of the four components together reconstitutes γ-secretase activity in yeast (Edbauer et al., 2003) or SF9 cells (Hayashi et al., 2004), both of which lack endogenous γ-secretase activity. Consistent with the existence of several distinct γ-secretases, the active enzyme contains four components, each with potentially unique properties (Serneels et al., 2009).
PS is the founding member of a novel GxGD-type aspartyl protease family that includes the prokaryotic type IV prepilin peptidase (TFPP) and the eukaryotic signal peptide peptidase (SPP) and SPP-like proteases (SPPLs) (Haass and Steiner, 2002). In contrast to PS, TFPP, SPP, and SPPL proteins are single-chain enzymes, performing their activity without additional partners (Golde et al., 2009; Wolfe, 2009). It is still unclear why PS needs several cofactors. The precise role for NCT within γ-secretase has been controversial. NCT may facilitate substrate recognition (Shah et al., 2005; Dries et al., 2009) or may instead act to stabilize the γ-secretase complex (Shirotani et al., 2004; Zhang et al., 2005); a critical residue thought to be involved in substrate recognition (E332) was shown to be essential for γ-secretase maturation but not for its activity in mouse cells (Chavez-Gutierrez et al., 2008). Thus, the exact role of NCT remains controversial.
To elucidate the contribution of NCT to γ-secretase, we analyzed γ-secretase activity in two independent NCT knock-out cell lines. We found that a complex containing PS1, Pen2, and Aph1a could cleave Notch and APP at the same sites used by holo-γ-secretase. The presence of catalytic activity demonstrated unequivocally that NCT is not required for γ-secretase substrate recognition, instead acting mainly to stabilize γ-secretase.
Materials and Methods
Plasmids.
pCS2+/N1ΔE-6MT, pCS2+/C99-6MT, and pcDNA3/PS1wt vectors have been described previously (Kopan et al., 1996; Schroeter et al., 2003).
Cell lines.
NCT-deficient Ncstntm1Pcw/Ncstntm1Pcw (NCTPW−/−) and wild-type (NCTPW+/+) mouse embryonic fibroblast (MEF) lines were generous gifts from Dr. Philip C. Wong (Johns Hopkins University, Baltimore, MD) (Li et al., 2003). PS1/PS2 double knock-out (PSDKO) and PS1/PS2 wild-type (PS+/+) MEF lines were generous gifts from Dr. Bart De Strooper (Katholieke Universiteit Leuven, Leuven, and Flanders Institute for Biotechnology, Leuven, Belgium) (Herreman et al., 1999). We established Ncstntm1Rroz/ Ncstntm1Rroz MEF using NCT+/− breeders generously provided by Dr. Richard Rozmahel (University of Toronto, Toronto, Canada) (Nguyen et al., 2006) using a previously described method (Li et al., 2003) but with a slight modification. Briefly, embryonic day (E)9.5 embryos were minced and resuspended in DMEM containing 0.025% trypsin/EDTA and incubated at 37°C for 20 min. DMEM supplemented with 10% fetal bovine serum (FBS) was added to neutralize the trypsin/EDTA, and the cells were further dissociated by pipetting, plated into 24-well plates, and subsequently immortalized with polyoma large T antigen. Immortalized NCT knock-out clones were confirmed by both genotyping and Western blot analysis.
Cell culture and plasmid transfection.
For most experiments, cells were maintained in DMEM supplemented with 10% FBS, 2 mm glutamine and incubated at 37°C and 5% CO2. To examine the generation of Notch intracellular domain (NICD) and amyloid intracellular domain (AICD), cells were transfected with pCS2+/N1ΔE-6MT and pCS2+/C99-6MT vectors or empty vector using Lipofectamine 2000 (Invitrogen) or FuGENE HD (Roche) transfection reagents, according to the manufacturers' instructions. The transfected cells were subsequently cultured in the presence or absence of proteasome inhibitors (4.5 μm lactacystin/0.15 μm MG262; Calbiochem) and γ-secretase inhibitors [either 1 μm N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester (DAPT) or 1 μm L-685,458; Calbiochem].
Aβ analysis.
pCS2+/C99-6MT-transfected cells were cultured in OPTI-MEM supplemented with 7% FBS, 2 mm glutamine, 10 mm nonessential amino acids, 10 μm phosphoramidon disodium (Sigma), with or without proteasome inhibitors and γ-secretase inhibitors. Both cells and conditioned media (CMs) were collected 20–22 h later. The CMs were supplemented with 1 mm AEBSF [4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride; Roche], centrifuged, and analyzed by Aβ ELISA (Invitrogen) according to the manufacturer's instructions, but with a slight modification. The incubation time for antibodies and stabilized chromogen and the time for each wash step were increased to make testing lower levels of Aβ more accurate. Each sample was run in duplicate. The CMs from cells transfected with empty vector were used as blanks.
Coculture of NCTRR−/− cells with ligand-expressing cells.
NCTRR−/− or PSDKO cells were coseeded with either CHO-DLL1-IRES-GFP (CHO: Chinese hamster ovary; IRES: internal ribosome entry site; GFP: green fluorescent protein) or CHO-GFP control cells (these two cell lines have been described previously) (Ong et al., 2008), cultured for 24 h, and then treated with or without lactacystin/MG262 and DAPT. The cells were collected after 20 h and lysed with lysis buffer A containing 8 m urea, 1% SDS, 50 mm Tris-HCl, pH 6.8, 2 mm EDTA, 2 mm DTT, and 1× protease inhibitor cocktail (Roche) (Zhao et al., 2004).
siRNA transfection.
ON-TARGETplus small interfering RNA (siRNA) smart pools targeting mouse PS1, PS2, Pen2, Aph1a, and Aph1b were purchased from Dharmacon (see supplemental Table 1, available at www.jneurosci.org as supplemental material for sequences). ON-TARGETplus nontargeting siRNA control (Dharmacon) was used as a negative control. siRNAs were transfected into NCT−/− cells using DharmaFect 1 (Dharmacon) according to the manufacturer's instructions. The cells were cultured in complete medium for 48 h and then transfected with pCS2+/N1ΔE-6MT vector. Knockdown efficiency was determined by either Western blotting or quantitative reverse transcription (qRT)-PCR.
qRT-PCR.
siRNA-transfected NCTRR−/− cells were cultured for 68 h. The total RNA was then isolated using RNeasy Micro kit (Qiagen) according to the manufacturer's instructions. The reverse transcription was performed using SuperScript II reverse transcriptase (Invitrogen) with oligo(dT)16 as the primer. Quantitative PCR was performed according to previously described methods (Lee et al., 2007) (see supplemental Table 2, available at www.jneurosci.org as supplemental material for primer sequences).
EDTA treatment.
NCTRR−/−, NCTPW−/−, PSDKO, and PS+/+ cells were washed once with HBSS buffer (without Ca or Mg) and incubated in 1.5 mm EDTA/HBSS buffer for 40 min at 37°C. For γ-secretase inhibition, cells were treated with 1 μm DAPT for 4 h before and during EDTA incubation.
Western blotting and antibodies.
Cells were lysed in lysis buffer A with brief sonication, and the protein concentration was determined using a BCA kit (Pierce). Twenty to 30 μg of total protein per sample was analyzed by SDS-PAGE/Western blot analysis. The following antibodies were used in this study: anti-V1744 (Cell Signaling Technology), anti-NCT (N1660, Sigma), anti-PS1-NTF (Santa Cruz Biotechnology), anti-PS2-CTF antibodies B24.2 and G2L [generous gifts from Dr. Bart De Strooper (Herreman et al., 1999) and Dr. Taisuke Tomita (Tomita et al., 1998), respectively], anti-Aph1a (Covance), anti-Notch1 ANK domain (mAN1) (Huppert et al., 2000), anti-β-actin (Sigma), and anti-myc (9E10).
Results
NICD is generated in NCT−/− cells but not in PSDKO cells
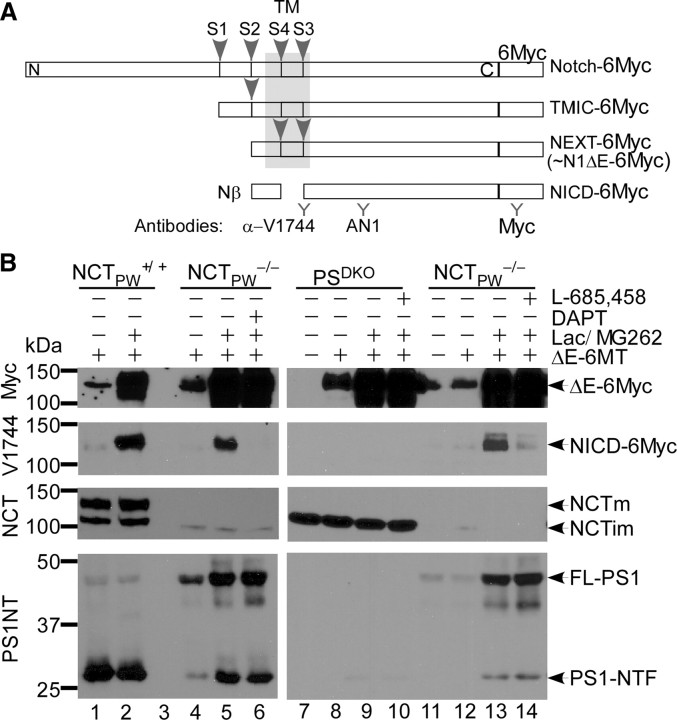
All GxGD proteases prefer substrates that have undergone ectodomain shedding, and all, including PS, contain a substrate recognition domain (Kornilova et al., 2005). We hypothesized that if the role of NCT was to stabilize γ-secretase, but not to bind substrates, we should be able to detect γ-secretase activity in the absence of NCT. To test this hypothesis, we transiently transfected wild-type (NCTPW+/+) and NCTPW−/− cells (from Dr. P.C. Wong) with ectodomain-truncated, Notch1-expressing vector (pCS2+/N1ΔE-6MT). The truncated Notch1 ΔE-6myc protein is a ligand-independent and direct substrate of γ-secretase, similar to Notch extracellular truncation (NEXT) (Fig. 1A). To enhance detection, transfected cells were cultured in the presence of proteasome inhibitors that reduce the turnover of the NICD (Tagami et al., 2008) and increase the sensitivity of detection for this cleavage product. Western blot analyses with an anti-myc antibody indicated that N1ΔE-6MT expression levels in NCTPW+/+ and NCTPW−/− cells were equivalent and that proteasome inhibition increased the amounts of N1ΔE-6MT (Fig. 1B, lanes 2, 5, and 6), presumably by decreasing degradation of uncleaved molecules. Consistent with previous reports (Zhang et al., 2005), we also observed PS1 N-terminal fragment (PS1-NTF) in NCTPW−/− cells, an indication that some presenilinase activity remained (Fig. 1B, lane 4). We then assessed N1ΔE-6MT cleavage between G1743-V1744 with an epitope-specific antibody (α-VLLS; i.e., V1744 antibody). When probed with anti-V1744 antibody, NICD was detected in NCTPW+/+ cells as expected. Significantly, in the presence of proteasome inhibitors, PS1-NTF and NICD accumulated in NCTPW−/− cells (Fig. 1B, lane 5). To examine whether the NICD generated in NCTPW−/− cells was produced by an aspartyl protease, we asked whether this NCT-independent protease was sensitive to a selective γ-secretase inhibitor (GSI), DAPT (N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester) (Weihofen et al., 2003). DAPT completely abolished the generation of NICD in NCTPW−/− cells (Fig. 1B, lane 6), suggesting that NICD is generated by a γ-secretase-like activity in the absence of NCT. Generation of NICD in NCTPW−/− cells was also blocked by a structurally distinct GSI, L-685,458 (Fig. 1B, lane 14). We therefore concluded that in the NCTPW−/− cell line, Notch is cleaved at the peptide bond between G1743 and V1744 [site 3 (S3)] by a GSI-sensitive activity.
Figure 1.
NICD is generated by a GSI-sensitive activity in NCT−/− cells but not in PSDKO cells. Representative Western blots of NICD levels in pCS2+/N1ΔE-6MT-transfected NCTPW+/+, NCTPW−/−, and PSDKO cells are shown. A, Schematic representation of Notch C-terminal fragments (CTFs). TMIC (transmembrane/intracellular), NEXT, and NICD denote Notch CTFs generated after S1, S2, and S3 site cleavages, respectively. B, Cells were transiently transfected with pCS2+/N1ΔE-6MT or pCS2+ empty vector. N1ΔE-6MT does not contain the extracellular domain, thereby making it a ligand-independent, direct substrate of γ-secretase. NICD accumulated in both NCTPW+/+ and NCTPW−/− cells cultured in the presence of proteasome inhibitors (lactacystin/MG262) but not in PSDKO cells; DAPT and L-685,458 blocked NICD generation. NCTm and NCTim denote mature and immature forms of NCT, respectively. FL-PS1, Full-length PS1.
To our knowledge, γ-secretase is the only enzyme that cleaves Notch1 at its S3 site, but the existence of other enzymes with γ-secretase-like activity has been proposed to compensate for PS loss (Huppert et al., 2005). To ask whether a Notch intramembrane protease exists in other γ-secretase-deficient cells, we examined NICD generation in PSDKO cells, which are deficient in both PS1 and PS2. Western blot analyses showed that both PSDKO and NCTPW−/− cells expressed high levels of N1ΔE-6MT, yet NICD was undetectable in samples from PSDKO cells under the same conditions that allow NICD accumulation in NCTPW−/− samples (Fig. 1B, lanes 9 and 13). The generation of NICD in PSDKO cells can be rescued by transfection of wild-type PS1 or PS2 but not an inactive aspartyl mutant PS1, confirming that the failure to generate NICD by PSDKO cells is not an indication of other defects (supplemental Fig. 2, available at www.jneurosci.org as supplemental material) (Schroeter et al., 2003). Overall, these results indicate that a γ-secretase-like activity in NCTPW−/− cells cleaves Notch to generate NICD and that this activity does not exist in PSDKO cells.
Generation of NICD is observed in two independent NCT−/− lines
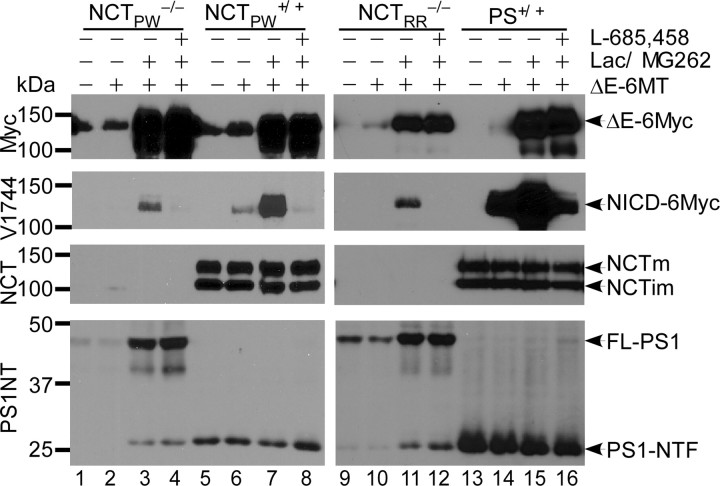
We noticed a faint cross-reacting band with mobility similar to that of immature NCT in NCTPW−/− cells (Fig. 1B). NCTPW−/− cells were derived from an NCT knock-out embryo in which the critical part of the DAP (DYIGS and peptidase homologous region) domain (exon 9, intron 9, and part of exon 10) was replaced with the neomycin resistance gene (Li et al., 2003). To test whether γ-secretase-like activity in this specific cell line was due to residual NCT expression, we analyzed a second, independently generated NCT knock-out mouse line (from Dr. Richard Rozmahel) in which exon 3 and a part of exon 4 had been deleted, and therefore splicing downstream of the deletion would shift the reading frame (Nguyen et al., 2006). We isolated NCTRR−/− MEF cells from E9.5 NCTRR−/− embryos and examined NCT production and γ-secretase activity in these cells. No NCT immunoreactivity was observed in NCTRR−/− cell extracts (Fig. 2, lanes 9–12). Importantly, as in NCTPW−/− cells, endoproteolysis of PS1 in NCTRR−/− was also observed, confirming that PS endoproteolysis does not require NCT. Accordingly, in the presence of proteasome inhibitors we observed NICD accumulation in NCTRR−/− cells (Fig. 2, lane 11). In this cell line, NICD production was again blocked by either L-685,458 (Fig. 2, lane 12) or DAPT (Fig. 3A). Overall, these data clearly demonstrate that generation of NICD by residual γ-secretase-like activity is independent of the specific NCT−/− line used.
Figure 2.
Generation of NICD is observed in two independent NCT−/− lines. Representative Western blots of NICD levels generated in pCS2+/N1ΔE-6MT-transfected NCTPW−/−, NCTRR−/−, NCTPW+/+, and PS+/+ cells are shown. NICD was detected in both NCTPW−/− and NCTRR−/− lysates and was blocked by the γ-secretase inhibitor L-685,458. Because NCTRR−/− cells express less N1ΔE-6MT compared with other cell lines, a longer exposure was used for detecting NICD. The two wild-type MEF lines (NCTPW+/+ and PS+/+) and wild-type MEFs isolated from littermates of NCTRR−/− mice displayed similar γ-secretase activity (lanes 5–8, 13–16, and data not shown). FL-PS1, Full-length PS1.
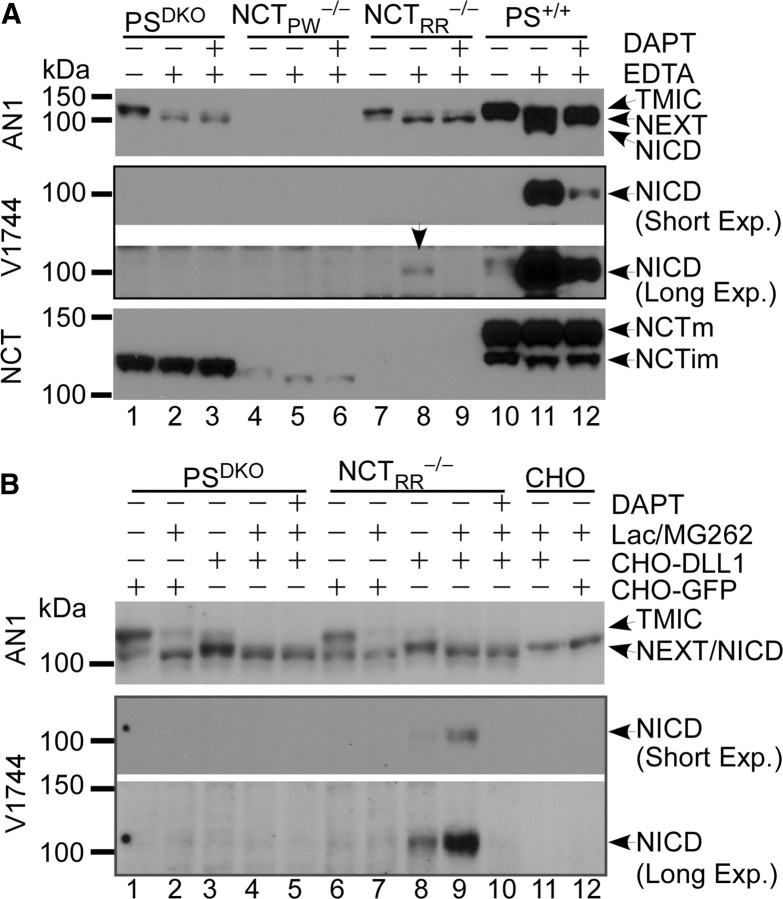
Figure 3.
NICD is generated from endogenous Notch1 in NCT−/− cells but not in PSDKO cells. A, Representative Western blots of NICD levels in EDTA-treated cells. Metalloprotease-mediated shedding occurs after calcium chelation-induced dissociation of endogenous Notch1. EDTA treatment led to reduced amounts of furin-cleaved Notch [transmembrane/intracellular (TMIC)] and increased amounts of NEXT, indicating that upon EDTA treatment Notch1 undergoes S2 site cleavage in PSDKO, NCTRR−/−, and PS+/+ cells. Although endogenous Notch1 levels are similar in both PSDKO and NCTRR−/− cells, NICD is produced from NEXT only in NCTRR−/− (arrow) cells but not in PSDKO cells. NCTPW−/− cells do not express endogenous Notch1. B, Representative Western blots of NICD levels in NCTRR−/− cells cocultured with Notch ligand-expressing cells. NICD is generated in NCTRR−/− cells from endogenous Notch1 receptors after activation by ligands presented by neighboring cells. Note that NICD was only detected in coculture of NCTRR−/− cells with CHO-DLL1 cells but not in the other cocultures or CHO cells alone. DAPT treatment blocked the generation of NICD. Long Exp., Long exposure; Short Exp., short exposure.
Endogenous Notch can be cleaved by the γ-secretase-like activity in NCT−/− cells but not in PSDKO cells
The γ-secretase-like activity that survived removal of NCT may only cleave ectopically expressed Notch substrates lacking an extracellular domain. To ask whether this enzyme could cleave endogenous Notch, we examined the cleavage of endogenous Notch1 receptors under conditions that induce ectodomain shedding at the cell surface. In the absence of ligands, a calcium-stabilized negative regulatory region prevents metalloprotease access to the S2 cleavage site (Gordon et al., 2007). Ligand binding or treatment with EDTA (Rand et al., 2000) is thought to result in a conformational change that leads to the exposure of the S2 cleavage site and subsequent cleavage by ADAM (a disintegrin and metalloproteinase) metalloproteases (Kopan and Ilagan, 2009), generating NEXT (Fig. 1A), a direct substrate of γ-secretase. Western blot analyses showed that all the MEF lines (except for NCTPW−/−) expressed endogenous Notch1. To test whether the generation of NICD from endogenous Notch1 can occur without NCT protein, PSDKO cells and NCTPW−/−, NCTRR−/−, and wild-type MEFs were treated with or without EDTA to induce S2 cleavage (see shift in mobility in Fig. 3A). Importantly, after treatment with EDTA, DAPT-sensitive NICD generation was detected in NCTRR−/− and wild-type cells (Fig. 3A, lanes 8–9 and 11–12). Moreover, after EDTA treatment, endogenous NICD was observed in NCT−/− cells even without proteasome inhibition (Fig. 3A,B). This result confirms that ectodomain shedding is required for the release of NICD in NCTRR−/− cells (Fig. 3A, compare lanes 7 and 8). In contrast, Notch1-expressing PSDKO cells accumulated the S2 cleavage product NEXT but did not produce NICD (Fig. 3A, lanes 1 and 2). Because removal of calcium with EDTA occurs at or near the cell surface and because both ADAM10 and Notch1 are mainly localized at the plasma membrane (van Tetering et al., 2009), this result suggests that the NCT-deficient γ-secretase-like protease performs its activity at or close to the plasma membrane.
To obtain physiologically relevant evidence that γ-secretase-like activity reached the cell surface, we asked whether the Notch1 receptors in NCTRR−/− cells could be activated by ligands presented on neighboring cells. We cocultured NCTRR−/− (or control PSDKO cells) with either ligand-expressing (CHO-DLL1) or control (CHO-GFP) cells in the presence or absence of proteasome inhibitors. Western blot analysis shows that coculture with CHO-DLL1 cells induced endogenous Notch1 S2 cleavage (Fig. 3B). As expected, if the enzyme reached the cell surface, endogenous NICD was detected in NCTRR−/− cells cocultured with CHO-DLL1 (Fig. 3B, lanes 8 and 9) but not in NCTRR−/− cells cocultured with CHO-GFP. DAPT blocked the generation of NICD in NCTRR−/− cells (Fig. 3B, lane10), and PSDKO cells cocultured with CHO-DLL1 cells did not release NICD, indicating that NICD was not generated by the CHO cells (Fig. 3B, lanes 4, 5, 11, and 12). Together, these results confirm that the γ-secretase-like activity in NCT−/− cells requires substrate ectodomain shedding and can reach the plasma membrane to mediate the cleavage of ligand-activated Notch.
C99 is converted into AICD and Aβ in NCT−/− cells but not in PSDKO cells
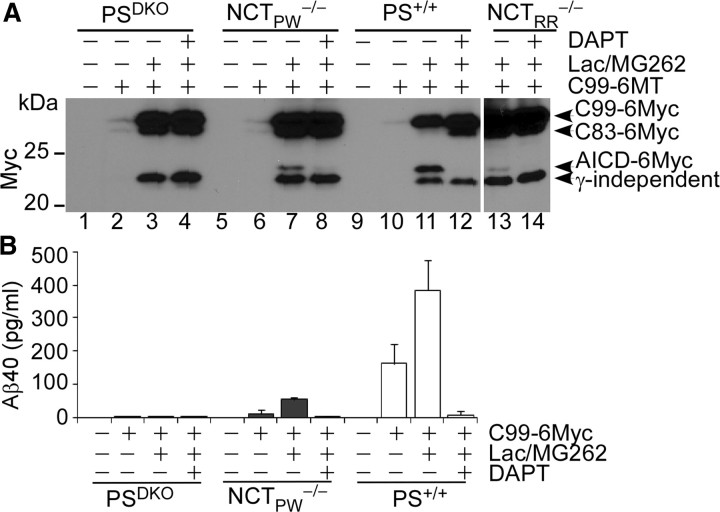
Another important substrate of γ-secretase is APP, which is involved in the pathogenesis of Alzheimer's disease. Since mutations in NCT were shown previously to differentially affect APP and Notch processing (Chen et al., 2001), we asked whether a truncated APP substrate (C99) could be processed in NCT−/− cells by the γ-secretase-like activity. We transiently transfected PSDKO, NCTPW−/−, NCTRR−/−, and wild-type MEF cells with C99-6myc-expressing vector. Western blot analyses confirmed that a DAPT-sensitive fragment (AICD-6myc) present in wild-type cells but not in PSDKO cells was also seen in two NCT-deficient cell lines (Fig. 4A, lanes 3, 7, and 13), suggesting that NCT−/− cells are able to cleave C99 (Fig. 4A; note that proteasome inhibition was required to detect the cleavage products). A longer exposure detected low amounts of AICD-myc in NCTPW−/− cells not treated with proteasome inhibitors (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). To address whether Aβ was also produced in NCT−/− cells, we performed ELISA analysis on conditioned media to determine the secreted Aβ levels. Aβ40 was detected in conditioned media from NCT−/− cells but not from PSDKO cells (Fig. 4B) and was abolished after DAPT addition (Fig. 4B). We were unable to detect Aβ42 levels with the Aβ42 ELISA kit, likely due to the low transfection efficiency of MEF cells. Collectively, these data indicate that not only can the residual γ-secretase activity cleave APP, it can proceed from the ε-cleavage site to the γ-cleavage site, a hallmark of γ-secretase (Tanzi and Bertram, 2005; Haass and Selkoe, 2007), even in the absence of NCT.
Figure 4.
C99 is cleaved to generate AICD and Aβ in NCT−/− cells but not in PSDKO cells. A, Representative Western blots of AICD levels in C99-6MT-transfected cells. PSDKO, NCTPW−/−, NCTRR−/−, and PS+/+ cells were transiently transfected with pCS2+/C99-6MT or empty vector and analyzed for AICD and Aβ generation. A DAPT-sensitive AICD-6Myc band was observed in NCT−/− cells and wild-type MEFs but not in PSDKO cells. A γ-secretase-independent, faster-migrating Myc-reactive band was observed in all samples (it was not sensitive to DAPT and was generated in PSDKO cells). B, Aβ40 is produced in NCT−/− cells but not in PSDKO cells. The CM from cells transfected with empty vector was used as the blank in the assay.
γ-Secretase-like activity in NCT−/− cells is PS1 dependent
The fact that two unrelated γ-secretase inhibitors abolished the γ-secretase-like activity in NCT−/− cell lines, and that PSDKO cells did not exhibit this activity, strongly implies that PS is the active enzyme in NCT−/− cells. To test this, we used siRNAs to knock down PS1 and PS2 (alone or together) in NCTRR−/− cells transfected with N1ΔE-6MT. Western blot analysis confirmed that PS1 siRNA markedly decreased both full-length PS1 and PS1-NTF (Fig. 5A, lanes 3 and 4), whereas control siRNA and PS2 siRNA did not affect PS1 protein level (Fig. 5A, lanes 1, 2, 5, and 6). Interestingly, PS1 knockdown alone was sufficient to significantly diminish NICD production, whereas knockdown of PS2 did not show an obvious effect (Fig. 5A, lanes 5–6). Since we could not detect PS2 in these cells with two different established antibodies (Tomita et al., 1998; Herreman et al., 1999), the efficiency of PS2 siRNA knockdown was confirmed by qRT-PCR (Fig. 5B). Accordingly, cotransfection of PS1 siRNA together with PS2 siRNA did not further reduce NICD amounts (Fig. 5A, lanes 7 and 8). Similar results were obtained with NCTPW−/− cells (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). These results suggest that PS1 or a PS1-containing enzyme in NCT−/− cells mediates NICD production.
Figure 5.
γ-Secretase-like activity in NCT−/− cells is PS1 dependent. A, Representative Western blots of NICD levels in PS siRNA-transfected NCTRR−/− cells. NCTRR−/− cells were transfected with PS1, PS2, PS1-plus-PS2, or nontargeting control siRNAs. The cells were subsequently transfected with pCS2+/N1ΔE-6MT and cultured in the presence of proteasome inhibitors. Cell lysates were then analyzed by Western blotting with antibodies against PS1NT, Myc, V1744, and β-actin. PS1 siRNA markedly decreased both full-length PS1 (FL-PS1) and PS1-NTF. Although the levels of N1ΔE-6myc are similar in every extract, NICD accumulation was strikingly reduced in PS1 siRNA-transfected cells and in PS1-plus-PS2 siRNA-transfected cells but not in siRNA control (Ctr)- or PS2 siRNA-transfected cells. B, Since the PS2 proteins in these NCT−/− cells were undetectable by Western blot analysis using two well-characterized anti-PS2 antibodies (data not shown), knockdown of PS2 was confirmed by qRT-PCR.
To ask whether PS2 can contribute to γ-secretase activity in NCT−/− cells, we overexpressed PS2 in NCTRR−/− cells. We first performed PS1 mRNA knockdown and then asked whether cotransfection of the substrate (pCS2+/N1ΔE-6MT) with either human PS1 or PS2 expression vector into these mouse PS1-depleted NCT−/− cells would restore the γ-secretase activity. While both PS1 and PS2 restored γ-secretase activity equally well in PSDKO cells, only human PS1, but not human PS2, restored γ-secretase activity in PS1 siRNA-transfected NCT−/− cells (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). These data confirmed that PS2 protein could not contribute to the γ-secretase activity in NCT−/− cells.
Pen2 and Aph1a are involved in the γ-secretase-like activity
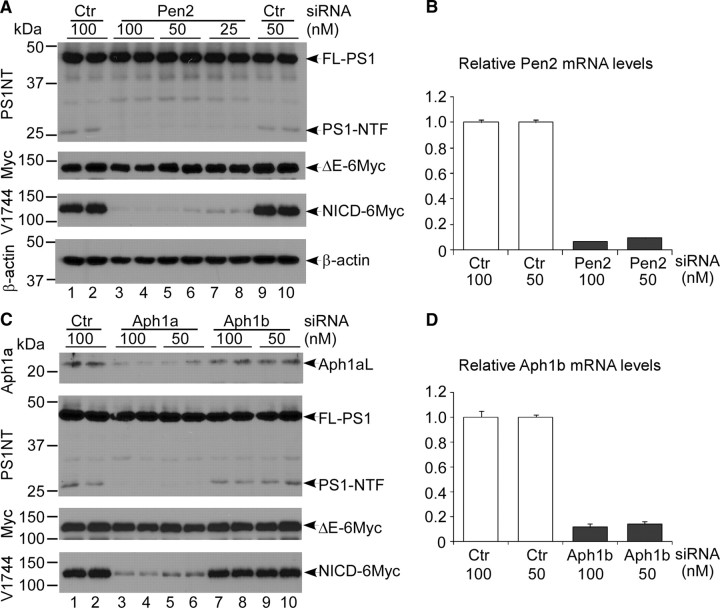
To ask whether PS1 acted as a single-molecule protease like SPP (Golde et al., 2009), we examined whether Pen2 and Aph1 were still required for the PS1 activity in the absence of NCT. We performed Pen2 or Aph1 knockdown in NCTRR−/− cells using siRNA pools and confirmed the efficiencies of siRNA knockdown by qRT-PCR (Fig. 6B,D). Western blot analyses demonstrated that knockdown of Pen2 did not decrease the amount of full-length PS1 but dramatically reduced PS1-NTF levels and, concomitantly, the amounts of NICD (Fig. 6A, lanes 3–8). Of the three murine Aph1 genes (Ma et al., 2005; Serneels et al., 2005), only knockdown of Aph1a in NCTRR−/− cells reduced PS1-NTF and NICD levels, whereas full-length PS1 levels remained unchanged (Fig. 6C, lanes 3–6). Aph1b siRNA did not impact PS1-NTF or NICD level (Fig. 6C, lanes 7–10). Similar results were obtained with NCTPW−/− cells (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). These results suggest that in NCT−/− cells, an unstable γ-secretase isomorph composed of three proteins (PS1, Pen2, and Aph1a) provides γ-secretase activity that correlates strongly with the amount of endoproteolytically processed PS1-NTF but not full-length PS1. Thus, PS1 activity requires endoproteolysis and the functions of Pen2 and Aph1a.
Figure 6.
Pen2 and Aph1a are involved in the γ-secretase activity in the absence of NCT. Representative Western blots of NICD levels in Pen2 and Aph1 siRNA-transfected NCTRR−/− cells are shown. A, Lysates from control (Ctr) siRNA- or Pen2 siRNA-transfected NCT−/− cells were analyzed by Western blotting. Pen2 siRNA markedly decreased PS1-NTF level but not that of full-length PS1 (FL-PS1). Whereas Pen2 depletion slightly affected N1ΔE-6MT expression, it significantly diminished NICD generation in a dose-dependent manner. Note that the amounts of a PS1 fragment (∼30 kDa) increased in Pen2 siRNA-treated samples. This PS1 fragment does not have γ-secretase activity. B, The efficiency of Pen2 knockdown (∼90%) was confirmed by qRT-PCR. C, Depletion of Aph1a in NCTRR−/− cells markedly decreased PS1-NTF and NICD generation, whereas Aph1b siRNA treatment did not significantly affect NICD levels. D, Knockdown of Aph1b was confirmed by qRT-PCR.
Trimeric γ-secretase retains 50% of the enzyme activity
To accurately evaluate the remaining γ-secretase activity in NCT−/− cells, we tried to establish a cell-free assay. However, although we could readily detect γ-secretase activity in solubilized membranes from wild-type cells, we failed to detect any γ-secretase-like activity in solubilized membranes from NCT−/− cells (data not shown). It is likely that the trimeric γ-secretase complex lacking NCT is highly unstable in the detergent solution, as it was unstable in blue native gels (data not shown). Therefore, we compared the relative activity of γ-secretase in NCT−/− cells and wild-type cells using a semiquantitative Western blot analysis of extracts isolated from cells cultured in the presence of proteasome inhibitors. As shown in supplemental Figure 4 (available at www.jneurosci.org as supplemental material), in cell lysates containing equivalent levels of PS1 NTF fragments, NCT−/− cells produced 50–55% of the NICD produced by wild-type lines. From these data, we can conclude that the trimeric enzyme is much more active than would be expected if NCT was providing the substrate recognition function in the complex. We cannot rule out an indirect contribution by NCT to overall activity due to increased enzyme stability.
Discussion
PS, NCT, Pen2, and Aph1 are the four proteins essential for reconstituting robust γ-secretase activity (De Strooper, 2003; Iwatsubo, 2004; Spasic and Annaert, 2008). With the exception of PS, which harbors the catalytic site of γ-secretase, the precise contributions of the other proteins to γ-secretase activity remain unclear, and PS remains the only GxGD protease that requires partners for its protease activity. Here, we have provided direct evidence that when PS1/Pen2/Aph1a are present, γ-secretase can assemble and acquire catalytic activity without NCT, cleaving both Notch and APP (C99) substrates. Furthermore, this three-protein enzyme remains biochemically similar to the four-protein enzyme: both are DAPT and L-685,458 sensitive (Fig. 1B), both generate NICD at the S3 site (Figs. 1–3), and both cleave APP at ε and γ to release AICD and Aβ40, respectively (Fig. 4). Importantly, ligand-mediated activation of endogenous Notch can also be observed in NCT−/− cells (Fig. 3B). However, this residual activity is not sufficient to permit survival of NCT−/− embryos (Li et al., 2003; Nguyen et al., 2006).
As this manuscript was being prepared, another group identified NCT-independent PS1 mutants (PS1 S438P or PS1 F411Y/S438P) (Futai et al., 2009), suggesting that perhaps this S438P mutation increased complex stability. We aligned PS proteins with their homologues and found that a Pro residue is located at a conserved position in TMD9 of SPP (P324; supplemental Fig. 5, available at www.jneurosci.org as supplemental material), suggesting that the α-helix-breaking Pro residue may increase stability of GxGD proteases. Interestingly, this analysis revealed that one of the two Caenorhabditis elegans PS proteins, Hop-1, also contains a Pro at the homologous position (P329) whereas the other (Sel-12) does not. Consistent with the hypothesis that γ-secretase complexes centered around Hop-1 may have reduced dependency on the Aph2/NCT gene (Goutte et al., 2000), Aph-2/Hop-1 double mutants are strongly affected but Aph-2/Sel-12 double mutants are not (Francis et al., 2002). This result indicates that Hop-1 is active without Aph-2, whereas Sel-12 is dependent on Aph-2 for full activity. NCT has been proposed to play a role in PS1 trafficking. In the absence of NCT, most PS1 proteins remain in the endoplasmic reticulum; PS1-NTFs were undetectable at the plasma membrane (Zhang et al., 2005). We have provided indirect evidence that at least some PS1 NTF-containing γ-secretase can reach the plasma membrane where it can activate a ligand-dependent Notch signal in the absence of NCT. Coupled with the genetic data (Francis et al., 2002) and with the analysis of NCT mutants (Chavez-Gutierrez et al., 2008), these observations favor a model in which NCT acts to stabilize γ-secretase but is not required for catalytic activity.
Although we cannot completely rule out the possibility that NCT contributes to γ-secretase activity and that this contribution requires E333 (Dries et al., 2009), our findings indicate that neither substrate recognition nor contribution to catalytic activity by NCT is necessary for γ-secretase activity. A distinct property of γ-secretase-mediated intramembrane cleavage is that it requires prior ectodomain shedding of the substrate; the large extracellular domain of NCT has been proposed to act as the gatekeeper that measures the size of the substrate ectodomain and the availability of a free N terminus (Hu et al., 2002; De Strooper, 2005; Shah et al., 2005). However, we found that NCT-deficient γ-secretase activity still requires prior ectodomain shedding of its substrates (Fig. 3) (Mumm et al., 2000). It is unlikely that an unknown protein may function as NCT in NCT−/− cells, and no additional NCT homologues have been found in the mouse or C. elegans genome. Indeed, a substrate-binding site has also been identified at the interface of PS1 NTF/CTF (Kornilova et al., 2005); this site may also be sensitive to the presence of a large extracellular domain. Additionally, the GxGD motif in PS has been reported to contribute to substrate identification (Yamasaki et al., 2006). Furthermore, a PS relative, SPPLP2, requires substrate shedding yet does not require putative substrate binding partners (Martin et al., 2009).
How γ-secretase assembly occurs is a critical question in γ-secretase biology. One model posits that PS binds initially to Aph1 and NCT to form a subcomplex, and then this subcomplex binds to Pen2 to initiate PS endoproteolysis and NCT glycosylation (Takasugi et al., 2003). Alternatively, PS and Pen2 form a subcomplex in which PS endoproteolysis occurs while NCT and Aph1 form another subcomplex. The two subcomplexes then bind together (Spasic and Annaert, 2008). In both models, γ-secretase activity is conferred only after the complete assembly of the four-protein complex. The observations reported here suggest that a trimeric complex containing PS1, Pen2, and Aph1a is active but unstable and perhaps rapidly converted to the four-protein complex in wild-type cells. Whether such a subcomplex occurs in the presence of NCT or only forms in its absence remains to be determined.
Why PS proteins require additional stabilizing components whereas SPP and SPPL do not and why PS2 is unable to act without NCT are interesting puzzles still to be resolved. The fact that γ-secretase activity could not be detected in PSDKO cells (Figs. 1, 3, and 4) and that PS1 siRNA greatly diminished the residual γ-secretase activity in NCT−/− cells (Fig. 5A; supplemental Figs. 2 and 3, available at www.jneurosci.org as supplemental material for sequences) confirms the centrality of PS for γ-secretase activity. Interestingly, although PS possesses both substrate-binding and catalytic sites and thus has the potential to act as an enzyme on its own, knockdown of Pen2 and Aph1a eliminated γ-secretase activity in NCT−/− cells (Fig. 6). Despite establishing that PS requires these two proteins, the current data cannot distinguish whether Pen2 and Aph1 are required for stabilizing PS1 or for enhancing its catalytic activity. If Aph1 or Pen2 are required only to stabilize PS1, it is conceivable that other unstable trimeric γ-secretase complexes can be formed in vitro. It is intriguing to suggest that isolation of such an active trimeric γ-secretase might facilitate the elucidation of its structure. It also appears that active Hop-1 trimeric γ-secretase complexes may exist in vivo and can be isolated from Aph-2 mutants.
In conclusion, the present data demonstrate that NCT is dispensable for PS1-containing γ-secretase activity and cell surface transport but critical for stabilizing γ-secretase. This study also provides a biochemical explanation for the differential requirement for Aph2 shown by the two C. elegans PS proteins, Hop-1 and Sel-12.
Footnotes
This study was supported by National Institutes of Health (NIH) Grant AG025973 (R.K.), Alzheimer's Association Grant IIRG-03-5283 (R.K.), and NIH/National Institute on Aging Grant P50-AG05681 (Washington University Alzheimer's Disease Research Center). We are grateful to Dr. Philip C. Wong for providing NCT−/− cells, Dr. Richard Rozmahel for the NCT+/− mice, Dr. Bart De Strooper for the PSDKO cells and PS2 antibody, and Dr. Taisuke Tomita for PS2 antibody. We thank Dr. David Holtzman for critical reading of this manuscript and all members of the Kopan laboratory for helpful discussions and technical assistance.
References
- Aster JC, Pear WS, Blacklow SC. Notch signaling in leukemia. Annu Rev Pathol. 2008;3:587–613. doi: 10.1146/annurev.pathmechdis.3.121806.154300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Wolpowitz D, Role LW, Talmage DA. Back signaling by the Nrg-1 intracellular domain. J Cell Biol. 2003;161:1133–1141. doi: 10.1083/jcb.200212085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Gutierrez L, Tolia A, Maes E, Li T, Wong PC, de Strooper B. Glu(332) in the Nicastrin ectodomain is essential for γ-secretase complex maturation but not for its activity. J Biol Chem. 2008;283:20096–20105. doi: 10.1074/jbc.M803040200. [DOI] [PubMed] [Google Scholar]
- Chen F, Yu G, Arawaka S, Nishimura M, Kawarai T, Yu H, Tandon A, Supala A, Song YQ, Rogaeva E, Milman P, Sato C, Yu C, Janus C, Lee J, Song L, Zhang L, Fraser PE, St George-Hyslop PH. Nicastrin binds to membrane-tethered Notch. Nat Cell Biol. 2001;3:751–754. doi: 10.1038/35087069. [DOI] [PubMed] [Google Scholar]
- Dejaegere T, Serneels L, Schafer MK, Van Biervliet J, Horre K, Depboylu C, Alvarez-Fischer D, Herreman A, Willem M, Haass C, Hoglinger GU, D'Hooge R, De Strooper B. Deficiency of Aph1B/C-γ -secretase disturbs Nrg1 cleavage and sensorimotor gating that can be reversed with antipsychotic treatment. Proc Natl Acad Sci U S A. 2008;105:9775–9780. doi: 10.1073/pnas.0800507105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B. Aph-1, Pen-2, and nicastrin with presenilin generate an active γ-secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- De Strooper B. Nicastrin: gatekeeper of the γ-secretase complex. Cell. 2005;122:318–320. doi: 10.1016/j.cell.2005.07.021. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Dries DR, Shah S, Han YH, Yu C, Yu S, Shearman MS, Yu G. Glu-333 of nicastrin directly participates in γ-secretase activity. J Biol Chem. 2009;284:29714–29724. doi: 10.1074/jbc.M109.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of γ-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, Parks AL, Xu W, Li J, Gurney M, Myers RL, Himes CS, Hiebsch R, Ruble C, Nye JS, Curtis D. aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell. 2002;3:85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- Futai E, Yagishita S, Ishiura S. Nicastrin is dispensable for γ-secretase protease activity in the presence of specific presenilin mutations. J Biol Chem. 2009;284:13013–13022. doi: 10.1074/jbc.M807653200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde TE, Wolfe MS, Greenbaum DC. Signal peptide peptidases: a family of intramembrane-cleaving proteases that cleave type 2 transmembrane proteins. Semin Cell Dev Biol. 2009;20:225–230. doi: 10.1016/j.semcdb.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC. Structural basis for autoinhibition of Notch. Nat Struct Mol Biol. 2007;14:295–300. doi: 10.1038/nsmb1227. [DOI] [PubMed] [Google Scholar]
- Goutte C, Hepler W, Mickey KM, Priess JR. aph-2 encodes a novel extracellular protein required for GLP-1-mediated signaling. Development. 2000;127:2481–2492. doi: 10.1242/dev.127.11.2481. [DOI] [PubMed] [Google Scholar]
- Haass C. Take five–BACE and the γ-secretase quartet conduct Alzheimer's amyloid β-peptide generation. EMBO J. 2004;23:483–488. doi: 10.1038/sj.emboj.7600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Haass C, Steiner H. Alzheimer disease gamma-secretase: a complex story of GxGD-type presenilin proteases. Trends Cell Biol. 2002;12:556–562. doi: 10.1016/s0962-8924(02)02394-2. [DOI] [PubMed] [Google Scholar]
- Hayashi I, Urano Y, Fukuda R, Isoo N, Kodama T, Hamakubo T, Tomita T, Iwatsubo T. Selective reconstitution and recovery of functional γ-secretase complex on budded baculovirus particles. J Biol Chem. 2004;279:38040–38046. doi: 10.1074/jbc.M405597200. [DOI] [PubMed] [Google Scholar]
- Herreman A, Hartmann D, Annaert W, Saftig P, Craessaerts K, Serneels L, Umans L, Schrijvers V, Checler F, Vanderstichele H, Baekelandt V, Dressel R, Cupers P, Huylebroeck D, Zwijsen A, Van Leuven F, De Strooper B. Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc Natl Acad Sci U S A. 1999;96:11872–11877. doi: 10.1073/pnas.96.21.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Ye Y, Fortini ME. Nicastrin is required for γ-secretase cleavage of the Drosophila Notch receptor. Dev Cell. 2002;2:69–78. doi: 10.1016/s1534-5807(01)00105-8. [DOI] [PubMed] [Google Scholar]
- Huppert S, Le A, Schroeter EH, Mumm JS, Saxena MT, Milner LA, Kopan R. Embryonic lethality in mice homozygous for a processing deficient allele of Notch1. Nature. 2000;405:966–970. doi: 10.1038/35016111. [DOI] [PubMed] [Google Scholar]
- Huppert S, Ilagan MXG, De Strooper B, Kopan R. Analysis of Notch function in presomitic mesoderm suggests a γ-secretase-independent role for presenilins in somite differentiation. Dev Cell. 2005;8:677–688. doi: 10.1016/j.devcel.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T. Assembly and activation of the γ-secretase complex: roles of presenilin cofactors. Mol Psychiatry. 2004;9:8–10. doi: 10.1038/sj.mp.4001438. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Schroeter EH, Weintraub H, Nye JS. Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain. Proc Natl Acad Sci U S A. 1996;93:1683–1688. doi: 10.1073/pnas.93.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornilova AY, Bihel F, Das C, Wolfe MS. The initial substrate-binding site of γ-secretase is located on presenilin near the active site. Proc Natl Acad Sci U S A. 2005;102:3230–3235. doi: 10.1073/pnas.0407640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukar TL, Ladd TB, Bann MA, Fraering PC, Narlawar R, Maharvi GM, Healy B, Chapman R, Welzel AT, Price RW, Moore B, Rangachari V, Cusack B, Eriksen J, Jansen-West K, Verbeeck C, Yager D, Eckman C, Ye W, Sagi S, et al. Substrate-targeting γ-secretase modulators. Nature. 2008;453:925–929. doi: 10.1038/nature07055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Basak JM, Demehri S, Kopan R. Bi-compartmental communication contributes to the opposite proliferative behavior of Notch1-deficient hair follicle and epidermal keratinocytes. Development. 2007;134:2795–2806. doi: 10.1242/dev.02868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ma G, Cai H, Price DL, Wong PC. Nicastrin is required for assembly of presenilin/γ -secretase complexes to mediate Notch signaling and for processing and trafficking of β-amyloid precursor protein in mammals. J Neurosci. 2003;23:3272–3277. doi: 10.1523/JNEUROSCI.23-08-03272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, Register RB, Sardana MK, Shearman MS, Smith AL, Shi XP, Yin KC, Shafer JA, Gardell SJ. Photoactivated γ-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- Ma G, Li T, Price DL, Wong PC. APH-1a is the principal mammalian APH-1 isoform present in γ-secretase complexes during embryonic development. J Neurosci. 2005;25:192–198. doi: 10.1523/JNEUROSCI.3814-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, Fluhrer R, Haass C. Substrate requirements for SPPL2b-dependent regulated intramembrane proteolysis. J Biol Chem. 2009;284:5662–5670. doi: 10.1074/jbc.M807485200. [DOI] [PubMed] [Google Scholar]
- Miele L, Miao H, Nickoloff BJ. NOTCH signaling as a novel cancer therapeutic target. Curr Cancer Drug Targets. 2006;6:313–323. doi: 10.2174/156800906777441771. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R. A ligand-induced extracellular cleavage regulates γ-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- Nguyen V, Hawkins C, Bergeron C, Supala A, Huang J, Westaway D, St George-Hyslop P, Rozmahel R. Loss of nicastrin elicits an apoptotic phenotype in mouse embryos. Brain Res. 2006;1086:76–84. doi: 10.1016/j.brainres.2006.02.122. [DOI] [PubMed] [Google Scholar]
- Ni CY, Murphy MP, Golde TE, Carpenter G. gamma-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- Ong CT, Sedy JR, Murphy KM, Kopan R. Notch and presenilin regulate cellular expansion and cytokine secretion but cannot instruct Th1/Th2 fate acquisition. PLoS One. 2008;3:e2823. doi: 10.1371/journal.pone.0002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand DM, Grimm MLM, Artavanis-Tsakonas S, Patriub V, Blacklow CS, Sklar CJ, Aster CJ. Calcium depletion dissociates and activates heterodimeric Notch receptors. Mol Cell Biol. 2000;20:1825–1835. doi: 10.1128/mcb.20.5.1825-1835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Pear WS, Aster JC. The multifaceted role of Notch in cancer. Curr Opin Genet Dev. 2007;17:52–59. doi: 10.1016/j.gde.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Schroeter EH, Ilagan MX, Brunkan AL, Hecimovic S, Li YM, Xu M, Lewis HD, Saxena MT, De Strooper B, Coonrod A, Tomita T, Iwatsubo T, Moore CL, Goate A, Wolfe MS, Shearman M, Kopan R. A presenilin dimer at the core of the γ-secretase enzyme: insights from parallel analysis of Notch 1 and APP proteolysis. Proc Natl Acad Sci U S A. 2003;100:13075–13080. doi: 10.1073/pnas.1735338100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Wolfe MS. Presenilin: running with scissors in the membrane. Cell. 2007;131:215–221. doi: 10.1016/j.cell.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Serneels L, Dejaegere T, Craessaerts K, Horre K, Jorissen E, Tousseyn T, Hebert S, Coolen M, Martens G, Zwijsen A, Annaert W, Hartmann D, De Strooper B. Differential contribution of the three Aph1 genes to γ-secretase activity in vivo. Proc Natl Acad Sci U S A. 2005;102:1719–1724. doi: 10.1073/pnas.0408901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serneels L, Van Biervliet J, Craessaerts K, Dejaegere T, Horre K, Van Houtvin T, Esselmann H, Paul S, Schafer MK, Berezovska O, Hyman BT, Sprangers B, Sciot R, Moons L, Jucker M, Yang Z, May PC, Karran E, Wiltfang J, D'Hooge R, et al. γ -Secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer's disease. Science. 2009;324:639–642. doi: 10.1126/science.1171176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE, 3rd, Sudhof T, Yu G. Nicastrin functions as a γ-secretase-substrate receptor. Cell. 2005;122:435–447. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Shirotani K, Edbauer D, Kostka M, Steiner H, Haass C. Immature nicastrin stabilizes APH-1 independent of PEN-2 and presenilin: identification of nicastrin mutants that selectively interact with APH-1. J Neurochem. 2004;89:1520–1527. doi: 10.1111/j.1471-4159.2004.02447.x. [DOI] [PubMed] [Google Scholar]
- Spasic D, Annaert W. Building γ-secretase: the bits and pieces. J Cell Sci. 2008;121:413–420. doi: 10.1242/jcs.015255. [DOI] [PubMed] [Google Scholar]
- Tagami S, Okochi M, Yanagida K, Ikuta A, Fukumori A, Matsumoto N, Ishizuka-Katsura Y, Nakayama T, Itoh N, Jiang J, Nishitomi K, Kamino K, Morihara T, Hashimoto R, Tanaka T, Kudo T, Chiba S, Takeda M. Regulation of Notch signaling by dynamic changes in the precision of S3 cleavage of Notch-1. Mol Cell Biol. 2008;28:165–176. doi: 10.1128/MCB.00863-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the γ-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Secretase inhibitors and modulators for Alzheimer's disease treatment. Expert Rev Neurother. 2009;9:661–679. doi: 10.1586/ern.09.24. [DOI] [PubMed] [Google Scholar]
- Tomita T, Tokuhiro S, Hashimoto T, Aiba K, Saido TC, Maruyama K, Iwatsubo T. Molecular dissection of domains in mutant presenilin 2 that mediate overproduction of amyloidogenic forms of amyloid β peptides. Inability of truncated forms of PS2 with familial Alzheimer's disease mutation to increase secretion of Aβ42. J Biol Chem. 1998;273:21153–21160. doi: 10.1074/jbc.273.33.21153. [DOI] [PubMed] [Google Scholar]
- van Tetering G, van Diest P, Verlaan I, van der Wall E, Kopan R, Vooijs M. The metalloprotease ADAM10 is required for notch1 S2 cleavage. J Biol Chem. 2009;284:31018–31027. doi: 10.1074/jbc.M109.006775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihofen A, Lemberg MK, Friedmann E, Rueeger H, Schmitz A, Paganetti P, Rovelli G, Martoglio B. Targeting presenilin-type aspartic protease signal peptide peptidase with γ-secretase inhibitors. J Biol Chem. 2003;278:16528–16533. doi: 10.1074/jbc.M301372200. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. γ-Secretase inhibition and modulation for Alzheimer's disease. Curr Alzheimer Res. 2008;5:158–164. doi: 10.2174/156720508783954767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS. Intramembrane-cleaving proteases. J Biol Chem. 2009;284:13969–13973. doi: 10.1074/jbc.R800039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Yamasaki A, Eimer S, Okochi M, Smialowska A, Kaether C, Baumeister R, Haass C, Steiner H. The GxGD motif of presenilin contributes to catalytic function and substrate identification of γ-secretase. J Neurosci. 2006;26:3821–3828. doi: 10.1523/JNEUROSCI.5354-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Luo WJ, Wang H, Lin P, Vetrivel KS, Liao F, Li F, Wong PC, Farquhar MG, Thinakaran G, Xu H. Nicastrin is critical for stability and trafficking but not association of other presenilin/γ-secretase components. J Biol Chem. 2005;280:17020–17026. doi: 10.1074/jbc.M409467200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Mao G, Tan J, Dong Y, Cui MZ, Kim SH, Xu X. Identification of a new presenilin-dependent ζ-cleavage site within the transmembrane domain of amyloid precursor protein. J Biol Chem. 2004;279:50647–50650. doi: 10.1074/jbc.C400473200. [DOI] [PubMed] [Google Scholar]