Summary
Background
Dengue virus infection causes a spectrum of clinical manifestations, usually classified according to the World Health Organization (WHO) guidelines into dengue fever (DF) and dengue hemorrhagic fever (DHF). Its ability to categorize severe dengue illness has recently been questioned.
Methods
We evaluated dengue case definitions in a prospective study at a pediatric hospital in Bangkok from 1994-2005. One thousand and thirteen children were enrolled within the first three days of fever and followed with standardized data collection. Cases were classified based on application of the strict WHO criteria. All dengue virus infections were laboratory confirmed. We retrospectively grouped patients based on whether they received significant intervention based on the fluid replacement and/or requirements for blood transfusion.
Results
Fifty eight percent (85/150), 15% (40/264), and 12% (73/599) of DHF, DF and other febrile illnesses (OFI) cases, respectively, received significant intervention. Sixty-eight percent of dengue cases requiring intervention met strict WHO criteria for DHF. In contrast, only 1% of OFI cases met WHO criteria for DHF. Plasma leakage and thrombocytopenia were the two components contributing to the specificity of the WHO case definition and identified dengue cases that required intervention. Hemorrhagic tendency did not reliably differentiate DF and DHF. In DF cases, thrombocytopenia and bleeding were associated with severity.
Conclusions
Dengue illness is heterogeneous in severity, and severe clinical features occurred in patients that were not characterized as DHF. The WHO case definition of DHF demonstrates 62% sensitivity and 92% specificity in identifying dengue illness requiring intervention without the need for laboratory confirmation of dengue virus infection in endemic areas.
Keywords: dengue hemorrhagic fever, dengue fever, WHO clinical guidelines, plasma leakage, clinical severity
Introduction
Dengue hemorrhagic fever (DHF) is the leading cause of viral hemorrhagic fever worldwide [1, 2]. The classification of dengue illness was developed by clinical experts based largely on experience in children in Thailand and was originally published by the WHO in 1975 and updated in 1997 [1, 2]. Dengue illness is classified into undifferentiated febrile illness, dengue fever (DF), and DHF. The case definition of DHF requires four diagnostic components: fever, hemorrhagic manifestation (positive tourniquet test, skin and, mucosal bleeding including gastrointestinal bleeding, epistaxis, menorrhagia), thrombocytopenia (≤100,000 cells/mm3), and evidence of plasma leakage (pleural effusion, ascites, or hemoconcentration ≥ 20%, hypoproteinemia) [2]. Dengue shock syndrome (DSS) is defined as DHF with circulatory failure [2].
The WHO classification has aided in the assessment of global dengue disease burden. and in the development of treatment algorithms, resulting in an improvement in the mortality rate of DHF [3]. However, the classification system categorizes cases based upon clinical manifestations and laboratory values. Its ability to categorize severe dengue illness has not been critically evaluated and recently been questioned [4]; several studies have reported that a number of severe dengue cases have failed to meet the case definition of DHF [4-12].
Here we address whether the WHO case definition of DHF can identify ‘severe” dengue cases as determined by the requirement for fluid replacement and blood transfusion. The sensitivity and specificity of each component of the WHO case definition in identifying severe cases were evaluated.
Methods
Participants and Procedures
Data collected from a prospective study of children in Bangkok with suspected dengue virus infection between 1994 and 2005 were analyzed [13]. Children between 6 months and 15 years of age with fever of less than 3 days duration and without an obvious source of infection were recruited. Exclusion criteria included known chronic disease or signs of shock on presentation. Subjects were monitored as in-patients until clinically stable for at least one day following defervescence. Data, including vital signs, hemorrhagic manifestations, presence of pleural effusions (detected by right lateral decubitus chest radiography) or ascites (detected by physical examinations), tourniquet test results [13], complete blood count and albumin were collected daily. Blood was obtained for dengue serology approximately 5-9 days after discharge.
The study was approved by the hospital Institutional Review Board, the Thai Ministry of Public Health, the US Army Surgeon General, and the University of Massachusetts Medical School.
Patients were managed following well-established clinical protocols, which generally follow WHO guidelines [1]. In lieu of early intravenous fluid treatment, patients were encouraged to drink. Intravenous fluid was initiated when any of the following were present: 1) signs suggestive of dehydration (dry mucous membranes, poor urine output) with poor oral intake, 2) signs of poor peripheral perfusion including persistent tachycardia, delayed capillary refill (more than 2 seconds), or narrow pulse pressure (less than 20 mmHg), 3) needs for blood or colloid solution transfusion.
A right lateral decubitus chest radiograph was performed one day after defervescence. The size of the effusion was expressed as pleural effusion index (PEI) = the vertical dimension of the fluid/the width of the hemithorax × 100 [13].
Laboratory Tests
Virus in plasma was identified by virus isolation in mosquitoes and/or by a serotype-specific RT-PCR as previously described [14] [15]. The serotype of isolated viruses were identified by typing ELISA [16]. Cases were classified as having primary or secondary dengue virus infection based on the ratio of dengue-specific IgG and IgM and by HAI test on paired samples as previously published [17].
Hematocrits were obtained by finger stick at least every 6 hours over the first 18 hours after defervescence. The percent hematocrit change was calculated: (highest hematocrit during hospitalization – hematocrit at convalescence)/hematocrit at convalescence X 100 [18].
Clinical Classification
Patients without virologic or serologic evidence of dengue virus infection were classified as other febrile illness (OFI). Dengue cases were classified into DF, DHF grade 1, 2, 3, or 4 based on strict application of the WHO case definitions [2]. In addition, case records from confirmed dengue cases were reviewed by a physician expert who did not participate in patient care (author SN) and dengue cases were classified as DF, DHF grade 1, 2, 3, or 4. Although the expert’s designation was based on the WHO case definitions, the expert physician took into consideration the patient’s clinical course and interventions that might have affected the parameters used in the classification. For example, the physician may have used the lowest hematocrit reading obtained prior to defervescence as a baseline or may not apply the strict hematocrit criteria if the clinical course was complicated by bleeding or if intravenous fluid was administered which may lower the hematocrit readings.
To evaluate the association between case designation and disease severity, we classified dengue cases based on intervention requirements. Patients were classified as dengue requiring intervention (DRI) or dengue not requiring intervention (DNRI) based on the requirement for significant interventions defined as follows: 1) intravenous fluid, or 2) fluid resuscitation (combined oral and intravenous fluid or oral fluid alone equal to or exceeding a combined volume of maintenance fluid and 5% volume deficit on any day during the hospitalization) [19, 20], or 3) transfusion of whole blood or blood products.
Statistical Analysis
Comparisons of continuous variables were performed using Student’s t-test. Chi-square was used to evaluate statistical differences in categorical variables between groups. All statistical analyses were performed using the SPSS 14.0.0 statistical package.
Role of the funding sources
The study sponsors had no role in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or the decision to submit the paper for publication.
Results
Characteristics of the Study Population
Among 1013 children recruited, 264 (26%), 150 (15%), and 599 (59%) were classified according to the strict WHO criteria and the laboratory diagnosis as having DF, DHF, or other (non-dengue) febrile illness (OFI), respectively (Table 1). There were 12, 128, 9, and 1 cases of DHF grade 1-4, respectively. Patients were classified according to the expert’s opinion into 265 (26%) DF, 149 (15%) DHF, and 599 (59%) OFI cases (Supplemental Table 1). The concordance between the strictly applied WHO criteria and the expert’s opinion in classifying dengue cases into DF and DHF was 88%. The expert classified 16% of WHO classified DHF cases as DF and 9% of WHO classified DF cases as DHF.
Table 1.
Clinical characteristics of study participants
| Clinical diagnosis | DF | DHF | OFI |
|---|---|---|---|
| Number of cases | 264 | 150 | 599 |
| Age –years (mean±SE) | 8·31 (0·18) | 8·73 (0·25) | 6·72 (0·12)* |
| Male/Female | 152/112 | 80/70 | 322/277 |
| Fever duration at presentation (mean ± SE) | 1·9 (0·1) ‡ | 2·1 (0·1) | 1·7 (0·0) |
| Duration of fever after study entry (d) (mean± SE) | 2·4(0·1) | 2·4 (0·1) | 1·9 (0·5) |
| Cases who received fluid intervention (case %) | 40 (15%) | 85 (57%) | 73 (12%) |
| Cases with narrow pulse pressure (<20 mmHg) or signs of shock) (cases (%)) | 3 (1%) | 10 (7%) | 6 (1%) |
Note - Average age of patients with OFI was lower than patients with dengue infection
(p<0·05). Mean durations of fever at presentation were greater in DHF group compared with DF and OFI groups
(P < 0·001). DF, dengue fever; DHF, dengue hemorrhagic fever; OFI, other febrile illness.
The dengue virus serotypes in this series were DENV-1 (35%), DENV-2 (24%), DENV-3 (26%), DENV-4 (13%) and unidentified (2%). The majority of WHO defined DHF cases (91%) were secondary virus infections.
WHO case definitions and disease severity
There were no deaths in this study. Among all 414 dengue cases, 125 (30%) were classified as dengue requiring intervention (DRI) (Table 2). 68% of DRI cases met the strict WHO case definitions for DHF (Table 3). This represented a 75% agreement between the strict WHO diagnosis of DHF and intervention requirement. 57% of the WHO-defined DHF cases required intervention compared to 15% of DF cases and 12% of OFI cases (Table 2). The proportions of cases with a low pulse pressure (< 20 mmHg) at any time during the hospitalization were higher in DHF (P < .01) (Table 1). Two out of 3 DF cases with low pulse pressure received blood transfusion. The remaining DF case with narrow pulse pressure had hemoconcentration without thrombocytopenia. Among 6 OFI patients with a narrow pulse pressure, small pleural effusions were found in 2 cases. The hypotension resolved without significant fluid resuscitation in all but one case.
Table 2.
Patient classifications based on WHO case definitions and fluid intervention
| Clinical diagnosis | No IV fluid, less than M+5% (cases (%)) | IV fluid + less than M+5% (cases (%)) | No IV fluid+M+5% (cases (%)) | IV fluid+M+5% (cases (%)) | Total (cases) |
|---|---|---|---|---|---|
| OFI | 526 (88) | 37 (6) | 33 (5.5) | 3 (0.5) | 599 |
| DNRI | DRI | DRI | DRI | ||
| DF | 224 (85) | 28 (10.5) | 8 (3) | 4 (1.5) | 264 |
| DHF | 65 (43) | 62 (42) | 3 (2) | 20 (13) | 150 |
| DHF grade 1 | 6 (50) | 5 (42) | 0 (0) | 1 (8) | 12 |
| DHF grade 2 | 59 (46) | 51 (40) | 3 (2) | 15 (12) | 128 |
| DHF grade 3 | 0 (0) | 5 (56) | 0 (0) | 4 (44) | 9 |
| DHF grade 4 | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 |
| Total | 815 (81) | 127 (12) | 44 (4) | 27 (3) | 1013 |
Note.-Patients were classified based on the fluid interventions received into 1) did not received any intravenous fluid and the total fluid on any day during hospitalization was below the combined volume of maintenance fluid and 5% deficit (M + 5%), 2) received intravenous fluid but the volume of daily fluid intake was below the volume of M + 5% deficit, 3) did not receive intravenous fluid intervention but fluid intake exceeded the combined volume at least one day during the illness, and 4) received intravenous fluid and the fluid intake exceeded the combined volume at least on day during the illness. Dengue patients in the last three groups were classified as dengue requiring intervention (DRI) in this analysis. The rest of dengue patients were classified as dengue not requiring intervention (DNRI).
Table 3.
Clinical and laboratory findings in dengue and non-dengue diagnostic categories and in cases classified based on requirement for significant intervention.
| Clinical finding | OFI % (n= 599) | DF % (n = 264) | DHF/DSS % (n = 150) | DHF (grade1,2) % (n = 140) | DSS (grade 3,4) % (n = 10) | No intervention % (n = 888) | DRI % (n = 125) |
|---|---|---|---|---|---|---|---|
| Bleeding manifestations | |||||||
| Positive tourniquet test (TT) | 23 | 71 | 84 | 80 | 100 | 39 | 88 |
| Spontaneous Bleeding | 50 | 68 | 91 | 91 | 80 | 56 | 86 |
| TT or spontaneous bleeding (Bleed) | 62 | 85 | 100 | 100 | 100 | 70 | 95 |
| Thrombocytopenia | |||||||
| Platelet count <100,000/mm3 (Plts) | 6 | 36 | 100 | 100 | 100 | 20 | 85 |
| Signs of plasma leakage | |||||||
| Pleural effusion (P) | 15 | 9 | 79 | 78 | 100 | 17 | 64 |
| Ascites (A) | 1 | 3 | 34 | 32 | 70 | 1 | 45 |
| Hemoconc. (H) | 13 | 15 | 69 | 67 | 90 | 17 | 53 |
| P or A or H (Leak) | 26 | 24 | 100 | 100 | 100 | 31 | 75 |
| Pleural effusion only | 12 | 7 | 24 | 26 | 0 | 12 | 11 |
| Combinations of findings | |||||||
| Plts and Bleed | 4 | 32 | 100 | 100 | 100 | 17 | 84 |
| Leak and Plts | 2 | 2 | 100 | 100 | 100 | 9 | 68 |
| Leak and Bleed | 17 | 20 | 100 | 100 | 100 | 24 | 74 |
| Leak and Plts and Bleed | 1 | 0 | 100 | 100 | 100 | 8 | 68 |
Note. Numbers denote the percentages of cases with the indicated findings in each diagnostic category. Spontaneous bleeding includes all signs of bleeding except bleeding elicited by trauma or tourniquet test. A positive tourniquet test was defined as petechiae of 20 or more spots in a 2.5 cm2 in the forearm area after applying pressure at midpoint between systolic and diastolic pressure for 5 minutes using a sphygmomanometer. Patients were classified dengue requiring intervention (DRI) if they received intravenous fluid or their total fluid intake on any day exceeded the combined volume of maintenance fluid and 5% deficit or received blood transfusion.
Contribution of individual components of DHF case definitions to clinical classifications
Bleeding manifestations were common in both DF and DHF (table 3). A positive tourniquet test differentiated dengue from OFI with 77% specificity. Hematemesis and melena were more common in DHF (23% and 11%, respectively) compared to DF (4% and 4%, respectively) (P < 0.01) (Supplemental Table 2). The specificities of bleeding in differentiating DHF from DF and DRI from DNRI were low (15% and 11% respectively) (Table 4). Seven study subjects received blood transfusions; five had DHF and two had DF (P = 0.12). The high incidence of hemorrhage in OFI was mostly due to petechiae and minor epistaxis which were detected by close observation.
Table 4.
The sensitivity and specificity of each component of the current WHO case definitions and the combinations of these components in classifying DHF from DF and non-DHF cases (DF and OFI combined), dengue (DHF+DF) from OFI, and in classifying disease severity in dengue cases based on intervention requirement
| Case definition components | ||||||||
|---|---|---|---|---|---|---|---|---|
| Classification | Parameter | Leak | Bleed | Plt | Leak + Bleed | Leak + Plt | Bleed+ Plt | Leak + Bleed+ Plt |
| DHF vs DF | Sensitivity | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Specificity | 76 | 15 | 64 | 80 | 98 | 68 | 100 | |
| DHF vs (DF + OFI) | Sensitivity | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Specificity | 75 | 31 | 89 | 82 | 98 | 87 | 96 | |
| (DHF + DF) vs OFI | Sensitivity | 52 | 90 | 59 | 49 | 37 | 57 | 36 |
| Specificity | 74 | 38 | 94 | 83 | 98 | 96 | 99 | |
| DRI vs DNRI | Sensitivity | 75 | 95 | 85 | 74 | 68 | 84 | 68 |
| Specificity | 58 | 11 | 52 | 62 | 75 | 55 | 78 | |
| DRI vs (DNRI + OFI) | Sensitivity | 75 | 95 | 85 | 74 | 68 | 84 | 68 |
| Specificity | 69 | 30 | 80 | 76 | 90 | 83 | 92 | |
Note.-Leak means plasma leakage (pleural effusion, ascites and/or hemoconcentration), Bleed means bleeding including a positive tourniquet test, plt means thrombocytopenia (platelet counts less than 100.000 cell/cumm). DRI: dengue requiring intervention, DNRI: dengue requiring intervention, OFI: other febrile illnesses.
The mean (SEM) of the minimum platelet counts in DHF, DF, and OFI were 46,763 (2145), 123,327 (3694), and 230,866 (5758), respectively (P < ·0.01). The specificity of thrombocytopenia (less than 100,000 cells/cu mm) in differentiating DHF from DF was 64%, and in differentiating DHF from DF and OFI was 89% (Table 4). The cut-off value that best differentiated DRI versus DNRI was 62,900 cells/mm3 with 69% sensitivity and 79% specificity (data not shown).
Pleural effusions were the most common sign of plasma leakage in DHF cases (79%) (Table 3). Ascites was detected in 34% of DHF cases and 96% of these cases also had pleural effusions (Table 3). Nine percent and 15% of DF and OFI cases had small pleural effusions (PEI = 4·41 ± 1·4% and 2·2± 0·1%, respectively) which were significantly smaller than those found in DHF cases (19·4 ± 1·6%; P < 0.001). Hemoconcentration (20% increase in hematocrit) was found in 69% of DHF cases and was the only sign of plasma leakage in 19% (Table 3). Thirteen percent and 11% of DF and OFI cases respectively had ≥20% hemoconcentration without any accompanying direct evidence of plasma leakage.
Pleural effusion alone provided sensitivity and specificity of 79% and 91% for discriminating DF from DHF (Supplemental Table 3). Inclusion of evidence of plasma leakage other than pleural effusion increased the sensitivity to 100%, but decreased the specificity to 76%. Pleural effusion yielded 64% sensitivity and 78% specificity for differentiating DRI versus DNRI. When other signs of plasma leakage were included, the sensitivity increased to 75% and the specificity declined to 58% (Supplemental Table 3).
Contribution of criteria to the WHO Classification
Plasma leakage and thrombocytopenia were the main diagnostic components contributing to the specificity of the case definitions in classifying dengue cases (76% and 64% specificity respectively), and pleural effusion was the key component identifying plasma leakage (Table 4 and Supplemental Table 3). The combination of thrombocytopenia and plasma leakage had a specificity of 98% in differentiating DHF from DF (Table 4). The specificity did not change significantly when hemorrhage was added to plasma leakage and thrombocytopenia (from 98% to 100%).
When dengue patients were classified based on the requirement for intervention, plasma leakage alone had a sensitivity of 75% and specificity of 58% (Table 4). The addition of hemorrhage did not significantly change the sensitivity (74%) and the specificity (62%). The sensitivity and the specificity for DRI of combined plasma leakage and thrombocytopenia criteria were 68% and 75%, respectively (Table 4). Thrombocytopenia alone or the combination of thrombocytopenia and plasma leakage differentiated dengue from OFI with 94% and 98% specificity respectively (Table 4). Only 1% of OFI met the definitions of DHF (plasma leakage, thrombocytopenia and hemorrhage).
A significant number of DF cases did receive significant intervention. To examine the characteristic differences among DF cases that differed in intervention requirement we compared the frequencies of components of the case definitions in DF cases classified as either DRI or DNRI. The prevalence of plasma leakage, bleeding and the combination of these two were not significantly different between the two groups (Figure 1). However, thrombocytopenia was more common in DF requiring intervention than those who did not (DRI-DF vs. DNRI-DF, 53% vs. 34%, respectively, P< 0.05). The frequencies of patients with combined thrombocytopenia and bleeding were also higher in DRI-DF (50%) than DNRI-DF (29%) cases, P< 0.01).
Figure 1.
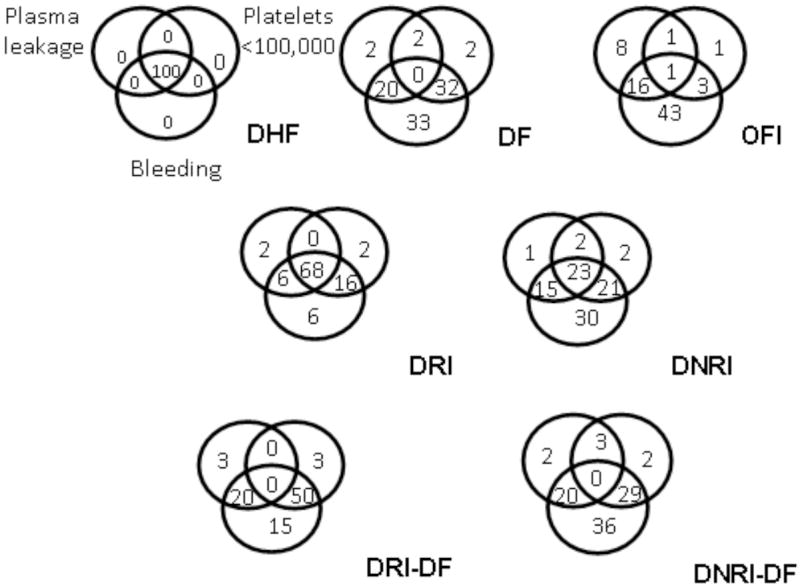
Frequencies of the components of case definitions for DHF: plasma leakage (left upper circles), thrombocytopenia (right upper circles), and bleeding including a positive tourniquet test (lower circles) in various patient populations. DHF = dengue hemorrhagic fever; DF = dengue fever; OFI = other febrile illnesses; DRI = dengue requiring intervention; DNRI = dengue not requiring intervention; DRI-DF = DF cases requiring intervention; DNRI-DF = DF cases not requiring intervention.
Discussion
Recent studies have questioned whether the WHO clinical classification scheme optimally identifies severe dengue cases [4, 5, 9, 12, 21-23]. To answer these questions we analyzed data collected over a 12 year period from a prospective study conducted at a referral center for dengue with a well established intervention guideline. The study design allowed us to collect serial clinical and laboratory data and to use the levels of interventions as a measure of disease severity independent of the WHO classification.
Our study showed that DHF as defined by the WHO criteria correlated strongly with the need for intervention. DHF constituted 68% of dengue cases that received significant intervention. However, 42% of DHF cases did not require intervention. In contrast, 15% of DF and 12% of OFI cases did receive significant intervention. This finding demonstrated the heterogeneity in severity within each disease category. Dehydration from fever and poor oral intake may be the common underlying cause of the requirement for fluid replacement in both DF and OFI cases. Furthermore, hemorrhage, sometimes severe, has been well recognized to occur in DF and has led to a separate category of “DF with unusual hemorrhage” within the WHO classification scheme. The majority of DF cases that received significant intervention had thrombocytopenia or thrombocytopenia and bleeding (DRI-DF, Figure 1. In addition, two severe cases that received blood transfusion were DF cases.
A number of studies have shown that a significant proportion of dengue patients with shock did not fulfill the WHO DHF case definition [4, 7, 9, 11, 12, 24]. In this study 76% (10/13 cases) of dengue cases with documented narrow pulse pressure were classified as DHF by the WHO case definitions. Two of the three cases of DF with hypotension had significant hemorrhage and required blood transfusion. This suggests that hemorrhage may contribute to severity in DF cases. Other possible explanations for the failure of the DHF case definition to detect shock cases include the lack of data on platelet counts, hematocrits or lateral decubitus chest radiogram at critical time points. Delays in treatment of dehydration and metabolic disturbances may result in disease severity irrespective of dengue case definitions. In addition, some severe cases might not have met the strictly applied criteria for DHF due to discordance between the presence of plasma leakage, bleeding, and the severity of thrombocytopenia.
Plasma leakage was a major component that contributed to the specificity of the case definition and correlated with intervention requirement in dengue cases. Our study demonstrated that the presence of significant (PEI > 4%) pleural fluid was the most sensitive and specific evidence of plasma leakage in DHF. We have previously demonstrated by serial ultrasonograms that in contrast to the progressive nature of plasma leakage in DHF, ultrasound evidence of plasma leakage in non-DHF cases was not progressive and rapidly resolved. [25].
Our study demonstrated that thrombocytopenia is an important discriminating factor for both DHF and disease severity. Thrombocytopenia also discriminated dengue from OFI with 94% specificity (Table 3). Importantly, thrombocytopenia is also a marker of severity in dengue patients who did not fulfill the WHO case definition of DHF. Studies have shown that platelet counts inversely correlated with plasma viral load, which has been shown to correlate with the extent of plasma leakage [26].
Hemorrhagic manifestations did not alter the sensitivity or specificity of the case definitions due to the high incidence in both DF and DHF, as has been previously reported in Vietnam [9]. Although the tourniquet test had low specificity in differentiating DHF from DF as previously described [5, 7], it distinguished dengue from OFI with a reasonable specificity (77%).
Even in a well-defined population and with frequent monitoring, the WHO case definition, when strictly applied, demonstrated only 88% concordance rate with diagnoses assigned by a physician expert. This discordance was largely related to differences in the determination of hemoconcentration; for application of strict WHO criteria, the peak hematocrit was compared to the convalescent hematocrit as a baseline whereas the pattern of hematocrits over the entire hospital course was utilized by the clinician. Significant discordance between the grading of DHF cases by the expert and by strict WHO criteria was also noted. The expert physician used clinical impression, the presence of rapid pulse, and signs of poor peripheral perfusion as indicators of severity while the grading based on WHO case definitions in this study relied only on documented pulse pressure, which may result in undergrading of some DHF cases.
Dengue is endemic in many countries where confirmatory laboratory tests for dengue virus infection may not be widely available. Simple and practical tools that help differentiate dengue from OFI and identify potentially severe dengue cases are indispensable for case management. Our study has demonstrated that tourniquet test and thrombocytopenia are useful for differentiating dengue from OFI (Table 3). Plasma leakage and thrombocytopenia individually or in combination further identified dengue patients at risk for severe illness requiring fluid or blood replacement.
We did not detect in our patients other severe manifestations such as encephalitis/encephalopathy and myocarditis reported elsewhere [21-23, 27-29]. It is possible that these manifestations were complications from shock which might have been prevented by early treatment in our study [30]. Alternatively, these severe manifestations might be due to associated infectious conditions, or represented distinct manifestations in different populations.
Our patient population likely differed from dengue cases seen in general practice. The early recruitment (less than three days of illness) and close observation in our study might have resulted in fewer severe dengue cases. Although we have found the current WHO case definition to be effective in identifying severe dengue cases our findings will need to be validated in other settings and in populations with different ethnicity.
Our findings have implications for the classification of dengue. First, the current WHO criteria identify the majority (68%), but not all, dengue patients who required intervention. It showed excellent specificity (99%-Table 4) in differentiating dengue from non-dengue illness. This supports the use of these criteria for case reporting in the absence of serological or virological confirmation of dengue infection, a practical issue for resource poor countries. Second, plasma leakage and thrombocytopenia are the two components of the case definitions that discriminate DHF from DF, and severe from milder cases. Although serial hematocrits and platelet counts remain important monitoring tools, clinical or laboratory indicators capable of predicting disease severity are needed. The development of such tools requires a proper classification of patients. For this purpose the current classification system appears to be suitable.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant NIH-P01AI34533 and the Military Infectious Disease Research Program. The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the view of the U.S. Government.
We thank the arbovirology and molecular sections of the Armed Forces Research Institute of Medical Sciences for diagnostic testing; doctors and nurses of Queen Sirikit National Institute of Child Health and the staff of the Armed Forces Research Institute of Medical Sciences for patient care and sample collection.
Footnotes
The authors have no conflicting financial interests.
References
- 1.Anonymous. Technical guides for diagnosis, treatment, surveillance, prevention and control of dengue haemorrhagic fever. Geneva: WHO (Southeast Asian and Western Pacific Regional Offices); 1975. [Google Scholar]
- 2.WHO. Dengue hemorrhagic fever: diagnosis, treatment, prevention and control. 2. Geneva: WHO; 1997. [Google Scholar]
- 3.Nimmannitya S. Clinical spectrum and management of dengue haemorrhagic fever. Southeast Asian J Trop Med Public Health. 1987 Sep;18(3):392–7. [PubMed] [Google Scholar]
- 4.Balmaseda A, Hammond SN, Perez MA, et al. Short report: assessment of the World Health Organization scheme for classification of dengue severity in Nicaragua. Am J Trop Med Hyg. 2005 Dec;73(6):1059–62. [PubMed] [Google Scholar]
- 5.Bandyopadhyay S, Lum LC, Kroeger A. Classifying dengue: a review of the difficulties in using the WHO case classification for dengue haemorrhagic fever. Trop Med Int Health. 2006 Aug;11(8):1238–55. doi: 10.1111/j.1365-3156.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- 6.Harris E, Videa E, Perez L, et al. Clinical, epidemiologic, and virologic features of dengue in the 1998 epidemic in Nicaragua. Am J Trop Med Hyg. 2000 Jul-Aug;63(1-2):5–11. doi: 10.4269/ajtmh.2000.63.5. [DOI] [PubMed] [Google Scholar]
- 7.Phuong CX, Nhan NT, Wills B, et al. Evaluation of the World Health Organization standard tourniquet test in the diagnosis of dengue infection in Vietnam. Trop Med Int Health. 2002;7:125–32. doi: 10.1046/j.1365-3156.2002.00841.x. [DOI] [PubMed] [Google Scholar]
- 8.Phuong CX, Nhan NT, Kneen R, et al. Evaluation of an algorithm for integrated management of childhood illness in an area of Vietnam with dengue transmission. Trop Med Int Health. 2004;9:573–81. doi: 10.1111/j.1365-3156.2004.01232.x. [DOI] [PubMed] [Google Scholar]
- 9.Phuong CX, Nhan NT, Kneen R, et al. Clinical diagnosis and assessment of severity of confirmed dengue infections in Vietnamese children: is the world health organization classification system helpful? Am J Trop Med Hyg. 2004 Feb;70(2):172–9. [PubMed] [Google Scholar]
- 10.Rigau-Perez JG. Surveillance for an emerging disease: dengue hemorrhagic fever in Puerto Rico, 1988-1997. Puerto Rico Association of Epidemiologists. P R Health Sci J. 1999 Dec;18(4):337–45. [PubMed] [Google Scholar]
- 11.Rigau-Perez JG, Laufer MK. Dengue-related deaths in Puerto Rico, 1992-1996: diagnosis and clinical alarm signals. Clin Infect Dis. 2006 May 1;42(9):1241–6. doi: 10.1086/501355. [DOI] [PubMed] [Google Scholar]
- 12.Setiati TE, Mairuhu AT, Koraka P, et al. Dengue disease severity in Indonesian children: an evaluation of the World Health Organization classification system. BMC Infect Dis. 2007;7:22. doi: 10.1186/1471-2334-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalayanarooj S, Vaughn DW, Nimmannitya S, et al. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997 Aug;176(2):313–21. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 14.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992 Mar;30(3):545–51. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen L, Gubler D. The use of mosquitoes to detect and propagate dengue viruses. Am J Trop Med Hyg. 1974 Nov;23(6):1153–60. doi: 10.4269/ajtmh.1974.23.1153. [DOI] [PubMed] [Google Scholar]
- 16.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000 Jan;181(1):2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 17.Innis BL, Nisalak A, Nimmannitya S, et al. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1989 Apr;40(4):418–27. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SN, Halstead SB. Shock associated with dengue infection. I. Clinical and physiologic manifestations of dengue hemorrhagic fever in Thailand, 1964. J Pediatr. 1966 Mar;68(3):448–56. doi: 10.1016/s0022-3476(66)80249-4. [DOI] [PubMed] [Google Scholar]
- 19.Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957 May;19(5):823–32. [PubMed] [Google Scholar]
- 20.Friedman AL. Pediatric hydration therapy: historical review and a new approach. Kidney Int. 2005 Jan;67(1):380–8. doi: 10.1111/j.1523-1755.2005.00092.x. [DOI] [PubMed] [Google Scholar]
- 21.Kularatne SA, Pathirage MM, Medagama UA, Gunasena S, Gunasekara MB. Myocarditis in three patients with dengue virus type DEN 3 infection. Ceylon Med J. 2006 Jun;51(2):75–6. doi: 10.4038/cmj.v51i2.1362. [DOI] [PubMed] [Google Scholar]
- 22.Poovorawan Y, Hutagalung Y, Chongsrisawat V, Boudville I, Bock HL. Dengue virus infection: a major cause of acute hepatic failure in Thai children. Ann Trop Paediatr. 2006 Mar;26(1):17–23. doi: 10.1179/146532806X90565. [DOI] [PubMed] [Google Scholar]
- 23.Solomon T, Dung NM, Vaughn DW, et al. Neurological manifestations of dengue infection. Lancet. 2000 Mar 25;355(9209):1053–9. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 24.Rigau-Perez JG, Bonilla GL. An evaluation of modified case definitions for the detection of dengue hemorrhagic fever. Puerto Rico Association of Epidemiologists. P R Health Sci J. 1999 Dec;18(4):347–52. [PubMed] [Google Scholar]
- 25.Srikiatkhachorn A, Krautrachue A, Ratanaprakarn W, et al. Natural history of plasma leakage in dengue hemorrhagic fever: a serial ultrasonographic study. Pediatr Infect Dis J. 2007 Apr;26(4):283–90. doi: 10.1097/01.inf.0000258612.26743.10. discussion 91-2. [DOI] [PubMed] [Google Scholar]
- 26.Libraty DH, Endy TP, Houng HS, et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis. 2002 May 1;185(9):1213–21. doi: 10.1086/340365. [DOI] [PubMed] [Google Scholar]
- 27.Cam BV, Fonsmark L, Hue NB, Phuong NT, Poulsen A, Heegaard ED. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg. 2001 Dec;65(6):848–51. doi: 10.4269/ajtmh.2001.65.848. [DOI] [PubMed] [Google Scholar]
- 28.de Macedo FC, Nicol AF, Cooper LD, Yearsley M, Pires AR, Nuovo GJ. Histologic, viral, and molecular correlates of dengue fever infection of the liver using highly sensitive immunohistochemistry. Diagn Mol Pathol. 2006 Dec;15(4):223–8. doi: 10.1097/01.pdm.0000213462.60645.cd. [DOI] [PubMed] [Google Scholar]
- 29.Solomon T. Viral encephalitis in southeast asia. Neurological Infections and Epidemiology. 1997;2:191–99. [Google Scholar]
- 30.Nimmannitya S, Thisyakorn U, Hemsrichart V. Dengue haemorrhagic fever with unusual manifestations. Southeast Asian J Trop Med Public Health. 1987 Sep;18(3):398–406. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


