Abstract
Context: Osteopathia striata with cranial sclerosis is an X-linked dominant condition caused by mutations in the WTX gene, resulting in linear striations in long bones in combination with cranial sclerosis. This condition is usually lethal in males.
Objective/Patient: Our aim was to determine the underlying genetic cause in a 37-yr-old male with this condition.
Design: DNA sequencing of peripheral blood and hair was performed to identify mutations in WTX. Quantitative PCR was performed to determine gene copy number variation.
Results: DNA sequenced from peripheral blood revealed the presence of two alleles at the 1108th position of the WTX gene. Subsequent DNA sequencing of hair follicles and quantitative PCR confirmed the presence of mosaicism.
Conclusion: A novel mutation (c.1108G>T) found in our patient results in a truncated protein (E370X). Our patient represents the first confirmed case of mosaicism in osteopathia striata with cranial sclerosis.
A 37-year-old male with a novel mutation of the WTX gene is the first confirmed case of mosaicism in osteopathia striata with cranial sclerosis.
Osteopathia striata with cranial sclerosis (OSCS; Online Mendelian Inheritance in Man 300373) is a rare X-linked dominant condition characterized by linear striations in long bones in combination with cranial sclerosis. This syndrome is also associated with macrocephaly, frontal bossing, ocular hypertelorism, broad nasal bridge, cleft palate, hearing loss, and mental retardation. Rarely it also causes cardiac, intestinal, and genitourinary malformations. This condition is recognized to be lethal in most male patients (1). Recently Jenkins et al. (2) identified mutations involving the WTX (also known as FAM123B) gene on the X chromosome, encoding an inhibitor of WNT signaling, to be responsible for this disease. We report a male patient with OSCS who has a novel mutation and is the first reported case of mosaicism in OSCS.
Patients and Methods
Clinical features
Our patient is a 37-yr-old male whose phenotype was described 10 yr ago (3). At birth he had hypertelorism, flat nasal bridge, clubfeet, cleft lip, and cleft palate. His physical examination was significant for macrocephaly with a prominent occipital bony protrusion, micrognathia, and bilaterally diminished hearing. He has evidence of multiple surgical procedures to correct these congenital abnormalities and ankylosis of the temporomandibular joints. He also has mild decrease in intellect. The phenotype of OSCS was confirmed by a computed tomography scan of the head showing thickening of the calvaria with dense sclerosis of the base of the skull and radiographs of the pelvis and femur demonstrating longitudinal striations. There is no family history of OSCS.
Genomic analyses
To uncover the patient’s underlying mutation, DNA was extracted from peripheral blood and hair from multiple sites after a written informed consent was obtained from the patient. The single coding exon of the WTX gene was amplified in 11 fragments using previously described primers (2,4). DNA sequencing was performed with Big Dye Terminator chemistry version 3.1 (Applied Biosystems, Foster City, CA) and visualized on an ABI PRISM 3100 genetic analyzer. Quantitative PCR was performed using the iTaq SYBR Green Supermix with ROX (Bio-Rad Laboratories, Hercules, CA) in the ABI-PRISM 7000 sequence detection system (Applied Biosystems). Gene copy number variation was determined by analyzing the data using the relative standard curve method.
Results
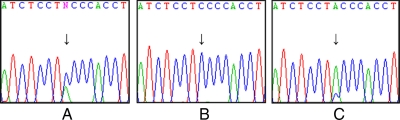
DNA sequenced from peripheral blood revealed the presence of two alleles at the 1108th position (Fig. 1A). Here, in addition to guanine expected in the wild-type sequence, thymine was also present (c.1108G>T). The different proportions of wild-type and mutant alleles suggested the presence of mosaicism or multiple gene copies. Contamination of genomic DNA was ruled out by sequencing an independent blood sample from the patient. Gene copy number variation (and Klinefelter syndrome) was also ruled out using quantitative PCR of WTX, which showed the patient to have only a single copy of the gene (data not shown). DNA from hair follicles from multiple sites of the patient’s body were subsequently sequenced and were found to have either the wild-type or mutant genotype (Fig. 1, B and C), confirming the suspicion of mosaicism in our patient.
Figure 1.
WTX sequence chromatograms of DNA extracted from peripheral blood (A), single hair follicle from left thigh (B), and single hair follicle from right forearm (C). The sequence is shown in reverse orientation. The presence of two alleles in C may represent contamination from epithelial cells surrounding the hair follicle.
Discussion
A novel mutation (c.1108G>T) found in our patient results in premature termination of translation and a truncated protein (E370X). The existence of mosaicism in OSCS has previously been postulated in a patient who had the phenotype of OSCS but in whom the deletion present in other affected members could not be detected (5). Our patient represents the first confirmed case of mosaicism in OSCS. The presence of normal WTX allele (i.e. mosaicism) did not confer a less severe phenotype in our patient compared with other surviving males with germline mutations.
The WTX gene is mutated in different types of cancer such as Wilms tumor (6), and infrequently in colorectal cancer (7) and acute myeloid leukemia (8). Previous studies had reported that germline mutations in WTX do not predispose to tumorigenesis (2,5). A postzygotic mutation resulting in mosaicism (as seen in our patient) is a somatic mutation; however, our patient has not developed cancer.
The existence of mosaicism in OSCS has important implications in genetic counseling. It is likely that additional patients with mosaicism will be described in the future when the existence of mosaicism in OSCS is better recognized.
Acknowledgments
The authors are grateful to the patient for his participation in this work. Thanks to Anthony M. Austin, Leah R. Padgett, and Amie K. Gray for their invaluable assistance in the laboratory.
Footnotes
This work was supported by National Institutes of Health Grant P01 AG18397.
Disclosure Summary: All authors have no conflict of interest.
First Published Online February 11, 2010
Abbreviation: OSCS, Osteopathia striata with cranial sclerosis.
References
- Behninger C, Rott HD 2000 Osteopathia striata with cranial sclerosis: literature reappraisal argues for X-linked inheritance. Genet Couns 11:157–167 [PubMed] [Google Scholar]
- Jenkins ZA, van Kogelenberg M, Morgan T, Jeffs A, Fukuzawa R, Pearl E, Thaller C, Hing AV, Porteous ME, Garcia-Miñaur S, Bohring A, Lacombe D, Stewart F, Fiskerstrand T, Bindoff L, Berland S, Adès LC, Tchan M, David A, Wilson LC, Hennekam RC, Donnai D, Mansour S, Cormier-Daire V, Robertson SP 2009 Germline mutations in WTX cause a sclerosing skeletal dysplasia but do not predispose to tumorigenesis. Nat Genet 41:95–100 [DOI] [PubMed] [Google Scholar]
- Lazar CM, Braunstein EM, Econs MJ 1999 Clinical vignette: osteopathia striata with cranial sclerosis. J Bone Miner Res 14:152–153 [DOI] [PubMed] [Google Scholar]
- Rivera MN, Kim WJ, Wells J, Driscoll DR, Brannigan BW, Han M, Kim JC, Feinberg AP, Gerald WL, Vargas SO, Chin L, Iafrate AJ, Bell DW, Haber DA 2007 An X chromosome gene, WTX, is commonly inactivated in Wilms tumor. Science 315:642–645 [DOI] [PubMed] [Google Scholar]
- Perdu B, de Freitas F, Frints SG, Schouten M, Schrander-Stumpel C, Barbosa M, Pinto-Basto J, Reis-Lima M, de Vernejoul MC, Becker K, Freckmann ML, Keymolen K, Haan E, Savarirayan R, Koenig R, Zabel B, Vanhoenacker FM, Van Hul W 2009 Osteopathia striata with cranial sclerosis due to WTX gene defect. J Bone Miner Res 25:82–90 [DOI] [PubMed] [Google Scholar]
- Ruteshouser EC, Robinson SM, Huff V 2008 Wilms tumor genetics: mutations in WT1, WTX, and CTNNB1 account for only about one-third of tumors. Genes Chromosomes Cancer 47:461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo NJ, Kim S, Lee SH 2009 Mutational analysis of WTX gene in Wnt/β-catenin pathway in gastric, colorectal, and hepatocellular carcinomas. Dig Dis Sci 54:1011–1014 [DOI] [PubMed] [Google Scholar]
- Owen C, Virappane P, Alikian M, Stasevich I, Summers K, Lillington D, Bonnet D, Burnett A, Mills K, Lister TA, Fitzgibbon J 2008 WTX is rarely mutated in acute myeloid leukemia. Haematologica 93:947–948 [DOI] [PubMed] [Google Scholar]