Abstract
Context: Teriparatide increases both bone formation and bone resorption.
Objective: We sought to determine whether combining teriparatide with an antiresorptive agent would alter its anabolic action.
Design and Setting: This was a randomized controlled trial conducted in a single university hospital.
Patients and Intervention: We randomized 93 postmenopausal women with low bone mineral density (BMD) to alendronate 10 mg daily (group 1), teriparatide 40 μg sc daily (group 2), or both (group 3) for 30 months. Teriparatide was begun at month 6.
Main Outcome Measures: BMD of the lumbar spine, proximal femur, proximal radius, and total body was measured by dual-energy x-ray absorptiometry (DXA) every 6 months. Lumbar spine trabecular BMD was measured at baseline and month 30 by quantitative computed tomography. Serum osteocalcin, N-terminal propeptide of type 1 collagen, and N-telopeptide levels were assessed frequently. Women who had at least one repeat DXA scan on therapy were included in the analyses (n = 69).
Results: DXA spine BMD increased more in women treated with teriparatide alone than with alendronate alone (18 ± 11 vs. 7 ± 4%; P < 0.001) or both (18±11 vs. 12 ± 9%; P = 0.045). Similarly, femoral neck BMD increased more in women treated with teriparatide alone than with alendronate alone (11 ± 5 vs. 4 ± 4%; P < 0.001) or both (11 ± 5 vs. 3 ± 5%; P < 0.001). Quantitative computed tomography spine BMD increased 1 ± 7, 61 ± 31, and 24 ± 24% in groups 1, 2, and 3 (P < 0.001 for all comparisons). Serum osteocalcin, N-terminal propeptide of type 1 collagen, and cross-linked N-telopeptides of type I collagen increased more with teriparatide alone than with both (P < 0.001 for each marker).
Conclusion: Alendronate reduces the ability of teriparatide to increase BMD and bone turnover in women.
Administration of alendronate reduces the ability of a 2-year course of teriparatide to increase bone mineral density of the lumbar spine and proximal femur as measured by various tests in women with postmenopausal osteoporosis.
Both alendronate and teriparatide increase bone mineral density (BMD) and reduce fracture risk in women with postmenopausal osteoporosis but do so by contrasting mechanisms (1,2,3,4,5,6). Whereas alendronate inhibits osteoclastic bone resorption and, as a result, also reduces bone formation (7), both intact human PTH 1-84 and its 34-amino acid amino-terminal fragment (teriparatide) increase bone formation and bone resorption. Thus, it seemed likely that combining PTH or teriparatide with alendronate would stimulate bone formation without increasing bone resorption and thereby increase BMD more than either agent alone. Surprisingly, in osteoporotic men, combining teriparatide with daily alendronate for 2 yr attenuated the ability of teriparatide to increase BMD of the spine, assessed by both dual-energy x-ray absorptiometry (DXA) and quantitative computed tomography, and the hip, although the effect on the hip was not observed until the second year of alendronate and teriparatide administration (8). Daily alendronate administration markedly attenuated the ability of teriparatide to increase circulating levels of biochemical markers of osteoblast activity (9). In postmenopausal women, combining intact PTH 1-84 with alendronate for 1 yr attenuated the ability of PTH to increase trabecular BMD of the spine as assessed by quantitative computed tomography but had no effect on spine or femoral neck BMD assessed by DXA and improved total hip BMD (10). The differences between the effects of combining teriparatide or intact PTH with alendronate in these two studies may reflect gender differences (female vs. male), differences in the study duration (1 vs. 2 yr), or differences between anabolic agents (intact PTH 1-84 vs. teriparatide). Because teriparatide is generally given for 2 yr, we evaluated the effects of combining teriparatide with alendronate for 2 yr in women with postmenopausal osteoporosis.
Subjects and Methods
Study subjects
We mailed 60,000 recruitment letters to women in the local area. Of 3471 women who returned the questionnaire, 293 were interested and eligible for further screening. Of these 293 women, 126 were disqualified because their bone density was too high, 16 on the basis of screening blood tests, and 48 for not keeping their screening appointment, leaving 103 who were eligible by bone density and screening laboratory criteria. Twenty-nine women decided not to participate and an additional 19 women were recruited from our clinic or bone density center. Thus, the final cohort consisted of 93 women.
All subjects were required to be 46–85 yr of age; have no menstrual periods for at least 2 yr; and have BMD of the lumbar spine in the posterior-anterior or lateral projection or the femoral neck at least 2 sd below the mean of young normal women. The remaining exclusionary criteria were identical with those used in our companion study in men (8).
Study protocol
Participants were randomly assigned by a computerized program to receive alendronate alone (10 mg orally once daily, group 1, n = 31), teriparatide alone (40 μg sc once daily, group 2, n = 31), or both (group 3, n = 31). Subjects were stratified into blocks based on age (above or <65 yr) and spine BMD (above or <2 sd below the mean for age). Alendronate was begun at the baseline visit and continued for 30 months in groups 1 and 3. Teriparatide was begun at the 6-month visit and continued for 24 months in groups 2 and 3. The study was not blinded because the Institutional Review Board at Massachusetts General Hospital believed it would be unethical to administer placebo injections for 2 yr. Calcium intake was estimated by a research dietitian and maintained at 1000–1200 mg daily through diet and/or supplements. All subjects received 400 U vitamin D daily.
Blood was collected at baseline and 1, 2, 3, 6, 7, 8, 9, 12, 18, 24, and 30 months to assess levels of serum osteocalcin (OC), N-terminal propeptide of type I collagen (P1NP), and cross-linked N-telopeptides of type I collagen (NTX), as well as routine chemistries, including serum calcium levels. Serum calcium was measured before and 4–6 h after teriparatide injection. Our laboratory’s normal range for calcium is 8.5–10.5 mg/dl. Twenty-four-hour urinary calcium excretion was measured every 6 months and also 1 month after starting teriparatide (month 7). BMD was measured by DXA at baseline and every 6 months and by quantitative computed tomography at baseline and month 30. A standardized questionnaire was administered at each visit to assess side effects since the last study visit. Compliance with study medications was assessed by medication diaries and by counting residual medication supplies. The study was approved by the Institutional Review Board of Massachusetts General Hospital, and all subjects provided written informed consent.
Teriparatide preparation and dose adjustments
Good manufacturing practices grade synthetic human PTH (1-34) (Teriparatide; Bachem, Inc., Torrance, CA) was vialed as a sterile lyophilized powder (with Mannitol, U.S.P.) under good manufacturing practices conditions by Ben-Venue Laboratories (Bedford, OH). Amino acid and HPLC analysis of the teriparatide preparation revealed that each vial contained 37 μg rather than the intended 40 μg.
The dose of teriparatide was reduced by 25% if any serum calcium value was above 10.5 mg/dl or if the investigators felt that the subject was experiencing a side effect of therapy. If hypercalcemia or symptoms persisted, the teriparatide dose was reduced by another 25%. If hypercalcemia or symptoms persisted after two dose reductions, teriparatide was discontinued. If the 24-h urinary calcium excretion was above 400 mg/d, dietary calcium and/or sodium was reduced by 25–50%. If hypercalciuria persisted, the teriparatide dose was reduced by 25–50% as described above. If hypercalciuria persisted after a 50% dose reduction, teriparatide was discontinued.
Measurements of BMD
BMD of the lumbar spine, proximal femur, distal one third radius shaft, and total body was measured by DXA using a QDR 4500A densitometer (Hologic, Waltham, MA.). For the radius shaft, two measurements were made at each visit, and the mean value was used in all analyses. Our short-term in vivo measurement sds are 0.005, 0.007, and 0.006 g/cm2 for lumbar spine, femoral neck, and total hip, respectively. Individual vertebrae with obvious deformities or areas of focal sclerosis were excluded from analyses. Total body scans were analyzed without the head region because it often contains artifacts (11). All bone density scans were analyzed by individuals who were blinded to study treatment.
Trabecular BMD of the lumbar spine was determined by quantitative computed tomography with General Electric LightSpeed QXi or LightSpeed Plus scanners (GE Healthcare, Waukesha, WI). Axial scans were obtained through the midbody of the first four lumbar vertebrae. Density of trabecular bone was determined by comparison with an internal hydroxyapatite standard, and values for vertebrae were then averaged. The precision error for this technique is 3–5 mg/cc (12).
Measurements of bone turnover
Serum NTX was measured using an enzyme-linked immunoassay (Wampole Laboratories, Princeton, NJ) with intra- and interassay coefficients of variation (CVs) of 4.6 and 6.9%. Serum OC was measured using an enzyme-linked immunoassay (ALPCO Diagnostics, Windham, NH) with intra- and interassay CVs of 3.1–4.7 and 3.5–5.6%. Serum P1NP was measured using a RIA (Orion Diagnostica, Espoo, Finland) with intra- and interassay CVs of 4.5 and 5.5%. All samples for each subject were run in the same assay unless an individual value needed to be repeated.
Statistical analysis
The predetermined primary end point was the difference in the change in BMD of the lumbar spine between groups 2 and 3. Our prespecified analysis was to use BMD data only while subjects were receiving active therapy, i.e. from month 0 to 30 in group 1 and from month 6 to 30 in group 2. For group 3, data from month 0 to 30 were compared with group 1 and data from month 6 to 30 were compared with group 2. Absolute changes from baseline to the final visit were compared by t tests. Baseline values were compared by ANOVA.
Because the goal of this study was to evaluate the mechanism by which teriparatide exerts its anabolic effect on bone, the data were analyzed according to protocol adherence rather than by intention to treat. Twenty-four women (two in group 1, 11 in group 2, and 11 in group 3) discontinued participation before any follow-up BMD measurements were made on their assigned study medication, and their data are excluded. Thus, data from 69 women (29 in group 1, 20 in group 2, and 20 in group 3) are included in the analyses. Eleven women (five in group 1, three in group 2, and three in group 3) discontinued study medication after at least one repeat BMD measurement was made while taking their assigned therapy. Results from those women are included in the analyses while on their assigned study medication. For women who stopped study medication before completing the study, the last observation was carried forward in the BMD analyses but not for the bone marker analyses. Unless specified otherwise, the statistical significance of comparisons was not affected if the 11 women who discontinued study medication prematurely are excluded.
For bone turnover markers, we compared the differences in area under the curve (AUC) of serum OC, P1NP, and NTX across the three treatment groups. AUC was calculated using the trapezoidal rule. The AUCs were then compared using a one-way ANOVA. If significant differences were found, pair-wise comparisons were performed. If the normality assumption was not held, Kruskal-Wallis test and Mann-Whitney U test were used. Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC). All P values are two sided. Unless otherwise noted, data are presented as the mean ± sd.
Results
Characteristics of the subjects
The baseline characteristics of the 69 subjects are shown in Table 1. There were no significant differences in baseline characteristics among groups. Baseline characteristics were also similar among all 93 randomized subjects (data not shown). No subject had received drug therapy for osteoporosis in the past.
Table 1.
Baseline characteristics of women with osteoporosis treated with alendronate alone (group 1), teriparatide alone (group 2), or both (group 3)
| Characteristic (normal range) | Group 1 Alendronate (n = 29) | Group 2 Teriparatide (n = 20) | Group 3 Both (n = 20) | P value |
|---|---|---|---|---|
| Age (yr) | 64 ± 6 | 65 ± 7 | 62 ± 7 | 0.28 |
| Height (cm) | 162 ± 6 | 163 | 161 ± 5 | 0.36 |
| Weight (kg) | 67 ± 11 | 67 ± 11 | 66 ± 12 | 0.92 |
| Body mass index | 25.6 ± 4.5 | 24.9 ± 3.6 | 25.4 ± 5.1 | 0.87 |
| Calcium intake (mg/d) | 1234 ± 530 | 1269 ± 724 | 1180 ± 585 | 0.91 |
| 25-Hydroxyvitamin D (ng/ml) (>15)a | 30 ± 16 | 27 ± 12 | 27 ± 11 | 0.64 |
| PTH (pg/ml) (10–60) | 33 ± 14 | 32 ± 12 | 35 ± 8 | 0.80 |
| Serum creatinine (mg/dl) (0.6–1.5) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.99 |
| Estimated GFR (ml/min)b (≥60) | 77 ± 24 | 76 ± 24 | 77 ± 28 | 0.99 |
| OC (ng/ml) (not available) | 28.4 ± 10.8 | 28.1 ± 10.4 | 29.7 ± 8.2 | 0.87 |
| P1NP (ng/ml) (2.1–86.1) | 48.7 ± 16.9 | 49.6 ± 13.6 | 48.2 ± 15.8 | 0.46 |
| N-telopeptide (nm BCE) (8.0–27.6) | 14.8 ± 4.0 | 15.7 ± 4.2 | 16.3 ± 7.3 | 0.60 |
| BMD (g/cm2) | ||||
| Posterior-anterior spine | 0.808 ± 0.120 | 0.815 ± 0.106 | 0.810 ± 0.143 | 0.98 |
| T score | −2.2 ± 1.1 | −2.1 ± 1.0 | −2.2 ± 1.4 | |
| Femoral neck | 0.646 ± 0.092 | 0.605 ± 0.073 | 0.660 ± 0.085 | 0.10 |
| T score | −1.9 ± 0.8 | −2.2 ± 0.7 | −1.7 ± 0.8 | |
| Total hip | 0.768 ± 0.109 | 0.746 ± 0.100 | 0.802 ± 0.097 | 0.22 |
| T score | −1.5 ± 0.9 | −1.6 ± 0.8 | −1.2 ± 0.8 | |
| One third radius | 0.597 ± 0.093 | 0.584 ± 0.085 | 0.617 ± 0.091 | 0.51 |
| T score | −1.7 ± 1.6 | −1.9 ± 1.5 | −1.3 ± 1.5 | |
| Total body | 0.839 ± 0.087 | 0.833 ± 0.080 | 0.849 ± 0.085 | 0.84 |
| T score | NA | NA | NA | |
| Quantitative computed tomography bone density (g/cm3) | 87 ± 21 | 85 ± 23 | 96 ± 23 | 0.26 |
| T score | −3.1 ± 0.7 | −3.1 ± 0.8 | −2.8 ± 0.9 |
Data shown are for all subjects in the per-protocol analyses. Values are the mean ± sd. The last column shows the P values determined by analysis of variance for all three groups. BCE, Bone collagen equivalents.
To convert to nanomoles per liter, multiply by 2.496. The normal range for 25-hydroxyvitamin D is given at the time the subjects were enrolled.
Glomerular filtration rate (GFR) estimated using the Cockcroft Gault equation.
Adherence to protocol medication
As noted above, 35 subjects (seven in group 1, 14 in group 2, and 14 in group 3) never started treatment or discontinued treatment prematurely. Most of the subjects who discontinued teriparatide did so due to discomfort or inconvenience related to the injections. All but two women in group 1 and one woman in group 3 took at least 90% of their alendronate doses while enrolled in the study. All but one woman in group 2 and three women in group 3 took at least 90% of their teriparatide doses while enrolled in the protocol.
Six women in group 2 had their teriparatide dose reduced by 25% and seven by 50%. Six women in group 3 had their teriparatide dose reduced by 25% and four by 50%. The mean dose of teriparatide that was administered was 28.5 μg/d in group 2 and 30.5 μg/d in group 3.
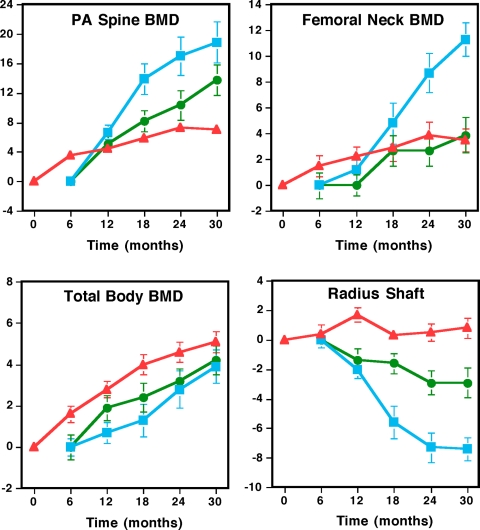
BMD (Fig. 1)
Figure 1.
BMD of the posterior-anterior (PA) spine, femoral neck, one third radius, and total body determined by DXA in women randomized to alendronate alone (n = 29; red triangles), teriparatide alone (n = 20; blue squares), or both (n = 20; green circles). Teriparatide was begun at month 6. Data are plotted as the mean percent change (±sem). In women treated with teriparatide alone or teriparatide plus alendronate, data are presented starting at month 6 when teriparatide was begun. Error bars that are not seen are contained within the symbols.
Posterior-anterior spine BMD increased 6.8 ± 4.2, 17.8 ± 10.9, and 11.9 ± 9.2%, respectively, in women treated with alendronate alone, teriparatide alone, or both. Posterior-anterior spine BMD increased more in women treated with teriparatide alone compared with combination therapy (P < 0.045) (Fig. 1 and Table 2). If the women who discontinued study medication prematurely are excluded, this difference was borderline significant (P = 0.09). Posterior-anterior spine BMD increased more with teriparatide alone or with combination therapy than with alendronate alone (P < 0.001 and P = 0.011, respectively). Femoral neck BMD increased more in women treated with teriparatide alone (10.8 ± 5.2%) than in women treated with alendronate alone (3.5 ± 4.4%) or with combination therapy (3.1 ± 4.5%) (P < 0.001 for each comparison). There was no significant difference in the change of femoral neck BMD between women treated with alendronate alone or alendronate plus teriparatide (P = 0.75; Fig. 1 and Table 2). Total hip BMD increased more in women treated with teriparatide alone (8.1 ± 5.7%) than in women treated with alendronate alone (3.1 ± 4.6%, P < 0.001) or with combination therapy (2.9 ± 4.8%, P = 0.004, Table 2). There was no significant difference in the change of total hip BMD between women treated with alendronate alone or alendronate plus teriparatide (P = 0.96). Radius shaft BMD decreased more in women treated with teriparatide alone (−7.1 ± 4.1%) than in women treated with alendronate alone (0.7 ± 3.3%, P < 0.001) or with combination therapy (−2.5 ± 4.0%, P = 0.002). Radius shaft BMD also decreased more in women treated with combination therapy than in women treated with alendronate alone (P = 0.006, Fig. 1 and Table 2). Total body BMD increased 4.8 ± 2.5, 3.6 ± 3.5, and 4.0 ± 3.2%, respectively, in women treated with alendronate alone, teriparatide alone, or both. There were no significant differences in changes of total body BMD between groups (group 1 vs. group 2, P = 0.11; group 1 vs. group 3, P = 0.42; group 2 vs. group 3, P = 0.53, Fig. 1 and Table 2). Spinal trabecular BMD, measured by quantitative computed tomography, increased more in women treated with teriparatide alone (61 ± 31%) than in women treated with alendronate alone (1 ± 7%) or with combination therapy (24 ± 24%) and more with combination therapy than alendronate monotherapy (P < 0.001 for each comparison, Table 2).
Table 2.
Mean percent change in BMD in women treated with alendronate alone (group 1), teriparatide alone (group 2), or both (group 3)
| Bone site | Group 1 mean percent change | Group 2 mean percent change | Group 3 mean percent change | Group 1 vs. group 2 | P value Group 1 vs. group 3 | Group 2 vs. group 3 |
|---|---|---|---|---|---|---|
| Lumbar spine | 6.8 ± 4.2 | 17.8 ± 10.9 | 11.9 ± 9.2 | <0.001 | 0.011 | 0.045 |
| Femoral neck | 3.5 ± 4.4 | 10.8 ± 5.2 | 3.1 ± 4.5 | <0.001 | 0.75 | <0.001 |
| Total hip | 3.1 ± 4.6 | 8.1 ± 5.7 | 2.9 ± 4.8 | <0.001 | 0.96 | 0.004 |
| Radial shaft | 0.7 ± 3.3 | −7.1 + 4.1 | −2.5 + 4.0 | <0.001 | 0.006 | 0.002 |
| Total body | 4.8 ± 2.5 | 3.6 ± 3.5 | 4.0 ± 3.2 | 0.11 | 0.42 | 0.53 |
| Spinal trabecular bone density on quantitative computed tomography | 1 ± 7 | 61 ± 31 | 24 ± 24 | <0.001 | <0.001 | <0.001 |
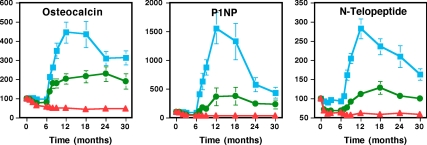
Bone turnover markers (Fig. 2)
Figure 2.
Serum OC, P1NP, and N-telopeptide concentrations in women randomized to alendronate alone (n = 29; red triangles), teriparatide alone (n = 20; blue squares), or both (n = 20; green circles). Teriparatide was begun at month 6. Data are plotted as the mean percent change (±sem). Error bars that are not seen are contained within the symbols.
Mean (±se) percent change in serum OC, P1NP, and NTX levels during the 30-month study period are shown in Fig. 2. In women treated with alendronate alone, mean serum OC and P1NP declined from baseline to month 6 and then remained stable, whereas mean serum NTX reached its nadir within 1–2 months. In women treated with teriparatide alone, mean serum OC, P1NP, and NTX reached peak values by month 12 (i.e. 6 months after starting teriparatide) and then declined gradually. In women treated with teriparatide plus alendronate, changes in all three markers mirrored those observed in women treated with alendronate alone from month 0 to 6, when both groups received only alendronate. During the first 6 months of combined therapy, mean serum OC and P1NP levels rose steeply and continued to increase throughout the study period, eventually reaching levels that were approximately 2–3 times baseline values. The mean serum NTX level also increased once teriparatide was added to alendronate therapy, but the increase was more gradual and much more modest than for OC or P1NP such that the mean NTX level barely exceeded the baseline level. For each marker, the mean AUC was highest in women treated with teriparatide alone, next highest in women treated with teriparatide plus alendronate, and lowest in women treated with alendronate alone, and all groups differed from one another (P < 0.001 for all comparisons between groups 1 and 2 and groups 1 and 3 and P = 0.007, P = 0.001, and P < 0.001 for comparisons of OC, P1NP, and NTX, respectively, between groups 2 and 3).
Adverse events
Hypercalcemia did not occur in group 1. Approximately 24 h after teriparatide dosing, five of 195 (2.5%) serum calcium values were elevated in group 2 and none of 173 values were elevated in group 3. When measured approximately 4 h after the last teriparatide dose, 24 of 160 (15%) serum calcium determinations were elevated in group 2 and five of 142 (3.5%) were elevated in group 3. Only one serum calcium level was above 12 mg/dl and 11 values were 11 mg/dl or greater. Urinary calcium excretion was above 400 mg/d in one of 148 (0.7%) collections in group 1, four of 148 (2.7%) collections in group 2, and seven of 140 (5%) collections in group 3. There were no significant differences in the frequency with which any adverse symptoms were reported (data not shown).
Discussion
In this study, we have shown that administration of alendronate, a potent inhibitor of bone resorption, reduced the ability of once daily teriparatide administration to increase lumbar spine and proximal femur BMD in postmenopausal women with low BMD when teriparatide is given for 24 months, as per its Food and Drug Administration-approved duration of use, although at a 50% higher mean daily dose than is currently approved in the United States. Alendronate also markedly reduced the ability of teriparatide to increase serum OC, P1NP, and NTX levels. These findings are virtually identical with those that we reported previously in men who underwent an identical protocol (8) but differ in important ways from findings of the parathyroid hormone and alendronate (PaTH) study in which postmenopausal women were treated with intact PTH-(1-84), alendronate, or both for 12 months (10). Whereas alendronate did attenuate PTH-(1-84)-induced increases in biochemical markers of bone turnover and of trabecular BMD of the spine (assessed by quantitative computed tomography) over 12 months, it had no effect on PTH-(1-84)-induced increases in spine or femoral neck BMD assessed by DXA. Because improvements in BMD of the proximal femur often require 18–24 months of teriparatide administration before they are seen by DXA (8,13,14,15), the failure of alendronate to attenuate increases in hip BMD after just 1 yr of combined therapy in the PaTH study is not surprising. The shorter duration of observation of the PaTH study likely explains some of the differences in its results with both our prior study in men (8) and our current study in women. Differences between the PaTH study and our two studies could also be related to the use of different anabolic agents or the duration of alendronate administration before adding either intact PTH or teriparatide. Because our findings in men and women were so similar (16), gender differences cannot explain the differences initially reported by Black et al. in women (10) and by our group in men (8).
Alendronate markedly attenuated teriparatide-induced increases in biochemical indices of bone turnover. Because increases in serum OC and P1NP reflect the ability of teriparatide to stimulate osteoblast activity (17), these findings are consistent with the hypothesis that bone resorption is required, at least in part, for teriparatide to stimulate new bone formation in women. This hypothesis is further supported by the observations that teriparatide failed to exert an anabolic effect in a patient with pyknodysostosis, a hereditary defect in osteoclastic bone resorption due to a deficiency in cathepsin K (18), in c-fos−/− mice that are unable to develop osteoclasts (19) and in tiludronate-treated sheep (20). There are, however, other interpretations of the current data and inconsistencies in various animal models. For example, alendronate could act directly on osteoblasts to reduce bone formation (21). In rats, estrogen, calcitonin, and bisphosphonates did not block the anabolic actions of teriparatide (22,23,24,25,26). The reasons for the differences between these animal models and the human data are unclear.
Intermittent PTH could increase osteoblast activity either through a direct stimulatory effect on osteoblasts by increasing the local production of skeletal growth factors (27,28,29,30,31) or indirectly by increasing bone resorption, thereby releasing preformed growth factors adsorbed to bone matrix (32). If intermittent PTH stimulates bone formation directly, reducing bone resorption should not impair its anabolic action on bone. If, however, the anabolic action of PTH is mediated, at least in part, by preformed growth factors that are released by bone resorption, suppressing bone resorption should impair the anabolic actions of teriparatide. Our findings that alendronate attenuates teriparatide-induced increases in OC and P1NP levels as well as spine and hip BMD are consistent with the hypothesis that the anabolic effect of teriparatide depends, at least in part, on its ability to induce bone resorption.
The effect of pretreating subjects with antiresorptive agents on the response to teriparatide has been examined in several studies. In postmenopausal women on long-term hormone therapy, adding teriparatide increased BMD of the lumbar spine, total hip, and total body more than simply continuing hormone therapy (33,34,35). Similarly, in postmenopausal women on long-term alendronate, adding teriparatide either daily or in 3-month cycles for 15 months increased BMD more than continuing alendronate alone (36). Because none of these studies included a group of subjects treated with teriparatide alone, however, these studies do not indicate whether combining an antiresorptive agent with teriparatide is superior, similar, or inferior to teriparatide monotherapy. In postmenopausal women treated with teriparatide for 2 yr, BMD increased less in women previously treated with antiresorptive agents than in women who had never received antiresorptive therapy (37). In a subgroup analysis of women in this study whose predominant antiresorptive treatment was alendronate, risedronate, etidronate, or a nonbisphosphonate, spine BMD increased more during teriparatide treatment in prior etidronate users, whereas increases in the other groups were similar (38). These results are limited, however, by mixtures of antiresorptive drugs in each group, lack of random assignment to antiresorptive agents, differences in lag times before starting teriparatide therapy, and large differences in duration of prior antiresorptive therapy.
Two other studies compared the response to teriparatide after treatment with different antiresorptive agents. Teriparatide increased bone formation and BMD more in postmenopausal women previously treated with raloxifene than in postmenopausal women previously treated with alendronate (39). Similarly, teriparatide increased bone formation and BMD more in postmenopausal women previously treated with risedronate than in postmenopausal women previously treated with alendronate (40). Because alendronate is a more potent antiresorptive agent than raloxifene or risedronate, these results are consistent with the idea that bone resorption helps to generate the anabolic effects of teriparatide. However, because the assignment of antiresorptive therapy regimens was not randomized in any of these studies, it is possible that differences in patient populations rather than differences in antiresorptive agents are responsible for subsequent differential responses to teriparatide. Recently the effects of adding vs. switching to teriparatide were assessed in women on long-standing alendronate or raloxifene (41). Whereas bone turnover markers increased much more in women who were switched to teriparatide, spine and total hip BMD (but not femoral neck BMD) increased more in women in whom teriparatide was added to alendronate. Changes in BMD at 18 months were similar with adding or switching to teriparatide in the women previously treated with raloxifene (41).
Some limitations of our study deserve mention. The dose of teriparatide that was administered (29–30 μg) was higher than that currently approved by the U.S. Food and Drug Administration (20 μg) but similar to the dose used in many clinical studies of teriparatide (3,4,6,42,43). Even though BMD increased more in women treated with 40 μg of teriparatide daily than in women treated with 20 μg/d, fracture rates were similar (6). Thus, only the lower dosage is approved for clinical use. It seems plausible, however, that alendronate might impair the anabolic actions of a lower dose of teriparatide more readily. To prevent an increase in bone resorption when teriparatide was added (44), alendronate was administered for 6 months before starting teriparatide. Different effects might have been observed if teriparatide and alendronate were begun concomitantly or if alendronate was stopped when teriparatide was begun. The lack of blinding of study medication led to a higher rate of discontinuation in the two groups treated with teriparatide, often before starting teriparatide. Although it did not appear that the discontinuations were related to the study outcomes, it is difficult to be certain that the lack of blinding had no impact on the results. Finally, the failure of BMD assessed by quantitative computed tomography to increase with alendronate monotherapy was surprising, although we observed similar results in men (8). Other than the PaTH study, which reported a 10% increase in trabecular BMD in alendronate-treated women (10), we are not aware of any other studies that have monitored trabecular BMD by quantitative computed tomography in subjects treated with alendronate.
In summary, as in men, alendronate impairs the ability of a 2-yr course of teriparatide to increase lumbar spine and proximal femur BMD in women with low BMD and diminishes teriparatide-induced loss from non-weight-bearing cortical bone sites. Alendronate also dramatically reduces the ability of teriparatide to increase biochemical markers of bone formation. Although the effects on fracture risk of these complex interactions are unknown, these findings may help inform clinicians considering treatment of postmenopausal osteoporotic women with teriparatide, particularly if a full 2-yr course of therapy is planned. Additional studies are needed to determine whether coadministration of teriparatide with other antiresorptive agents has similar effects on BMD and bone formation.
Acknowledgments
We thank Ms. Robbin Cleary and Ms. Sarah Zhang for performing the bone density measurements and the nursing and dietary staff of the Mallinckrodt General Clinical Research Center for their dedicated care of the patients.
Footnotes
This work was supported by National Institutes of Health Grants 5 P50 AR44855, K24 DK02759, and RR-1066.
Disclosure Summary: J.S.F. has previously received lecture fees and served as a consultant to Eli Lilly and Co. and Merck. R.M.N. has previously received lecture fees from Eli Lilly and Co. and Merck; has previously consulted for Merck and Radius Inc.; and currently consults for Eli Lilly, Novartis, and TransPharma. J.J.W. and H.L. have nothing to declare.
First Published Online February 17, 2010
Abbreviations: AUC, Area under the curve; BMD, bone mineral density; CV, coefficient of variation; DXA, dual-energy x-ray absorptiometry; NTX, cross-linked N-telopeptides of type I collagen; OC, osteocalcin; P1NP, N-terminal propeptide of type I collagen; PaTH, parathyroid hormone and alendronate.
References
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE 1996 Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348:1535–1541 [DOI] [PubMed] [Google Scholar]
- Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ 1998 Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures. Results from the Fracture Intervention Trial. JAMA 280:2077–2082 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Klibanski A, Arnold AL, Toth TL, Hornstein MD, Neer RM 1998 Prevention of estrogen deficiency-related bone loss with human parathyroid hormone-(1-34): a randomized, controlled trial. JAMA 280:1067–1073 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Klibanski A, Schaefer EH, Hornstein MD, Schiff I, Neer RM 1994 Parathyroid hormone for the prevention of bone loss induced by estrogen deficiency. N Engl J Med 331:1618–1623 [DOI] [PubMed] [Google Scholar]
- Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs Jr RW, Dequeker J, Favus M, The Alendronate Phase III Osteoporosis Treatment Study Group 1995 Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 333:1437–1443 [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH 2001 Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441 [DOI] [PubMed] [Google Scholar]
- Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, Wesolowski G, Russell RG, Rodan GA, Reszka AA 1999 Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci USA 96:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM 2003 The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349:1216–1226 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Leder BZ, Burnett SM, Wyland JJ, Lee H, de la Paz AV, Gibson K, Neer RM 2006 Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J Clin Endocrinol Metab 91:2882–2887 [DOI] [PubMed] [Google Scholar]
- Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ 2003 The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215 [DOI] [PubMed] [Google Scholar]
- Taylor A, Konrad PT, Norman ME, Harcke HT 1997 Total body bone mineral density in young children: influence of head bone mineral density. J Bone Miner Res 12:652–655 [DOI] [PubMed] [Google Scholar]
- Rosenthal DI, Ganott MA, Wyshak G, Slovik DM, Doppelt SH, Neer RM 1985 Quantitative computed tomography for spinal density measurement. Factors affecting precision. Invest Radiol 20:306–310 [DOI] [PubMed] [Google Scholar]
- Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR, Blosch CM, Mathisen AL, Morris SA, Marriott TB 2007 Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med 146:326–339 [DOI] [PubMed] [Google Scholar]
- Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP 2000 Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 85:3069–3076 [DOI] [PubMed] [Google Scholar]
- McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF 2005 Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165:1762–1768 [DOI] [PubMed] [Google Scholar]
- Leder BZ, Neer RM, Wyland JJ, Lee HW, Burnett-Bowie SA, Finkelstein JS 2009 Effects of teriparatide treatment and discontinuation in postmenopausal women and eugonadal men with osteoporosis. J Clin Endocrinol Metab 94:2915–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD 1997 Biochemical markers in the assessment of bone disease. Am J Med 103:427–436 [DOI] [PubMed] [Google Scholar]
- Chavassieux P, Asser Karsdal M, Segovia-Silvestre T, Neutzsky-Wulff AV, Chapurlat R, Boivin G, Delmas PD 2008 Mechanisms of the anabolic effects of teriparatide on bone: insight from the treatment of a patient with pycnodysostosis. J Bone Miner Res 23:1076–1083 [DOI] [PubMed] [Google Scholar]
- Demiralp B, Chen HL, Koh AJ, Keller ET, McCauley LK 2002 Anabolic actions of parathyroid hormone during bone growth are dependent on c-fos. Endocrinology 143:4038–4047 [DOI] [PubMed] [Google Scholar]
- Delmas PD, Vergnaud P, Arlot ME, Pastoureau P, Meunier PJ, Nilssen MH 1995 The anabolic effect of human PTH (1-34) on bone formation is blunted when bone resorption is inhibited by the bisphosphonate tiludronate—is activated resorption a prerequisite for the in vivo effect of PTH on formation in a remodeling system? Bone 16:603–610 [DOI] [PubMed] [Google Scholar]
- Idris AI, Rojas J, Greig IR, Van't Hof RJ, Ralston SH 2008 Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif Tissue Int 82:191–201 [DOI] [PubMed] [Google Scholar]
- Hock JM, Hummert JR, Boyce R, Fonseca J, Raisz LG 1989 Resorption is not essential for the stimulation of bone growth by hPTH-(1-34) in rats in vivo. J Bone Miner Res 4:449–458 [DOI] [PubMed] [Google Scholar]
- Hock JM, Gera I 1992 Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res 7:65–72 [DOI] [PubMed] [Google Scholar]
- Baumann BD, Wronski TJ 1995 Response of cortical bone to antiresorptive agents and parathyroid hormone in aged ovariectomized rats. Bone 16:247–253 [DOI] [PubMed] [Google Scholar]
- Wronski TJ, Yen CF, Qi H, Dann LM 1993 Parathyroid hormone is more effective than estrogen or bisphosphonates for restoration of lost bone mass in ovariectomized rats. Endocrinology 132:823–831 [DOI] [PubMed] [Google Scholar]
- Ma YL, Bryant HU, Zeng Q, Schmidt A, Hoover J, Cole HW, Yao W, Jee WS, Sato M 2003 New bone formation with teriparatide [human parathyroid hormone-(1-34)] is not retarded by long-term pretreatment with alendronate, estrogen, or raloxifene in ovariectomized rats. Endocrinology 144:2008–2015 [DOI] [PubMed] [Google Scholar]
- Canalis E, Centrella M, Burch W, McCarthy TL 1989 Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest 83:60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy TL, Centrella M, Canalis E 1989 Parathyroid hormone enhances the transcript and polypeptide levels of insulin-like growth factor I in osteoblast enriched cultures from fetal rat bone. Endocrinology 124:1247–1253 [DOI] [PubMed] [Google Scholar]
- Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP 2002 Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res 17:1570–1578 [DOI] [PubMed] [Google Scholar]
- Hurley MM, Okada Y, Xiao L, Tanaka Y, Ito M, Okimoto N, Nakamura T, Rosen CJ, Doetschman T, Coffin JD 2006 Impaired bone anabolic response to parathyroid hormone in Fgf2−/− and Fgf2+/− mice. Biochem Biophys Res Commun 341:989–994 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Ogata N, Shinoda Y, Akune T, Kamekura S, Terauchi Y, Kadowaki T, Hoshi K, Chung UI, Nakamura K, Kawaguchi H 2005 Insulin receptor substrate-1 is required for bone anabolic function of parathyroid hormone in mice. Endocrinology 146:2620–2628 [DOI] [PubMed] [Google Scholar]
- Oreffo RO, Mundy GR, Seyedin SM, Bonewald LF 1989 Activation of the bone derived latent TGF-β complex by isolated osteoclasts. Biochem Biophys Res Commun 158:817–823 [DOI] [PubMed] [Google Scholar]
- Cosman F, Nieves J, Woelfert L, Formica C, Gordon S, Shen V, Lindsay R 2001 Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res 16:925–931 [DOI] [PubMed] [Google Scholar]
- Lindsay R, Nieves J, Formicfa C, Henneman E, Woelfert L, Shen V, Dempster D, Cosman F 1997 Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 350:550–555 [DOI] [PubMed] [Google Scholar]
- Ste-Marie LG, Schwartz SL, Hossain A, Desaiah D, Gaich GA 2006 Effect of teriparatide [rhPTH(1-34)] on BMD when given to postmenopausal women receiving hormone replacement therapy. J Bone Miner Res 21:283–291 [DOI] [PubMed] [Google Scholar]
- Cosman F, Nieves J, Zion M, Woelfert L, Luckey M, Lindsay R 2005 Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med 353:566–575 [DOI] [PubMed] [Google Scholar]
- Obermayer-Pietsch BM, Marin F, McCloskey EV, Hadji P, Farrerons J, Boonen S, Audran M, Barker C, Anastasilakis AD, Fraser WD, Nickelsen T 2008 Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res 23:1591–1600 [DOI] [PubMed] [Google Scholar]
- Boonen S, Marin F, Obermayer-Pietsch B, Simões ME, Barker C, Glass EV, Hadji P, Lyritis G, Oertel H, Nickelsen T, McCloskey EV 2008 Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 93:852–860 [DOI] [PubMed] [Google Scholar]
- Ettinger B, San Martin J, Crans G, Pavo I 2004 Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19:745–751 [DOI] [PubMed] [Google Scholar]
- Miller PD, Delmas PD, Lindsay R, Watts NB, Luckey M, Adachi J, Saag K, Greenspan SL, Seeman E, Boonen S, Meeves S, Lang TF, Bilezikian JP 2008 Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab 93:3785–3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman F, Wermers RA, Recknor C, Mauck KF, Xie L, Glass EV, Krege JH 2009 Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab 94:3772–3780 [DOI] [PubMed] [Google Scholar]
- Slovik DM, Rosenthal DI, Doppelt SH, Potts Jr JT, Daly MA, Campbell JA, Neer RM 1986 Restoration of spinal bone in osteoporotic men by treatment with human parathyroid hormone (1-34) and 1,25-dihydroxyvitamin D. J Bone Miner Res 1:377–381 [DOI] [PubMed] [Google Scholar]
- Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA 2003 The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18:9–17 [DOI] [PubMed] [Google Scholar]
- Cosman F, Nieves J, Woelfert L, Shen V, Lindsay R 1998 Alendronate does not block the anabolic effect of PTH in postmenopausal osteoporotic women. J Bone Miner Res 13:1051–1055 [DOI] [PubMed] [Google Scholar]