Abstract
Context: Cushing syndrome (CS) is a rare but potentially fatal feature of McCune-Albright syndrome (MAS). Optimal management, prognostic features, and long-term follow-up of this disorder have not been described.
Setting: The study was conducted at an academic tertiary care center.
Patients: A total of 112 patients participating in a natural history study at the National Institutes of Health (NIH) were evaluated, and 21 published cases were reviewed.
Interventions: Subjects received observation, medical management, or bilateral adrenalectomy.
Main Outcome Measures: We measured prevalence, prognostic factors, and natural history.
Results: The prevalence of CS among NIH patients was 7.1%. The median age at diagnosis was 3 months. Clinical features included “Cushingoid facies” (66.7%), failure to thrive (60.0%), low birth weight (50.0%), liver disease (36.7%), and heart disease (26.7%). Six patients (20.0%) died, four after adrenalectomy. Death was more likely in patients with comorbid heart disease (odds ratio, 13.3; P < 0.05). Of 23 survivors, 13 underwent adrenalectomy, and 10 exhibited spontaneous resolution. Two patients with spontaneous resolution who were tested later in life (3 and 15 yr after resolution) continued to have low-level, autonomous adrenal function with biochemical adrenal insufficiency. Compared to MAS patients without CS, patients with CS were more likely to have a cognitive/developmental disorder (44.4 vs. 4.8%; P < 0.001; odds ratio, 8.8).
Conclusions: Comorbid heart and liver disease were poor prognostic markers and may indicate the need for prompt adrenalectomy. The high incidence of cognitive disorders indicates a need for close developmental follow-up and parental counseling. Patients with spontaneous resolution of CS may develop adrenal insufficiency, and they require long-term monitoring.
Cushing’s syndrome is present in up to 7.1% of patients with McCune-Albright syndrome; it may remit spontaneously and is associated with later cognitive dysfunction.
McCune-Albright syndrome (MAS) is caused by postzygotic, activating mutations of the α-subunit of the stimulatory G protein (Gsα) that is coupled to many cell surface hormone receptors (1,2,3). The clinical manifestations of the disorder are variable due to the somatic nature of the mutations and the mosaic distribution of affected tissues. These are now recognized to extend well beyond the classic triad of precocious puberty, fibrous dysplasia of bone, and café-au-lait skin pigmentation (4,5,6), and include nonendocrine manifestations such as renal phosphate wasting (7), hepatobiliary dysfunction (8,9), and heart disease (9,10). Hyperfunctioning endocrinopathies include gonadotropin-independent precocious puberty, hyperthyroidism, GH excess, hyperprolactinemia, and hypercortisolism (9).
Hypercortisolism, or Cushing syndrome, affects a minority of patients with MAS and has a quite heterogeneous natural history, ranging from spontaneous resolution to need for adrenalectomy or even death (11). Due to the small number of reported patients, it may be difficult for the clinical practitioner to recognize which patients can be safely monitored or treated medically vs. those patients at high risk of morbidity and mortality who may benefit from prompt adrenalectomy. In addition, long-term outcomes of these patients have not been reported. To address questions regarding diagnosis, prognosis, management, and long-term outcome of this disorder, we analyzed our eight cases of MAS and cortisol excess and reviewed the 21 cases from the literature and one additional case from personal communication.
Patients and Methods
A database of patients with MAS enrolled in a natural history study at the National Institutes of Health (NIH) was queried to identify patients with Cushing syndrome, cortisol excess, or adrenal abnormalities. The study was approved by the institutional review board of the National Institute of Dental and Craniofacial Research, and subjects and/or their guardians provided informed consent/assent. A PubMed search identified an additional 21 patients with the diagnoses of MAS and Cushing syndromes (9,11,12,13,14,15,16,17,18,19,20,21,22,23,24). One additional patient was identified by personal communication (Stratakis, C.; Table 1, patient 9). A case-control analysis was performed comparing patients with MAS and Cushing syndrome (NIH + literature, n = 30) to NIH patients with MAS without Cushing syndrome (n = 104).
Table 1.
Patients with Cushing syndrome and MAS
(Continued)
Table 1A.
Continued
F/U, Follow-up; PFD, polyostotic fibrous dysplasia; PP, precocious puberty; LVH, left ventricular hypertrophy; FTT, failure to thrive; SGA, small for gestational age; ARDS, acute respiratory distress syndrome; OCD, obsessive compulsive disorder; NR, not reported; Dev., developmental; PC, personal communication; AST, aspartate aminotransferase; CS, Cushing syndrome; ADHD, attention deficit and hyperactivity disorder; Dex, dexamethasone; Phos, phosphate; GI, gastrointestinal; HTN, hypertension; Resp, respiratory; UFC, urinary free cortisol.
a Age (in years) at diagnosis of Cushing syndrome (line 1), at diagnosis of MAS (line 2), and at last reported follow-up (line 3). b Cushing syndrome was first feature of MAS. c Fig. 1F; d Fig. 1, A–C; e Fig. 1, D and E.
Statistical analyses were performed using GraphPad InStat Version 3 (GraphPad Software, Inc., San Diego, CA).
Results
Natural history of Cushing syndrome in MAS
Eight patients with hypercortisolism were identified in the NIH cohort of 112 patients (7.1%). The characteristics of MAS patients from NIH, personal communication, and the medical literature are summarized in Tables 1 and 2. The median age at diagnosis was 3.1 months (range, birth to 44 months). A history of low birth weight for gestational age (<10th centile) was noted in 15 patients (50%). The most frequent clinical features of cortisol excess at the time of diagnosis were “Cushingoid facies” (66.7%), failure to thrive (60.0%), hypertension (33.3%), nephrocalcinosis (30.0%), hirsutism (26.6%), hyperglycemia (20.0%), and linear growth failure with normal or excess weight gain (10%). Additional clinical features included liver disease such as cholestasis or elevated transaminases (36.7%) and heart disease such as cardiomyopathy (26.70%).
Table 2.
Clinical features of patients with Cushing syndrome
| Age at diagnosis (yr) | |
| Mean | 0.5 |
| Median | 0.3 |
| Range | 0–3.7 |
| Small for gestational age | 15 (50.0) |
| Clinical features at diagnosis | |
| Round face | 20 (66.7) |
| Failure to thrive | 18 (60.0) |
| Hypertension | 10 (33.3) |
| Hirsutism | 8 (26.6) |
| Nephrocalcinosis | 9 (30.0) |
| Hyperglycemia | 6 (20.0) |
| Linear growth arrest | 3 (10.0) |
| Method of diagnosis | |
| Laboratory (elevated cortisols with no suppression to dexamethasone) | 25 (83.3) |
| Clinical | 2 (6.7) |
| Not reported | 3 (10.0) |
| Outcome | |
| Death | 6 (20.0) |
| Early death (before adrenalectomy) | 2 (33.3) |
| Late death (after adrenalectomy) | 4 (66.7) |
| Medical therapy | 4 (13.3) |
| Successful (resolution) | 2 (50.0) |
| Unsuccessful (death) | 2 (50.0) |
| Adrenalectomy | 17 (56.7) |
| Successful (resolution) | 13 (76.5) |
| Unsuccessful (death) | 4 (23.6) |
| Spontaneous resolution | 10 (33.3) |
| Without medical therapy | 8 (80) |
| After medical therapy | 2 (20) |
| Not reported | 1 (3.3) |
| MAS features | |
| Fibrous dysplasia | 28 (93.3) |
| Café-au-lait spots | 28 (93.3) |
| Precocious puberty | 22 (73.3) |
| Hyperthyroid | 17 (56.7) |
| Renal phosphate wasting | 5 (16.7) |
| GH excess | 3 (10.0) |
| Other clinical feature | |
| Liver disease | 11 (36.7) |
| Heart disease | 8 (26.7) |
Data are expressed as number of patients (percentage), except for age.
Diagnosis of Cushing syndrome was based on elevated serum or urine cortisol with failure to suppress to dexamethasone in 25 patients (83.3%). Detailed information on laboratory evaluation of Cushing syndrome was available in six NIH patients, all of whom had lack of cortisol suppression to high-dose dexamethasone and/or ACTH levels below the lower limit of normal, consistent with cortisol excess of adrenal origin. In two patients evaluated at NIH, the diagnosis of Cushing syndrome was made retrospectively based on clinical criteria. The method of diagnosis was not reported in three cases from the literature (25,26).
Six patients (20.0%) died, four of whom had undergone prior bilateral adrenalectomy. In one patient from the dermatology literature (17), management and outcome were not reported. Among the 23 known survivors (76.7%), 13 underwent adrenalectomy, and 10 exhibited resolution of Cushing syndrome without adrenalectomy. Two of the patients who spontaneously improved had previously been treated medically with cortisol-lowering drugs (ketoconazole and metyrapone). The most common pathological finding in patients who underwent adrenalectomy was nodular hyperplasia (11 of 14).
Predicting clinical outcome in Cushing syndrome
We sought correlations between the clinical features of MAS (e.g. hyperthyroidism) and outcome (death, adrenalectomy, or spontaneous resolution). The presence of heart disease (including all cardiac disorders listed in Table 1) was a poor prognostic marker for survival [Fischer’s exact test, P = 0.018; odds ratio (OR), 13.3; 95% confidence interval (CI), 1.6–107]. There was a trend toward lower likelihood of spontaneous resolution in patients with liver disease (P = 0.098; OR, 0.12; 95% CI, 0.01–1.2). No other significant associations between clinical features of MAS and outcome were found.
The mean age at diagnosis of patients who died was 2.4 ± 1.6 months (range, birth to 4 months) vs. 7.1 ± 10.1 months (range, 1 wk to 3.7 yr) for survivors; however, this difference was not statistically significant.
Comparison of MAS patients with and without Cushing syndrome
A case-control analysis of MAS patients without Cushing syndrome (Table 3) revealed that the presence of Cushing syndrome was correlated with an increased number of common clinical features of MAS, including café-au-lait skin pigmentation (Fig. 1, D and E), fibrous dysplasia, precocious puberty (Fig. 1E), hyperthyroidism, renal phosphate wasting, and GH excess. Patients with Cushing syndrome had a mean of 3.4 of these features, vs. a mean of 2.9 in patients without Cushing syndrome (P = 0.041, unpaired t test). Specific features of MAS that were associated with Cushing syndrome were café-au-lait spots (P = 0.0013; OR, 7.8; 95% CI, 1.8–34.8), precocious puberty (P = 0.036; OR, 2.7; 95% CI, 1.1–6.7), and hyperthyroidism (P = 0.0035; OR, 3.7; 95% CI, 1.6–8.7). In addition, MAS patients with Cushing syndrome who survived beyond 1 yr of age were more likely to have a cognitive disorder, including specific learning or speech disorders, or global developmental delay (P = 0.0006; OR, 8.8; 95% CI, 2.6–30.0). Interestingly, three of the NIH patients with Cushing syndrome had speech apraxia, vs. 0 of 104 patients without Cushing syndrome (P = 0.01; OR, 26.6; 95% CI, 1.3–530.8).
Table 3.
Comparison of MAS patients with vs. without Cushing syndrome
| Cortisol excess | No cortisol excess | P value | OR (95% CI) | |
|---|---|---|---|---|
| Male | 10 (33.3) | 40 (38.5) | 0.67 | 0.8 (0.3–1.9) |
| Female | 20 (66.7) | 64 (61.5) | ||
| Café-au-lait | 28 (93.3) | 66 (63.5) | 0.0013 | 7.8 (1.8–34.8) |
| Fibrous dysplasia | 28 (93.3) | 103 (99.0) | 0.13 | 0.1 (0.01–1.6) |
| Precocious puberty | 22 (73.3) | 52 (50.0) | 0.036 | 2.75 (1.1–6.7) |
| Hyperthyroid | 17 (56.7) | 27 (26.0) | 0.0035 | 3.7 (1.6–8.7) |
| Phosphate wasting | 5 (16.7) | 34 (32.7) | 0.11 | 0.41 (0.1–1.2) |
| GH excess | 3 (10.0) | 19 (18.3) | 0.40 | 0.5 (0.1–1.8) |
| Cognitive disordera | 8 (44.4) | 5 (4.8) | 0.0006 | 8.8 (2.6–30.0) |
| Total no. of MAS manifestationsb | 3.4 ± 1.1c | 2.9 ± 1.4c | 0.041 | |
| Age (yr) | 28.8 ± 16.9c | 10.2 ± 6.3c | <0.0001 |
Data are expressed as number of patients (percentage) unless explained otherwise. Significant differences shown in bold.
Among children who survived to at least 1 yr of age.
Not including Cushing syndrome.
Mean ± sd.
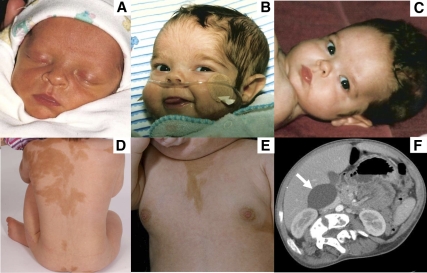
Figure 1.
A–C, Patient 6 as neonate with no clinical signs of cortisol excess (A); at age 3 months, with frequent infections and hirsutism of forehead (B); and at age 6 months, with resolved hirsutism (C). D and E, Patient 7 at age 5 yr demonstrating typical café-au-lait skin pigmentation (D) and at age 15 months demonstrating breast development from precocious puberty (E). F, Computed tomography of the abdomen in patient 5 demonstrating a large adrenal cyst (white arrow). This patient has persistent autonomous adrenal function and poor adrenal reserve.
Persistent autonomous adrenal function after clinical resolution of Cushing syndrome
Patients 4 and 5 exhibited spontaneous resolution of hypercortisolism followed by residual autonomous adrenal function with low adrenal reserve, a phenomenon not previously reported in the literature.
Patient 4 was diagnosed with Cushing syndrome at age 4 months. At 6 months of age, her serum cortisol levels were markedly elevated without diurnal variation [2400 h cortisol values, 19.7–40 μg/dl (531–1094 nmol/liter); 0800 h cortisol values, 20.3–27.8 μg/dl (548–751 nmol/liter)], ACTH was low [2–3 pg/ml (0.44–0.67 pmol/ liter)], and she had a lack of cortisol suppression to dexamethasone. Clinical features of Cushing syndrome resolved by 1 yr of age, and serum cortisol values gradually decreased, but with persistent lack of diurnal variation. Evaluation at NIH at age 3 5/12 yr revealed serum cortisol values of 1.8–2.1 μg/dl (50–58 nmol/liter) at 2400 h and 2.3–3.3 μg/dl (63–91 nmol/liter) at 0800 h, demonstrating a continued lack of diurnal variation. ACTH remained undetectable [<5 pg/ml (<1.1 pmol/liter)], indicating continued autonomous adrenal function. ACTH stimulation testing (250 μg Cosyntropin) showed a peak cortisol response of 18 μg/dl (496 nmol/liter) at 60 min, indicating borderline adrenal reserve.
Patient 5 was diagnosed with Cushing syndrome at age 18 months, at which time her morning cortisol was 27.4 μg/dl (755 nmol/liter) and nighttime cortisol was 21.1 μg/dl (581 nmol/liter). Evaluation at NIH at age 15 8/12 yr revealed serum cortisol values of 4.7–5 μg/dl (129–138 nmol/liter) at 2400 h and 4.7–5.2 μg/dl (129–143 nmol/liter) at 0800 h, demonstrating persistent lack of diurnal variation. ACTH was undetectable, indicating autonomous adrenal function. Computed tomography imaging of the adrenal glands showed a 5-cm cyst of the right adrenal gland (Fig. 1F). ACTH stimulation testing (250 μg Cosyntropin) showed a peak cortisol response of 15.4 μg/dl (424 nmol/liter) at 60 min, meeting the biochemical criteria for inadequate adrenal reserve. However, the patient did not have any signs or symptoms of adrenal insufficiency and had, in fact, had major orthopedic surgery 2 yr earlier without developing adrenal crisis. Nonetheless, it was felt prudent to educate her parents on the management of adrenal insufficiency and prescribe injectable hydrocortisone for surgery, severe illness, or injury.
Discussion
Cushing syndrome in the context of MAS is a rare disorder, and publications to date have largely consisted of case reports. In this study, we analyzed eight previously unpublished cases with long-term follow-up, as well as the published cases. In addition, we included a large, well-characterized case-control cohort of patients with MAS without cortisol excess. Our goal was to provide useful guidelines on the diagnosis, prognosis, management, and long-term outcome of this disorder.
It is possible that the NIH cohort may be biased toward a more severe spectrum of MAS and that the prevalence of 7.1% reported here may be an overestimate of the true prevalence of Cushing syndrome in MAS. Conversely, lack of access to medical records or follow-up on cases taken from the literature may have led to an underestimation of other clinical features, such as nephrocalcinosis or cognitive delay.
This study showed that hypercortisolism may be the first recognized manifestation of MAS in some cases (often appearing before the development of the café-au-lait skin pigmentation). Therefore, prompt and appropriate care of the infant or young child with MAS and Cushing syndrome depends on having a high index of suspicion for the diagnosis. In the child in whom the diagnosis is suspected, a rapid assessment for significant comorbidities should be performed, including hyperthyroidism and cardiac and liver disease. Evaluation for complications of glucocorticoid excess should be conducted, including hypertension, hyperglycemia, and nephrocalcinosis. If present, nephrocalcinosis is best managed by correcting the glucocorticoid excess, whereas hypertension and hyperglycemia can be medically managed pending definitive therapy. Patients with significant cortisol excess are immunocompromised, and prophylaxis for pneumocystis carinii pneumonia should be considered in all patients before definitive therapy.
The clinical features of cortisol excess in patients with MAS were generally similar to those seen in Cushing syndrome of other etiologies. One notable exception is that the majority of MAS patients with Cushing syndrome demonstrated failure of both linear growth and weight gain (failure to thrive), in contrast to the excessive weight gain typically associated with cortisol excess. It is possible that, in some cases, this failure to gain weight was related to comorbidities such as hyperthyroidism or heart disease.
Cortisol excess is almost certainly present in utero in the majority of MAS patients with Cushing syndrome, as evidenced by low birth weight and early age of presentation. As with other endocrinopathies in MAS, cortisol excess is caused by tonic activation of Gsα in the adrenal cortex, leading to unregulated overproduction of cAMP, and thus cortisol. Cushing syndrome is unique among MAS-related endocrinopathies, however, in its tendency to spontaneously resolve. This phenomenon of spontaneous resolution may reflect the fact that the Gsα mutation is found within cells in the fetal zone of the adrenal cortex, which undergoes rapid apoptosis in the postnatal period. During gestation, the bulk of the adrenal gland consists of the steroidogenic “fetal zone,” which broadly expresses Gsα (27,28). The fetal zone undergoes rapid degeneration after birth and is gone by 1 yr of age (29). In the cases of Cushing syndrome in MAS that resolve spontaneously, this may reflect the appropriate (albeit delayed) involution of fetal adrenal cells.
We report long-term follow-up on two patients with spontaneous resolution of hypercortisolism, in whom biochemical testing revealed continued autonomous adrenal function even after resolution of overt Cushing syndrome. More importantly, ACTH stimulation testing on these patients showed low peak cortisols, suggesting the possibility of adrenal insufficiency under stress. Such patients should be educated on the signs and symptoms of adrenal insufficiency and may require stress-dosing of glucocorticoids for illness or surgery.
The prognosis of patients with Cushing syndrome in this study was most strongly associated with the presence of comorbid heart disease, which was associated with increased odds of death. There was a weaker association of comorbid liver disease with outcomes of death or adrenalectomy. Because both heart and liver disease in MAS have in some cases been shown to be a direct consequence of Gsα mutations in the affected tissues, it is possible that the poor outcome of Cushing syndrome in this group is merely a reflection of a greater total body burden of Gsα mutation and, thus, sicker patients. Similarly, the greater number of MAS-associated endocrinopathies seen in patients with Cushing syndrome may reflect a greater burden of mutation-carrying cells. Nonetheless, recognition of comorbid heart or liver disease in children with MAS and cortisol excess should prompt consideration of early adrenalectomy when medically feasible. Although patients who have had adrenalectomy, if not appropriately treated, are at risk for adrenal crisis and death, in only one subject was this identified as a possible cause of death. Nonetheless, this possibility remains, and appropriate education of families is essential.
The high incidence of developmental problems among survivors of Cushing syndrome has not been previously noted, and again this raises the question of the effects of exposure to excess glucocorticoid in utero or during early postnatal life. In addition, the finding of speech apraxia in three of 10 NIH patients is quite striking, given that the prevalence of this disorder in the general population is estimated at only 0.125–1.3% (30). In children who developed Cushing syndrome later than the neonatal period, minor declines in cognitive function have been observed after treatment, particularly in younger children, but overall IQ scores remained in the normal range (31). Studies of cognitive function in children at risk for congenital adrenal hyperplasia or premature birth who were exposed to dexamethasone in utero have not shown significant differences in cognitive outcome related to glucocorticoid exposure (32,33,34,35). However, affected infants with Cushing syndrome due to MAS may have been exposed to significantly higher levels of glucocorticoid than those used therapeutically for lung maturation and congenital adrenal hyperplasia. An alternative explanation of cognitive impairment in MAS is the possibility of mutated Gsα in the central nervous system as part of the mosaic distribution, with Cushing syndrome survivors being more affected due to greater total body mutation burden. Transgenic mice in which a similarly activating Gsα mutation (Q227L) was selectively expressed in the brain showed a neurobehavioral phenotype including short- and long-term memory deficits, and impaired associative and spatial learning (36,37). Interestingly, these animals actually exhibit decreased cAMP levels in the cortex and hippocampus (as opposed to the increase that might be expected from constitutively activated Gsα). This was found to be due to compensatory increases in enzymes that break down cAMP: phosphodiesterases 1 and 4 (37,38); and the phosphodiesterase 4 inhibitor, rolipram, was found to reverse some aspects of the neurobehavioral phenotype. cAMP levels are not known to be decreased in the brains of patients with MAS, but it is worth considering whether phosphodiesterase inhibitors may be helpful in those patients with significant cognitive or neuropsychiatric dysfunction. This approach should be considered with caution, however, because phosphodiesterase inhibitors could theoretically worsen other manifestations of MAS, such as hyperthyroidism.
In summary, Cushing syndrome occurs in up to 7.1% of patients with MAS and may be characterized by low birth weight or failure to thrive, as well as more classic features of cortisol excess. Comorbid heart and liver disease appear to be poor prognostic markers and may indicate the need for prompt adrenalectomy. The high incidence of cognitive disorders in survivors indicates a need for close developmental follow-up. Patients with spontaneous resolution of Cushing syndrome may develop adrenal insufficiency, and adrenal function should be monitored long term in this group.
Footnotes
This work was supported by the intramural research programs of the National Institute of Dental and Craniofacial Research, the National Institute of Child Health and Development, and the National Institute of Diabetes, Digestive and Kidney Diseases.
Clinical Trial no. NCT00001727.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 15, 2010
Abbreviations: CI, Confidence interval; Gsα, α-subunit of the stimulatory G protein; MAS, McCune-Albright syndrome; OR, odds ratio.
References
- Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM 1991 Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med 325:1688–1695 [DOI] [PubMed] [Google Scholar]
- Schwindinger WF, Francomano CA, Levine MA 1992 Identification of a mutation in the gene encoding the α subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci USA 89:5152–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu CE, Collins MT 2008 McCune-Albright syndrome. Orphanet J Rare Dis 3:12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuillan PP 1997 McCune-Albright syndrome. Curr Ther Endocrinol Metab 6:235–239 [PubMed] [Google Scholar]
- Diaz A, Danon M, Crawford J 2007 McCune-Albright syndrome and disorders due to activating mutations of GNAS1. J Pediatr Endocrinol Metab 20:853–880 [DOI] [PubMed] [Google Scholar]
- Zacharin M 2007 The spectrum of McCune Albright syndrome. Pediatr Endocrinol Rev 4(Suppl 4):412–418 [PubMed] [Google Scholar]
- Collins MT, Chebli C, Jones J, Kushner H, Consugar M, Rinaldo P, Wientroub S, Bianco P, Robey PG 2001 Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. J Bone Miner Res 16:806–813 [DOI] [PubMed] [Google Scholar]
- Silva ES, Lumbroso S, Medina M, Gillerot Y, Sultan C, Sokal EM 2000 Demonstration of McCune-Albright mutations in the liver of children with high γGT progressive cholestasis. J Hepatol 32:154–158 [DOI] [PubMed] [Google Scholar]
- Shenker A, Weinstein LS, Moran A, Pescovitz OH, Charest NJ, Boney CM, Van Wyk JJ, Merino MJ, Feuillan PP, Spiegel AM 1993 Severe endocrine and nonendocrine manifestations of the McCune-Albright syndrome associated with activating mutations of stimulatory G protein GS. J Pediatr 123:509–518 [DOI] [PubMed] [Google Scholar]
- Ringel MD, Schwindinger WF, Levine MA 1996 Clinical implications of genetic defects in G proteins. The molecular basis of McCune-Albright syndrome and Albright hereditary osteodystrophy. Medicine (Baltimore) 75:171–184 [DOI] [PubMed] [Google Scholar]
- Kirk JM, Brain CE, Carson DJ, Hyde JC, Grant DB 1999 Cushing’s syndrome caused by nodular adrenal hyperplasia in children with McCune-Albright syndrome. J Pediatr 134:789–792 [DOI] [PubMed] [Google Scholar]
- Aarskog D, Tveteraas E 1968 McCune-Albright’s syndrome following adrenalectomy for Cushing’s syndrome in infancy. J Pediatr 73:89–96 [DOI] [PubMed] [Google Scholar]
- Bareille P, Azcona C, Stanhope R 1999 Multiple neonatal endocrinopathies in McCune-Albright syndrome. J Paediatr Child Health 35:315–318 [DOI] [PubMed] [Google Scholar]
- Boston BA, Mandel S, LaFranchi S, Bliziotes M 1994 Activating mutation in the stimulatory guanine nucleotide-binding protein in an infant with Cushing’s syndrome and nodular adrenal hyperplasia. J Clin Endocrinol Metab 79:890–893 [DOI] [PubMed] [Google Scholar]
- Brogan P, Khadilkar VV, Stanhope R 1998 Occult T3 toxicosis in McCune-Albright syndrome. Horm Res 50:105–106 [DOI] [PubMed] [Google Scholar]
- Danon M, Robboy SJ, Kim S, Scully R, Crawford JD 1975 Cushing syndrome, sexual precocity, and polyostotic fibrous dysplasia (Albright syndrome) in infancy. J Pediatr 87:917–921 [DOI] [PubMed] [Google Scholar]
- Davies JH, Barton JS, Gregory JW, Mills C 2001 Infantile McCune-Albright syndrome. Pediatr Dermatol 18:504–506 [DOI] [PubMed] [Google Scholar]
- Halioui-Louhaichi S, Azzabi O, Nefzi L, Ben Hariz M, Ben Mrad N, Ben Ammar B, Maherzi A 2005 [Treatment with metyrapone of Cushing’s syndrome revealing McCune-Albright syndrome]. Arch Pediatr 12:1120–1123 [DOI] [PubMed] [Google Scholar]
- Kessel D, Hall CM, Shaw DG 1992 Two unusual cases of nephrocalcinosis in infancy. Pediatr Radiol 22:470–471 [DOI] [PubMed] [Google Scholar]
- Lala R, Matarazzo P, Andreo M, Defilippi C, de Sanctis C 2002 Impact of endocrine hyperfunction and phosphate wasting on bone in McCune-Albright syndrome. J Pediatr Endocrinol Metab 15(Suppl 3):913–920 [PubMed] [Google Scholar]
- Listernick R 2004 A 14-week-old girl with severe respiratory distress, hyperpigmentation. Pediatr Ann 33:218–222 [DOI] [PubMed] [Google Scholar]
- Mauras N, Blizzard RM 1986 The McCune-Albright syndrome. Acta Endocrinol Suppl (Copenh) 279:207–217 [DOI] [PubMed] [Google Scholar]
- Post EM CL, Hitch I, Oliphant M, Dracker R, Richman RA 1983 Congenital Cushing syndrome with polyostotic fibrous dysplasia. Pediatr Res 17:169A [Google Scholar]
- Yoshimoto M, Nakayama M, Baba T, Uehara Y, Niikawa N, Ito M, Tsuji Y 1991 A case of neonatal McCune-Albright syndrome with Cushing syndrome and hyperthyroidism. Acta Paediatr Scand 80:984–987 [DOI] [PubMed] [Google Scholar]
- Benjamin DR, McRoberts JW 1973 Polyostotic fibrous dysplasia associated with Cushing syndrome. Arch Pathol 96:175–178 [PubMed] [Google Scholar]
- Fragoso MC, Domenice S, Latronico AC, Martin RM, Pereira MA, Zerbini MC, Lucon AM, Mendonca BB 2003 Cushing’s syndrome secondary to adrenocorticotropin-independent macronodular adrenocortical hyperplasia due to activating mutations of GNAS1 gene. J Clin Endocrinol Metab 88:2147–2151 [DOI] [PubMed] [Google Scholar]
- Bocian-Sobkowska J, Malendowicz LK, WoŸniak W 1997 Comparative stereological study on zonation and cellular composition of adrenal glands of normal and anencephalic human fetuses. I. Zonation of the gland. Histol Histopathol 12:311–317 [PubMed] [Google Scholar]
- Breault L, Chamoux E, Lehoux JG, Gallo-Payet N 2000 Localization of G protein α-subunits in the human fetal adrenal gland. Endocrinology 141:4334–4341 [DOI] [PubMed] [Google Scholar]
- Bocian-Sobkowska J, WoŸniak W, Malendowicz LK 1998 Postnatal involution of the human adrenal fetal zone: stereologic description and apoptosis. Endocr Res 24:969–973 [DOI] [PubMed] [Google Scholar]
- Shriberg LD, Aram DM, Kwiatkowski J 1997 Developmental apraxia of speech. I. Descriptive and theoretical perspectives. J Speech Lang Hear Res 40:273–285 [DOI] [PubMed] [Google Scholar]
- Keil MF, Merke DP, Gandhi R, Wiggs EA, Obunse K, Stratakis CA 2009 Quality of life in children and adolescents 1-year after cure of Cushing syndrome: a prospective study. Clin Endocrinol (Oxf) 71:326–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bahlburg HF, Dolezal C, Baker SW, Carlson AD, Obeid JS, New MI 2004 Cognitive and motor development of children with and without congenital adrenal hyperplasia after early-prenatal dexamethasone. J Clin Endocrinol Metab 89:610–614 [DOI] [PubMed] [Google Scholar]
- Hirvikoski T, Nordenström A, Lindholm T, Lindblad F, Ritzén EM, Wedell A, Lajic S 2007 Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J Clin Endocrinol Metab 92:542–548 [DOI] [PubMed] [Google Scholar]
- Dalziel SR, Lim VK, Lambert A, McCarthy D, Parag V, Rodgers A, Harding JE 2005 Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ 331:665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley PA 1995 Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol 173:322–335 [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Patterson SL, Kelly MP, Kreibich A, Kandel ER, Abel T 2006 Chronically increased Gsα signaling disrupts associative and spatial learning. Learn Mem 13:745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Cheung YF, Favilla C, Siegel SJ, Kanes SJ, Houslay MD, Abel T 2008 Constitutive activation of the G-protein subunit Gαs within forebrain neurons causes PKA-dependent alterations in fear conditioning and cortical Arc mRNA expression. Learn Mem 15:75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Isiegas C, Cheung YF, Tokarczyk J, Yang X, Esposito MF, Rapoport DA, Fabian SA, Siegel SJ, Wand G, Houslay MD, Kanes SJ, Abel T 2007 Constitutive activation of Gαs within forebrain neurons causes deficits in sensorimotor gating because of PKA-dependent decreases in cAMP. Neuropsychopharmacology 32:577–588 [DOI] [PMC free article] [PubMed] [Google Scholar]