Abstract
Context: Vitamin D insufficiency has now reached epidemic proportions and has been linked to increased body fat and decreased muscle strength. Whether vitamin D insufficiency is also related to adipose tissue infiltration in muscle is not known.
Objective: The objective of the study was to examine the relationship between serum 25-hydroxyvitamin D (25OHD) and the degree of fat infiltration in muscle.
Design: This was a cross-sectional study.
Outcome Measures and Subjects: Measures were anthropometric measures, serum 25OHD radioimmunoassay values, and computed tomography (CT) values of fat, muscle mass, and percent muscle fat in 90 postpubertal females, aged 16–22 yr, residing in California.
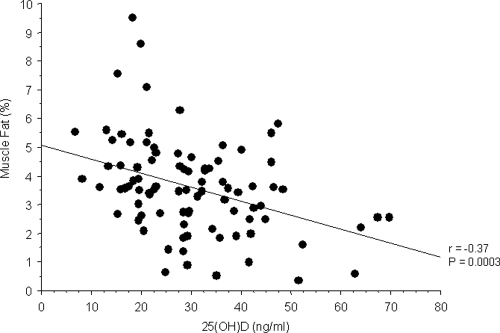
Results: Approximately 59% of subjects were 25OHD insufficient (≤29 ng/ml), of which 24% were deficient (≤20 ng/ml), whereas 41% were sufficient (≥30 ng/ml). A strong negative relationship was present between serum 25OHD and CT measures of percent muscle fat (r = −0.37; P < 0.001). In contrast, no relationship was observed between circulating 25OHD concentrations and CT measures of thigh muscle area (r = 0.16; P = 0.14). Multiple regression analysis indicated that the relation between 25OHD and muscle adiposity was independent of body mass or CT measures of sc and visceral fat. Percent muscle fat was significantly lower in women with normal serum 25OHD concentrations than in women with insufficient levels and deficient levels (3.15 ± 1.4 vs. 3.90 ± 1.9; P = 0.038).
Conclusions: We found that vitamin D insufficiency is associated with increased fat infiltration in muscle in healthy young women.
Vitamin D insufficiency is associated with increased fat infiltration in muscle, a relation that is independent of body mass or other measures of adiposity.
Vitamin D, a key regulator of bone metabolism, is also known to be significantly associated with muscle strength (1). A lack of vitamin D can cause myopathy (2,3), which tends to be more marked in the proximal muscles (4). In the elderly, vitamin D deficiency is linked to muscle weakness, increased body sway, and increased susceptibility to falls and fractures, which are improved by the administration of vitamin D with calcium (5,6,7,8,9,10,11,12,13). Vitamin D levels have also been shown to be significantly associated with muscle strength in healthy postmenarchal girls, suggesting that muscle contractility may be affected by vitamin D status (1). The mechanisms underlying the effect of vitamin D on muscle strength are not fully understood but could be related to an independent effect on muscle mass, or alternatively, to enhancement of muscle function mediated through the effect of vitamin D. Nonetheless, vitamin D action requires activation of the vitamin D receptor, which is widely distributed in various tissues including skeletal muscle (14,15).
Available data indicate that a higher muscle lipid content, measured as muscle attenuation with computed tomography (CT), is associated with lower levels of muscle strength and physical performance, independent of muscle mass (16,17). Additionally, several studies in patients with neuromuscular disorders have demonstrated skeletal muscle attenuation determined by CT to be strongly reciprocally associated with muscle lipid content (18,19,20).
In the current study, we propose that 25-hydroxyvitamin D (25OHD) concentrations are reciprocally related to adipose tissue infiltration in muscle independently of muscle mass. To test this hypothesis, we examined the relation between vitamin D and skeletal muscle lipid content and muscle mass using CT in a cohort of healthy young women living in California who were recently studied and found to have a reciprocal relation between vitamin D status and measures of adiposity (21).
Subjects and Methods
Study subjects
The sample of this study was the same 90 postpubertal healthy females who have been more thoroughly described in a previous investigation on the relation of vitamin D to body fat and bone (21). This study was approved by the institutional review board at our institution, and informed consent was obtained from all parents and/or subjects.
Candidates were excluded if they had a diagnosis of any underlying disease or chronic illness, had been ill for longer than wk during the 6 months before examination, had been admitted to the hospital at any time during the prior 3 yr, or were taking any medications including oral contraceptives. Candidates who were pregnant, had ever been pregnant, or had absence of menses for more than 4 consecutive months were also excluded from the study. To decrease the seasonal variability in biochemical determinations, all appointments were scheduled between May and October. In addition, all subjects had normal kidney function and normal liver function tests, and there was no evidence of liver abnormalities detected by CT. All participants had reached sexual maturity, defined as Tanner V of breast development (22), and skeletal maturity, defined as epiphyseal closure in the phalanges and metacarpals using the radiographic atlas of Greulich and Pyle (23).
Levels of physical activity in 83 participants were examined using a 7-d physical activity recall questionnaire. Participants were asked to indicate the number of times in the past week that they had engaged in strenuous, moderate, and mild forms of physical activity for more than 15 min and the daily time spent watching television and/or on the computer. This measure represents frequency, intensity, and duration elements of physical activity and inactivity with a test-retest reliability coefficient of 0.81 (24,25).
Muscle and fat measurements
CT measurements of muscle and sc fat were determined using a Hilite Advantage scanner (General Electric Healthcare, Milwaukee, WI) with a standardized reference phantom for simultaneous calibration. At the level of the umbilicus, measurements of the abdominal visceral fat (VF; square centimeters) and sc fat (SF; square centimeters) were obtained. For the purpose of this study, SF was defined as the amount of adipose tissue located between the skin and rectus muscles of the abdomen, the external oblique muscles, the broadest muscles of the back, and the erector muscles of the spine at the level of the umbilicus. VF was defined as the intraabdominal adipose tissue surrounded by the rectus muscles of the abdomen, the external oblique muscles, the lumbar quadrate muscle, the psoas muscles, and the lumbar spine at the same level. The coefficient of variation (CV) for repeated measures of VF and SF has been reported to range from 1.5 to 3.5% (26). Additionally, measures of sc thigh fat and thigh muscle area were obtained at the midshafts of the femurs, and values from both the right and left leg measurements were averaged for analysis; the CVs for repeated CT measurements of sc fat and muscle in the thigh were previously reported to fall between 1 and 2% (27). Measures of thigh muscle area included the vastus muscles, adductors, rectus femoris, sartorius, gracilis, and semitendinosis and semimembranosus muscles.
The tissue densities of muscle and fat were determined by converting CT values expressed as Hounsfield units into measures of tissue density using an external phantom (28). All muscle and fat measures were obtained from a 2-cm2 region of the rectus femoris and a 2-cm2 region of adjacent sc fat. Based on the tissue densities of fat and muscle, the percentage of adipose tissue in the muscle was calculated according to the formula: (M−x)/(M−y) × 100, where M represents the density of muscle without fat, x represents the density of the muscle, and y represents the density of fat. For the purpose of this study, the highest muscle density was used as the reference value representing muscle tissue with no fat infiltration. The CV for muscle attenuation from single-slice CT scans of the midthigh have previously been reported to be 0.51% for test-retest variability and 3.3% for within-subject variance (18). The inter- and intraclass correlation coefficients for measurements of adiposity in soft tissues using CT and a reference phantom are outstanding at greater than 0.98 (29). The time taken to complete the CT scans was approximately 10 min and the effective radiation dose was approximately 0.1 mSv (30).
Biochemical determinations
Serum levels of 25OHD were assayed using a RIA as described by Hollis et al. (31). The lower limit of detection was 5 ng/ml (12.5 nmol/liter). Goat anti-25OHD was a gift from Dr. Bruce Hollis, the Director of Pediatric Nutritional Sciences at the Medical University of South Carolina.125I-25-(OH)D3 and donkey antigoat secondary antibody were purchased from Diasorin (Stillwater, MN). This assay recognizes 25OHD2 and 25OHD3 equally and shows no bias when compared with HPLC (32). Calculated assay precision for within-assay variation averages of 6% and interassay of 16%. For the purpose of this study and according to the current consensus, subjects were divided into a 25OHD sufficient, or normal, group (≥30 ng/ml) and an insufficient group (≤29 ng/ml). Intact PTH (1–84) was measured with an electrochemiluminescent assay (33). The sensitivity of the assay is 1.2 pg/ml (0.127 pmol/liter) and intra- and interassay variations are 1.9–4 and 2.6–6.5%, respectively. To minimize interassay variability, all samples were analyzed simultaneously.
Statistical analysis
Statistical analysis was carried out using Statview (version 5.0.1; SAS Institute Inc., Cary, NC). Data were analyzed using simple linear regression analysis, multiple regression analysis, and unpaired t tests. All values are expressed as mean ± sd.
Results
Age, anthropometric characteristics, 25OHD, and CT measures of muscle and muscle fat, separated based on 25OHD concentrations, are described in Table 1. Thirty-seven women (41%) had 25OHD concentrations 30 ng/ml or greater, whereas 57 women (59%) had insufficient 25OHD concentrations (<29 ng/ml), of which 22 women (24%) were vitamin D deficient (≤20 ng/ml). Compared with women with normal 25OHD values, vitamin D-insufficient subjects were significantly shorter and heavier and had greater body mass index (BMI) and abdominal SF and VF.
Table 1.
25OHD values, age, anthropometric characteristics, and CT measures of muscle for 90 women separated by 25OHD concentration groups
| All (n = 90) | Sufficient (n = 37) | Insufficient (n = 53) | P values | |
|---|---|---|---|---|
| 25OHD (ng/ml) | 30.1 ± 13.0 (6.7–69.6) | 42.4 ± 10.1 (30.0–69.6) | 21.5 ± 5.9 (6.7–29.6) | <0.001 |
| Age (yr) | 19.4 ± 1.5 (16.3–22.8) | 19.2 ± 1.6 (16.3–22.8) | 19.5 ± 1.4 (17.0–22.87) | 0.408 |
| Weight (kg) | 68.3 ± 17.5 (45.5–126.0) | 63.9 ± 11.9 (45.6–113.0) | 71.3 ± 20.0 (45.5–126.0) | 0.046 |
| Height (cm) | 162.9 ± 4.7 (153.9–171.8) | 164.1 ± 3.9 (156.8–170.3) | 162.1 ± 5.1 (153.9–171.8) | 0.048 |
| BMI | 25.7 ± 6.3 (16.7–44.5) | 23.7 ± 4.6 (16.7–43.9) | 27.1 ± 7.1 (17.6–44.5) | 0.014 |
| SF (cm2) | 252.8 ± 152.7 (59.1–799.7) | 203.3 ± 98.9 (59.1–431.9) | 288.1 ± 174.0 (63.9–799.7) | 0.029 |
| VF (cm2) | 36.46 ± 42.89 (2.8–240.8) | 24.74 ± 33.88 (5.6–218.2) | 44.81 ± 46.83 (2.8–240.8) | 0.009 |
| Thigh musculature (cm2) | 87.49 ± 33.92 (33.5–177.4) | 92.98 ± 33.50 (36.5–151.9) | 83.66 ± 34.01 (33.5–177.4) | 0.202 |
| Subcutaneous thigh fat (cm2) | 118.9 ± 61.0 (14.4–324.0) | 104.8 ± 45.5 (26.6–219.5) | 128.7 ± 69.4 (14.4–324.0) | 0.067 |
| Muscle fat (%) | 3.59 ± 1.70 (0.0–9.5) | 3.15 ± 1.37 (0.4–5.8) | 3.90 ± 1.85 (0.0–9.5) | 0.038 |
| Inactivity (h/wk)a | 7.8 ± 5.1 (1.5–31.8) | 6.8 ± 10.4 (3.0–16.0) | 8.4 ± 36.5 (1.5–31.8) | 0.161 |
Measures of Inactivity were obtained in 83 subjects: 34 in the sufficient and 49 in the insufficient group.
CT measures of muscle attenuation were significantly lower in the 25OHD-insufficient group when compared with women with normal 25OHD values. The percentage of fat infiltration of the muscle derived from the attenuation values was also greater in women with lower 25OHD concentrations. In contrast, there were no differences in CT values for muscle area in the thighs between women in the two vitamin D groups. This was true whether the right or left thighs were analyzed independently (data not shown) or both extremities analyzed together.
Significant inverse correlations were observed between 25OHD and BMI, SF, VF, sc thigh fat, the degree of inactivity, and percent muscle fat; the strongest with percent muscle fat (Table 2 and Fig. 1). In contrast, neither age nor CT measures of muscle area were associated with 25OHD levels. Measures of muscle fat were unrelated to BMI, SF, VF, sc thigh fat, and measures of inactivity but inversely related to muscle area (Table 2).
Table 2.
Relations between 25OHD, anthropometric characteristics, and CT measures of muscle and fat in 90 women
| r | P | |
|---|---|---|
| 25OHD (ng/ml) | ||
| Age (yr) | −0.17 | 0.101 |
| BMI (kg/m2) | −0.35 | <0.001 |
| SF (cm2) | −0.36 | <0.001 |
| VF (cm2) | −0.28 | 0.007 |
| Thigh muscle area (cm2) | 0.16 | 0.138 |
| Subcutaneous thigh fat (cm2) | −0.32 | 0.002 |
| Inactivity (h/wk)a | −0.27 | 0.013 |
| Muscle fat (%) | ||
| Age (yr) | 0.28 | 0.007 |
| BMI (kg/m2) | −0.09 | 0.411 |
| SF (cm2) | −0.08 | 0.459 |
| VF (cm2) | −0.04 | 0.690 |
| Thigh muscle area (cm2) | −0.24 | 0.024 |
| Subcutaneous thigh fat (cm2) | −0.05 | 0.634 |
| Inactivity (h/wk)a | 0.17 | 0.122 |
Measures of inactivity were obtained in 83 subjects.
Figure 1.
Relation between vitamin D concentrations and CT measures of muscle fat infiltration.
Multiple regression analysis indicated that the relation between 25OHD levels and percent muscle fat was independent of body mass and measures of inactivity (Table 3). Similar results were found when measures of sc and visceral adiposity replaced BMI as an independent variable in this model (data not shown).
Table 3.
Multiple regression analysis with serum levels of vitamin D as the dependent variable in 83 women
| β | se | P value | R2 | |
|---|---|---|---|---|
| 25OHD (ng/ml) | ||||
| Muscle fat (%) | −2.94 | 0.72 | <0.001 | |
| BMI (kg/m2) | −0.65 | 0.20 | 0.001 | 0.29 |
| Inactivity (h/wk) | −0.29 | 0.25 | 0.254 | |
A significant inverse correlation was found between 25OHD and PTH (r = −0.27; P = 0.01), and PTH values were higher in the insufficient than in the sufficient group (2.28 ± 0.88 and 1.92 ± 0.90, respectively; P = 0.025).
Discussion
In this study, we used CT to investigate whether muscle mass and muscle adiposity in healthy young adult females are associated with vitamin D. Our data demonstrated that serum 25OHD was inversely related to the percent fat of skeletal muscle, a relation that was independent of body mass or CT measures of sc and visceral fat. Compared with women with normal 25OHD values, vitamin D-insufficient women had approximately 24% greater muscle fat infiltration than women with normal serum 25OHD concentration. In contrast, we found no relation between thigh muscle area and serum levels of vitamin D and no significant differences in the cross-sectional area of thigh muscles between women with and without vitamin D insufficiency.
It should be noted that none of the young women had symptoms of overt vitamin D insufficiency or complained of muscle weakness, yet we were able to show the association of 25OHD with fat accumulation in skeletal muscle. However, muscle adiposity is known to strongly influence muscle strength, and available data indicate that im fat accumulation increases with reduced physical activity and decreases with exercise (16,34,35). It has also been shown that exercise training in both lean and obese subjects significantly improved the ability to oxidize lipids in skeletal muscle (34,36). Although we did not measure muscle strength, a recent study by Ward et al. in a group of healthy postmenarchal adolescents (1) demonstrated serum 25OHD to be positively related to muscle power, force, velocity, and jump height. Because these associations, like those found in the current study, were independent of BMI, they cannot be ascribed to overall body adiposity.
In the current study, we found no relation between vitamin D levels and the cross-sectional area of the muscles in the thigh. However, vitamin D supplementation has been reported to enhance muscle strength without any apparent changes in muscle mass (11,37,38). Indeed, whereas muscle mass and strength are often associated, this association is relatively weak (39), and muscle strength can significantly improve without significant changes in muscle mass (40,41,42). Whereas measures of inactivity were positively related to weight and reciprocally related to vitamin D, neither CT measures of muscle area nor muscle adiposity was associated with time spent watching television and on the computer. Hence, it is possible that the muscle fat content in our subjects was a direct consequence of vitamin D insufficiency rather than the result of reduced vitamin D associated with increased adiposity due to inactivity.
Although the mechanism(s) underlying the vitamin D-muscle fat link is unknown, it is possible that that im adipose tissue accumulation could regulate muscle metabolism. Vitamin D has been shown to mediate protein synthesis and cellular ATP accumulation (43), increase troponin C (44), and increase actin and sarcoplasmic protein expression (45) in striated muscles; whether these and/or other cellular events are influenced by im fat requires further study. Regardless of the mechanism by which vitamin D influences skeletal muscle composition, im lipids play an important role in the function of myocytes. Skeletal muscle fat infiltration impairs skeletal muscle mitochondrial function (46) and decreases the rate of mitochondrial oxidative phosphorylation (47). Furthermore, muscle triglyceride content reduces insulin-stimulated glucose uptake within muscle (48,49). Other studies indicate that im adipose tissue deposition is associated with insulin resistance and diabetes (50,51,52) and accompanied by decreased glucose use, decreased signaling through the phosphatidylinositol 3-kinase/Akt pathway, and decreased insulin-stimulated glycogen synthesis (52,53). Additionally, low levels of circulating 25OHD have also been associated with glucose intolerance (54,55) and insulin resistance (56) in adults, suggesting a possible link between vitamin D status, muscle fat content, and glucose homeostasis.
The high prevalence of vitamin D insufficiency in this young population, living in a sun-rich area, even during spring and summer months when vitamin D synthesis is greatest, is surprising and likely multifactorial. Close to 60% of women studied had insufficient 25OHD concentrations (<29 ng/ml), and close to 25% were vitamin D deficient (≤20 ng/ml). These findings are, however, consistent with international data suggesting that even in the sunniest areas of the world, vitamin D deficiency is common. Studies in Turkey, Lebanon, Saudi Arabia, United Arab Emirates, India, and Australia show 30–50% of children and young adults to have 25OHD levels less than 20 ng/ml (57). Moreover, a recent study found that a substantial proportion (60%) of healthy adolescents living in a sunny Brazilian climate had vitamin D insufficiency (58).
There are limitations to our study, including its cross-sectional design and the inability to establish a causal relation between skeletal muscle adipose tissue infiltration and vitamin D. We used a single CT measure obtained at the midthigh as a surrogate of muscle volume to minimize radiation exposure and were able to measure only a relatively small depot of skeletal muscle adipose tissue. However, previous studies noted a relatively strong correlation between the skeletal muscle fat content at different sites (59). Moreover, our study subjects were not recruited from the community at large and were limited to young females. Future studies are needed to determine whether vitamin D influences fat infiltration in the muscle in males and the elderly. Nevertheless, serum 25OHD is related to overall physical fitness in postmenopausal women (60), and ample data indicate that inadequate vitamin D status is related to muscle weakness and the risk of falls in older persons, regardless of gender (35). Lastly, studies are needed to determine whether the increased muscular adiposity (17,31,49) and decreased muscle strength and mobility (57,58,59,60) with aging are associated with vitamin D status. This is particularly relevant because vitamin D insufficiency is highly prevalent in the elderly population (61,62).
In conclusion, our data show that vitamin D insufficiency is significantly and inversely associated with the degree of fat infiltration in skeletal muscle, a relation that is independent of body mass. This reciprocal association between vitamin D status and muscle fat was not previously reported and is unexplained and intriguing. Further studies are needed to characterize the possible mechanistic relationship between muscle fat and vitamin D.
Acknowledgments
The authors express their gratitude to Dr. Timothy Reinhardt for providing the measures of serum 25OHD.
Footnotes
This work was supported by National Institutes of Health Grant 1R01 AR052744-01, Department of the Army Grant DAMD17-01-1-0817, Canadian Institutes of Health Research Grant MT-10839, Natural Science and Engineering Research Council/Dairy Farmers of Canada, and Natural Sciences and Engineering Research Council and Dimensional Fund Advisors Canada.
Disclosure Summary: V.G., A.K., A.O.M., T.A.L.W., and R.K. have nothing to declare.
First Published Online February 17, 2010
Abbreviations: BMI, Body mass index; CT, computed tomography; CV, coefficient of variation; 25OHD, 25-hydroxyvitamin D; SF, sc fat; VF, visceral fat.
References
- Ward KA, Das G, Berry JL, Roberts SA, Rawer R, Adams JE, Mughal Z 2009 Vitamin D status and muscle function in post-menarchal adolescent girls. J Clin Endocrinol Metab 94:559–563 [DOI] [PubMed] [Google Scholar]
- Crocombe S, Mughal MZ, Berry JL 2004 Symptomatic vitamin D deficiency among non-Caucasian adolescents living in the United Kingdom. Arch Dis Child 89:197–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladhani S, Srinivasan L, Buchanan C, Allgrove J 2004 Presentation of vitamin D deficiency. Arch Dis Child 89:781–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Andersen H, Charles P, Eriksen EF 2000 Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int 66:419–424 [DOI] [PubMed] [Google Scholar]
- Bischoff HA, Stähelin HB, Dick W, Akos R, Knecht M, Salis C, Nebiker M, Theiler R, Pfeifer M, Begerow B, Lew RA, Conzelmann M 2003 Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res 18:343–351 [DOI] [PubMed] [Google Scholar]
- Bischoff HA, Stahelin HB, Urscheler N, Ehrsam R, Vonthein R, Perrig-Chiello P, Tyndall A, Theiler R 1999 Muscle strength in the elderly: its relation to vitamin D metabolites. Arch Phys Med Rehabil 80:54–58 [DOI] [PubMed] [Google Scholar]
- Boland R 1986 Role of vitamin D in skeletal muscle function. Endocr Rev 7:434–448 [DOI] [PubMed] [Google Scholar]
- Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ 1992 Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 327:1637–1642 [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE 1997 Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 337:670–676 [DOI] [PubMed] [Google Scholar]
- Mowé M, Haug E, Bøhmer T 1999 Low serum calcidiol concentration in older adults with reduced muscular function. J Am Geriatr Soc 47:220–226 [DOI] [PubMed] [Google Scholar]
- Sorensen OH, Lund B, Saltin B, Andersen RB, Hjorth L, Melsen F, Mosekilde L 1979 Myopathy in bone loss of ageing: improvement by treatment with 1 α-hydroxycholecalciferol and calcium. Clin Sci (Lond) 56:157–161 [DOI] [PubMed] [Google Scholar]
- Stein MS, Wark JD, Scherer SC, Walton SL, Chick P, Di Carlantonio M, Zajac JD, Flicker L 1999 Falls relate to vitamin D and parathyroid hormone in an Australian nursing home and hostel. J Am Geriatr Soc 47:1195–1201 [DOI] [PubMed] [Google Scholar]
- Al-Said YA, Al-Rached HS, Al-Qahtani HA, Jan MM 2009 Severe proximal myopathy with remarkable recovery after vitamin D treatment. Can J Neurol Sci 36:336–339 [DOI] [PubMed] [Google Scholar]
- Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stähelin HB, Dick W 2001 In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J 33:19–24 [DOI] [PubMed] [Google Scholar]
- Simpson RU, Thomas GA, Arnold AJ 1985 Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem 260:8882–8891 [PubMed] [Google Scholar]
- Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB 2007 Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr 85:377–384 [DOI] [PubMed] [Google Scholar]
- Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB 2005 Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 60:324–333 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R 2000 Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol 89:104–110 [DOI] [PubMed] [Google Scholar]
- Liu M, Chino N, Ishihara T 1993 Muscle damage progression in Duchenne muscular dystrophy evaluated by a new quantitative computed tomography method. Arch Phys Med Rehabil 74: 507–514 [DOI] [PubMed] [Google Scholar]
- Nordal HJ, Dietrichson P, Eldevik P, Grønseth K 1988 Fat infiltration, atrophy and hypertrophy of skeletal muscles demonstrated by X-ray computed tomography in neurological patients. Acta Neurol Scand 77:115–122 [DOI] [PubMed] [Google Scholar]
- Kremer R, Campbell PP, Reinhardt T, Gilsanz V 2009 Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab 94:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J 1978 Physical growth and development. In: Textbook of pediatrics. 2nd ed. Edinburgh, Scotland: Churchill Livingstone. [Google Scholar]
- Greulich WW, Pyle SI 1959 Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford, CA: Stanford University Press [Google Scholar]
- Morris FL, Naughton GA, Gibbs JL, Carlson JS, Wark JD 1997 Prospective ten-month exercise intervention in premenarcheal girls: positive effects on bone and lean mass. J Bone Miner Res 12:1453–1462 [DOI] [PubMed] [Google Scholar]
- Genant HK, Engelke K, Fuerst T, Glüer CC, Grampp S, Harris ST, Jergas M, Lang T, Lu Y, Majumdar S, Mathur A, Takada M 1996 Noninvasive assessment of bone mineral and structure: state of the art. J Bone Miner Res 11:707–730 [DOI] [PubMed] [Google Scholar]
- Arfai K, Pitukcheewanont PD, Goran MI, Tavare CJ, Heller L, Gilsanz V 2002 Bone, muscle, and fat: sex-related differences in prepubertal children. Radiology 224:338–344 [DOI] [PubMed] [Google Scholar]
- Gilsanz V, Wren TA, Dorey F, Judex S, Rubin C 2005 Brief periods of low level, high frequency mechanical stimulation enhance the axial and appendicular musculoskeletal system of young women. Submitted for publication [Google Scholar]
- Bushberg J, Seibert J, Leidholdt E, Boone J 1994 X-ray computed tomography. In: Passano W, ed. The essentials of medical imaging. Baltimore, MD: Williams, Wilkins; 239–289 [Google Scholar]
- Speliotes EK, Massaro JM, Hoffmann U, Foster MC, Sahani DV, Hirschhorn JN, O'Donnell CJ, Fox CS 2008 Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol 23:894–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalender WA 1992 Effective dose values in bone mineral measurements by photon absorptiometry and computed tomography. Osteoporos Int 2:82–87 [DOI] [PubMed] [Google Scholar]
- Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL 1993 Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem 39:529–533 [PubMed] [Google Scholar]
- Carter GD, Carter R, Jones J, Berry J 2004 How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem 50:2195–2197 [DOI] [PubMed] [Google Scholar]
- Seehofer D, Rayes N, Ulrich F, Müller C, Lang M, Neuhaus P, Steinmüller T 2001 Intraoperative measurement of intact parathyroid hormone in renal hyperparathyroidism by an inexpensive routine assay. Langenbecks Arch Surg 386:440–443 [DOI] [PubMed] [Google Scholar]
- Berggren JR, Boyle KE, Chapman WH, Houmard JA 2008 Skeletal muscle lipid oxidation and obesity: influence of weight loss and exercise. Am J Physiol Endocrinol Metab 294:E726–E732 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB 2001 Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol 90:2157–2165 [DOI] [PubMed] [Google Scholar]
- Durheim MT, Slentz CA, Bateman LA, Mabe SK, Kraus WE 2008 Relationships between exercise-induced reductions in thigh intermuscular adipose tissue, changes in lipoprotein particle size, and visceral adiposity. Am J Physiol Endocrinol Metab 295:E407–E412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaar HJ, Samson MM, Jansen PA, de Vreede PL, Manten JW, Duursma SA 2000 Muscle strength, functional mobility and vitamin D in older women. Aging (Milano) 12:455–460 [DOI] [PubMed] [Google Scholar]
- Gloth 3rd FM, Smith CE, Hollis BW, Tobin JD 1995 Functional improvement with vitamin D replenishment in a cohort of frail, vitamin D-deficient older people. J Am Geriatr Soc 43:1269–1271 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB 2006 The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition Study, for the Health ABC Study. J Gerontol A Biol Sci Med Sci 61:1059–1064 [DOI] [PubMed] [Google Scholar]
- Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ 1988 Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol 64:1038–1044 [DOI] [PubMed] [Google Scholar]
- Sipilä S, Suominen H 1995 Effects of strength and endurance training on thigh and leg muscle mass and composition in elderly women. J Appl Physiol 78:334–340 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, Kritchevsky SB, Pahor M, Newman AB 2008 Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol 105:1498–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge SJ, Haddad JG 1975 25-Hydroxycholecalciferol stimulation of muscle metabolism. J Clin Invest 56:1100–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointon JJ, Francis MJO, Smith R 1979 Effect of vitamin D deficiency on sarcoplasmic reticulum function and troponin C concentration of rabbit skeletal muscle. Clin Sci 57:257–263 [DOI] [PubMed] [Google Scholar]
- de Boland AR, Albornoz LE, Boland R 1983 The effect of cholecalciferol in vivo on proteins and lipids of skeletal muscle from rachi. Calcif Tissue Int 35:798–805 [DOI] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Shulman GI 2006 Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55(Suppl 2):S9–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE 1999 Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J 13:2051–2060 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE 1997 Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 46:1579–1585 [DOI] [PubMed] [Google Scholar]
- Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins ABJ, Storlien LH 1997 Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46:983–988 [DOI] [PubMed] [Google Scholar]
- Albu JB, Kovera AJ, Allen L, Wainwright M, Berk E, Raja-Khan N, Janumala I, Burkey B, Heshka S, Gallagher D 2005 Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr 82:1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljkovic-Gacic I, Gordon CL, Goodpaster BH, Bunker CH, Patrick AL, Kuller LH, Wheeler VW, Evans RW, Zmuda JM 2008 Adipose tissue infiltration in skeletal muscle: age patterns and association with diabetes among men of African ancestry. Am J Clin Nutr 87:1590–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulver MW, Dohm GL 2004 The molecular mechanism linking muscle fat accumulation to insulin resistance. Proc Nutr Soc 63:375–380 [DOI] [PubMed] [Google Scholar]
- Shulman, GI 2004 Unraveling the cellular mechanism of insulin resistance in humans: new insights from magnetic resonance spectroscopy. Physiology (Bethesda) 19:183–190 [DOI] [PubMed] [Google Scholar]
- Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E 1995 Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract 27:181–188 [DOI] [PubMed] [Google Scholar]
- Hyppönen E, Power C 2006 Vitamin D status and glucose homeostasis in the 1958 British birth cohort: the role of obesity. Diabetes Care 29:2244–2246 [DOI] [PubMed] [Google Scholar]
- Chiu KC, Chu A, Go VL, Saad MF 2004 Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am J Clin Nutr 79:820–825 [DOI] [PubMed] [Google Scholar]
- Holick MF 2007 Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- Peters BS, dos Santos LC, Fisberg M, Wood RJ, Martini LA 2009 Prevalence of vitamin D insufficiency in Brazilian adolescents. Ann Nutr Metab 54:15–21 [DOI] [PubMed] [Google Scholar]
- Larson-Meyer DE, Smith SR, Heilbronn LK, Kelley DE, Ravussin E, Newcomer BR 2006 Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring) 14:73–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JW, Alekel DL, Ritland LM, Van Loan M, Gertz E, Genschel U 2009 Serum 25-hydroxyvitamin D is related to indicators of overall physical fitness in healthy postmenopausal women. Menopause 16:1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloth 3rd FM, Gundberg CM, Hollis BW, Haddad JG Jr, Tobin JD 1995 Vitamin D deficiency in homebound elderly persons. JAMA 274:1683–1686 [DOI] [PubMed] [Google Scholar]
- Goldray D, Mizrahi-Sasson E, Merdler C, Edelstein-Singer M, Algoetti A, Eisenberg Z, Jaccard N, Weisman Y 1989 Vitamin D deficiency in elderly patients in a general hospital. J Am Geriatr Soc 37:589–592 [DOI] [PubMed] [Google Scholar]