Abstract
Context: Whether a child with low bone mineral density (BMD) at one point in time will continue to have low BMD, despite continued growth and maturation, is important clinically. The stability of a characteristic during growth is referred to as “tracking.”
Objective: We examined the degree of tracking in bone mineral content (BMC) and BMD during childhood and adolescence and investigated whether tracking varied according to age, sexual maturation, and changes in growth status.
Design: We conducted a longitudinal study with measurements at baseline and annually for 3 yr.
Setting: The Bone Mineral Density in Childhood Study was conducted at five clinical centers in the United States.
Study Participants: A total of 1554 girls and boys, ages 6–16 yr at baseline, participated in the study.
Main Outcome Measures: Whole body, spine, hip, and forearm BMC and BMD were measured by dual-energy x-ray absorptiometry, and age-, sex-, and race-specific Z-scores were calculated. Deviation from tracking was calculated as the Z-score at yr 3 minus baseline.
Results: Correlations between Z-scores at baseline and yr 3 ranged from 0.76–0.88. Among children with a Z-score below −1.5 at baseline, 72–87% still had a Z-score below −1 after 3 yr. Age, sexual maturation, and deviations in growth status (P < 0.01) were associated with deviation from tracking; however, tracking was strongly evident even after adjusting for the effects of age, maturation, and growth.
Conclusions: Bone density showed a high degree of tracking over 3 yr in children and adolescents. Healthy children with low bone density will likely continue to have low bone density unless effective interventions are instituted.
The strong degree of tracking found in this study provides support for use of bone mineral status measurements in growing children and adolescents.
Osteoporosis is a major public health problem. Approximately 10 million people in the United States have osteoporosis, and another 34 million have low bone mass (1). Many have speculated that the origins of osteoporosis begin in childhood (2) because bone mass accrued during childhood and adolescence determines peak bone mass in young adulthood. In turn, variation in peak bone mass is thought to predict osteoporotic fracture risk later in life (3,4). Others have challenged this notion, citing that compensations in bone mass occur in response to recent conditions and physical challenges (5), and that bone formed early in life is completely replaced during growth due to skeletal modeling and remodeling (6). About 40% of adult total body bone mineral content (BMC) is acquired during the 2 yr around peak height velocity in adolescence (7). Thus, it remains unclear whether bone mass accrued during childhood has any effect on BMC and risk of osteoporosis and fractures in adulthood.
Bone mineral status, defined as a child’s BMC or bone mineral density (BMD) relative to age- and sex-specific norms, is used as the indicator of bone mineral accrual to account for the increases in bone mass and density throughout childhood and adolescence. The continuity or stability of bone mineral status throughout childhood and adolescence is referred to as “tracking” (8). Establishing whether and to what degree bone mineral status tracks is important because tracking forms the cornerstone for screening and targeting of interventions to improve bone mineral status. Because childhood and adolescence are such dynamic periods of bone accrual, it is unclear how well BMC and BMD track during these periods of growth and maturation. A better understanding of tracking may provide greater opportunities for early screening and targeting of interventions to prevent osteoporosis later in life.
There is some evidence that BMC and BMD track during growth and maturation. BMC and BMD of the total body, hip, and spine measured in pre- and early puberty are correlated (r = 0.54–0.81) with measurements obtained 7 to 8 yr later (9,10,11). Most studies to date, however, have been constrained by small sample sizes (e.g. ≤25 subjects/group) (12,13,14), and/or measurement of BMD by techniques not widely used in clinical practice (13,15). Although mean values of BMC and BMD have been compared over time, it is important to examine the persistence of values that are considered to be “low” because these are of clinical concern. A large sample size is needed to adequately examine the tails of a distribution (i.e. the lowest or highest values). Furthermore, the potential variations in tracking of bone mineral status with age, stage of sexual maturation, and accelerated or delayed linear growth and weight gain have received little attention. A better understanding of factors that affect the degree of tracking will help elucidate the clinical utility of bone mineral status measures.
The objectives of this study were to estimate the degree of tracking in bone mineral status over a 3-yr period during childhood and adolescence and to investigate whether tracking varied according to age, sexual maturity, and changes in height and weight status. We assessed the degree of tracking for a variety of skeletal sites to help inform selection of bone measurements that should be monitored clinically.
Subjects and Methods
Subjects
The Bone Mineral Density in Childhood Study (BMDCS) is a multicenter, longitudinal study of bone accrual in 1554 healthy children and adolescents. Detailed information about the study participants, inclusion/exclusion criteria, and study procedures have been published previously (16). In brief, 1554 healthy subjects were enrolled: girls ages 6 to 15 yr, and boys ages 6 to 16 yr. All measurements were obtained at baseline and annually thereafter. Presented here are data from the baseline and three annual follow-up visits.
Measurements
Bone mass and density were measured by dual-energy x-ray absorptiometry (DXA) scans of the spine, hip, forearm, and whole body (Hologic QDR 4500/Delphi/Discovery; Hologic, Inc., Bedford, MA). There were small differences in calibration of densitometers among centers as reported previously (16), and we adjusted all DXA measures to remove center differences. Z-scores were calculated for each bone measurement using sex- and race-specific LMS curves. LMS curves were based on 4 yr of data (baseline, yr 1 to 3) from the BMDCS cohort and were generated using the LMS program version 1.16 (17). Worm plots were used to assess goodness of fit (18).
Weight was measured on a digital scale, and height was measured using a stadiometer. Z-scores for height, weight, and body mass index (BMI) were calculated using the CDC 2000 growth charts.
Ethnicity (Hispanic/Latino vs. non-Hispanic/Latino) and race were elicited by questionnaire using National Institutes of Health definitions. Participants were categorized as black or non-black for data analysis purposes.
Sexual maturation was determined by physical examination performed by a physician or nurse practitioner with established expertise in pediatric endocrinology. The stage of breast development (girls) and testicular volume by orchidometer (boys) were evaluated based upon the criteria of Tanner (19).
Statistical analyses
Tracking was assessed by calculating the Pearson correlation coefficients between Z-scores at baseline with those in subsequent years. Correlation coefficients were calculated separately according to sex and race. We examined the persistence of low and high bone mineral status by calculating the proportion of subjects who had “LOW” (Z-score <−1.5) or “HIGH” (Z-score >1.5) values at baseline who continued to have LOW or HIGH values after 1, 2, and 3 yr of follow-up. We used a Z-score cutoff of −1.5 to define LOW, which differs from that of the International Society of Clinical Densitometry of −2, so that we had larger sample sizes at the extremes, giving more stable estimates. The results were similar using a Z-score cutoff of −2 (data not shown).
Sometimes extremely low or high values are due to measurement error or variability within the individual. In these situations, when the measurement is repeated, the values are often closer to the sample mean (20). This phenomenon is referred to as regression toward the mean. Assessing the magnitude of regression toward the mean aids interpretation of extreme results and, thereby, the degree of tracking. To quantify regression toward the mean, we categorized children according to their bone Z-scores at baseline and calculated the mean Z-score by baseline category for each study year.
We investigated whether the degree of tracking varied according to age, maturation, and change in growth status, defined as the change in height- and weight-for-age Z-scores. First, we examined the correlations between Z-scores at baseline and yr 3 for each bone measure according to age and Tanner stage at baseline. We then calculated deviation from tracking, which was the difference between the Z-score at yr 3 and the Z-score at baseline, and examined the correlation between deviation from tracking and changes in height and weight Z-scores over the 3-yr period. Multiple regression analyses were conducted to determine which of these factors independently predicted deviation from tracking. Baseline bone Z-scores were included in the regression models to account for regression toward the mean. Regression analyses were conducted separately by sex so that we could better account for differences in ages of sexual maturation and peak growth velocity between boys and girls.
Because height and weight are important determinants of BMD and are known to track over time, it is useful to know whether bone mineral status tracks even when accounting for the effects of height and weight. We used multiple regression to predict bone Z-scores at yr 3 as a function of baseline bone Z-scores and sequentially fitted height and weight Z-scores at baseline and change in Z-scores over the 3-yr period.
Results
Of 1554 children enrolled in the study, 1477 returned for their follow-up visit in yr 1, 1443 in yr 2, and 1401 in yr 3. The analysis subset was restricted for follow-up visits within ±2.0 months of the anniversary date of the baseline visit, resulting in 1459 subjects in yr 1, 1400 subjects in yr 2, and 1370 subjects in yr 3. The proportion of subjects with a missed or out of window visit for yr 3 was greater for blacks [16.2% (60 of 371)] than non-blacks [7.9% (93 of 1183)]. Non-blacks without a yr 3 visit were slightly younger (−0.6 yr; P = 0.08) and had a slightly higher lumbar spine bone density Z-score at baseline (+0.26; P = 0.03) compared with those with a yr 3 visit. Blacks without a yr 3 visit tended to be older (+0.5 yr; P = 0.20) with a slightly lower whole body BMC Z-score (−0.25; P = 0.10) and lower weight Z-score (−0.28; P = 0.02) at baseline compared with those with a yr 3 visit.
The correlation coefficients between Z-scores for BMC and BMD and growth measures determined at baseline with values obtained in subsequent years were high overall and decreased only slightly each year (Table 1). Similar trends were seen for boys and girls, and blacks and non-blacks. Results for lumbar spine, total hip, femoral neck, and 1/3 radius BMC were similar to those for BMD and thus not shown. The correlations for bone Z-scores were as good as those of height, weight, and BMI Z-scores; most correlation coefficients differed by less than 5%.
Table 1.
Correlation coefficients between Z-scores at baseline and Z-scores at yr 1–3 by sex and race
| Variables | White
|
Black
|
||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 1 | Year 2 | Year 3 | |
| Whole body BMC | ||||||
| Boys | 0.95 (545) | 0.88 (513) | 0.80 (490) | 0.96 (163) | 0.91 (143) | 0.82 (139) |
| Girls | 0.96 (566) | 0.90 (532) | 0.84 (519) | 0.95 (162) | 0.89 (145) | 0.84 (151) |
| Lumbar spine BMD | ||||||
| Boys | 0.94 (549) | 0.87 (534) | 0.80 (520) | 0.94 (165) | 0.87 (150) | 0.80 (150) |
| Girls | 0.94 (569) | 0.89 (551) | 0.84 (540) | 0.95 (171) | 0.88 (160) | 0.85 (158) |
| Total hip BMD | ||||||
| Boys | 0.94 (549) | 0.89 (531) | 0.84 (514) | 0.95 (164) | 0.88 (145) | 0.83 (141) |
| Girls | 0.94 (568) | 0.88 (550) | 0.84 (540) | 0.95 (171) | 0.89 (160) | 0.87 (159) |
| Femoral neck BMD | ||||||
| Boys | 0.94 (549) | 0.89 (531) | 0.83 (514) | 0.95 (164) | 0.90 (145) | 0.85 (141) |
| Girls | 0.92 (568) | 0.86 (550) | 0.83 (540) | 0.95 (171) | 0.90 (160) | 0.88 (159) |
| 1/3 radius BMD | ||||||
| Boys | 0.87 (531) | 0.81 (513) | 0.76 (510) | 0.90 (161) | 0.83 (145) | 0.81 (141) |
| Girls | 0.89 (551) | 0.85 (528) | 0.82 (524) | 0.89 (170) | 0.85 (159) | 0.84 (159) |
| Height | ||||||
| Boys | 0.95 (551) | 0.88 (534) | 0.82 (520) | 0.96 (165) | 0.89 (150) | 0.84 (145) |
| Girls | 0.96 (572) | 0.91 (554) | 0.86 (544) | 0.93 (171) | 0.85 (162) | 0.78 (161) |
| Weight | ||||||
| Boys | 0.93 (551) | 0.85 (534) | 0.76 (520) | 0.94 (165) | 0.88 (150) | 0.81 (145) |
| Girls | 0.94 (572) | 0.88 (554) | 0.81 (544) | 0.94 (171) | 0.87 (162) | 0.80 (161) |
| BMI | ||||||
| Boys | 0.93 (551) | 0.85 (534) | 0.78 (520) | 0.92 (165) | 0.88 (150) | 0.82 (145) |
| Girls | 0.92 (572) | 0.86 (554) | 0.80 (544) | 0.92 (171) | 0.85 (162) | 0.80 (161) |
Data are expressed as correlation coefficients (number of subjects). All correlation coefficients are statistically significant (P value <0.0001). Results (not shown) were similar for BMC.
We examined the persistence of values at the tails of the distribution by categorizing children according to their baseline Z-score as LOW (<−1.5) and HIGH (>1.5) (Table 2), each constituting approximately 7% of the total sample. Among those categorized as LOW, 65–77% still had a LOW bone Z-score after 1 yr, and this decreased to 44–62% after 3 yr. Using a more lenient criteria of a Z-score of less than −1 at follow-up, 72–87% of children remained LOW after 3 yr. In contrast, only 3–4% of those who had a Z-score of more than −1.5 at baseline had a Z-score of less than −1.5 after 3 yr. Among children classified as HIGH at baseline, 57–77% still had a HIGH Z-score after 1 yr, and this decreased to 50–62% after 3 yr. Using a criterion of a Z-score greater than 1 at follow-up, 81–85% of children remained HIGH after 3 yr. Only 2–5% of those who had a Z-score of less than 1.5 at baseline had a Z-score of more than 1.5 after 3 yr. Overall, there was no difference in the persistence of LOW or HIGH Z-scores between boys and girls across skeletal sites. Race-specific analyses were not performed because there were too few blacks to allow reliable analyses. Of note, the persistence of LOW and HIGH bone Z-scores was of similar magnitude to the persistence of height, weight, and BMI Z-scores.
Table 2.
Persistence of LOW (below −1.5) and HIGH (>1.5) Z-scores at baseline over 3 yr
| Z-score at follow-up | LOW Z-score
|
HIGH Z-score
|
||||
|---|---|---|---|---|---|---|
| Year 1 (NY1Y0/NY0) | Year 2 (NY2Y0/NY0) | Year 3 (NY3Y0/NY0) | Year 1 (NY1Y0/NY0) | Year 2 (NY2Y0/NY0) | Year 3 (NY3Y0/NY0) | |
| Whole body BMC | ||||||
| Below −1.5 or >1.5 | 77 (74/96) | 57 (52/91) | 52 (45/87) | 77 (71/92) | 72 (64/89) | 61 (50/82) |
| Below −1 or >1 | 97 (93/96) | 88 (80/91) | 77 (67/87) | 100 (92/92) | 94 (84/89) | 89 (73/82) |
| Lumbar spine BMD | ||||||
| Below −1.5 or >1.5 | 77 (72/94) | 74 (67/91) | 60 (52/87) | 77 (80/104) | 64 (64/100) | 62 (59/95) |
| Below −1 or >1 | 98 (92/94) | 91 (83/91) | 87 (76/87) | 98 (102/104) | 73 (73/100) | 81 (77/95) |
| Total hip BMD | ||||||
| Below −1.5 or >1.5 | 75 (62/83) | 68 (54/80) | 62 (48/78) | 70 (79/113) | 63 (66/105) | 50 (52/105) |
| Below −1 or >1 | 98 (81/83) | 90 (72/80) | 86 (67/78) | 96 (108/113) | 90 (95/105) | 84 (88/105) |
| Femoral neck BMD | ||||||
| Below −1.5 or >1.5 | 71 (63/89) | 64 (54/84) | 57 (48/84) | 74 (78/105) | 61 (61/100) | 56 (54/96) |
| Below −1 or >1 | 94 (84/89) | 92 (77/84) | 82 (69/84) | 97 (102/105) | 92 (92/100) | 88 (85/96) |
| 1/3 radius BMD | ||||||
| Below −1.5 or >1.5 | 65 (67/103) | 48 (49/102) | 44 (46/104) | 59 (50/85) | 62 (50/81) | 51 (37/73) |
| Below −1 or >1 | 87 (90/103) | 77 (79/102) | 72 (75/104) | 89 (76/85) | 83 (67/81) | 85 (62/73) |
| Height | ||||||
| Below −1.5 or >1.5 | 65 (22/34) | 53 (16/30) | 44 (14/32) | 79 (55/70) | 72 (48/67) | 64 (43/67) |
| Below −1 or >1 | 94 (32/34) | 87 (26/30) | 81 (25/31) | 98 (69/70) | 88 (59/67) | 90 (60/67) |
| Weight | ||||||
| Below −1.5 or >1.5 | 50 (10/20) | 45 (9/20) | 40 (8/20) | 70 (64/92) | 63 (55/87) | 59 (52/88) |
| Below −1 or >1 | 85 (17/20) | 65 (13/20) | 60 (12/20) | 98 (90/92) | 92 (80/87) | 89 (78/88) |
| BMI | ||||||
| Below −1.5 or >1.5 | 46 (11/24) | 29 (7/24) | 39 (9/23) | 72 (84/116) | 58 (65/112) | 61 (66/111) |
| Below −1 or >1 | 100 (24/24) | 58 (14/24) | 52 (12/23) | 96 (112/116) | 95 (106/112) | 91 (101/111) |
Data are expressed as percentage (number of subjects/total number of subjects). NY1Y0, Number of subjects that had a LOW (or HIGH) Z-score at base line and at yr 1; NY2Y0, number of subjects that had a LOW (or HIGH) Z-score at base line and at yr 2; NY3Y0, number of subjects that had a LOW (or HIGH) Z-score at base line and at yr 3; NY0, number of subjects that had a LOW (or HIGH) Z-score at baseline. The sample size for N0 varies because some subjects did not have visits in all follow-up years. All races and sexes are combined.
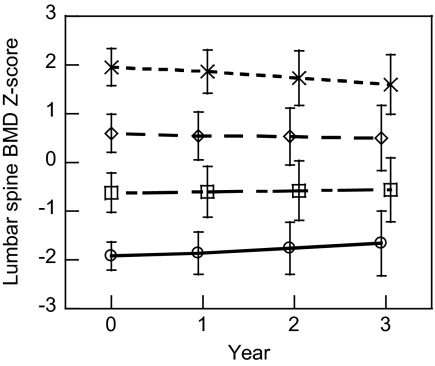
To investigate the degree of regression toward the mean, we categorized subjects according to their baseline Z-score for lumbar spine BMD and calculated the mean Z-score for each category over time (Fig. 1). Those classified in the highest category at baseline experienced a small decrease in mean Z-score over time (P < 0.001), but mean values still remained higher than those in the category below (P < 0.001). Similarly, those classified in the lowest category at baseline experienced a mean increase in Z-score over time (P < 0.001). The yearly change in mean Z-score was fairly steady over time. Those in middle categories displayed little or no change in mean Z-score over time. Results were similar for all other skeletal sites (results not shown).
Figure 1.
Mean (±sd) lumbar spine BMD Z-score over time. Subjects were grouped according to their lumbar spine BMD Z-score at baseline: above 1.5 (n = 108), 0 to 1.5 (n = 702), −1.5 to 0 (n = 628), and below −1.5 (n = 96). Group means significantly differ at all times points (P < 0.05).
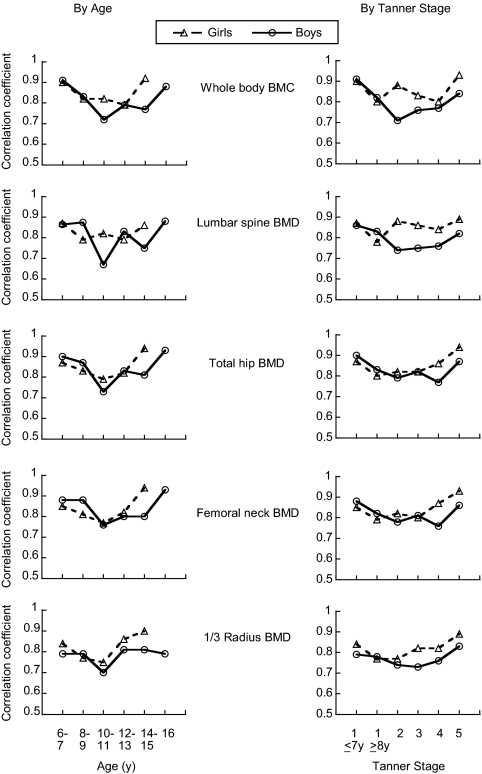
The degree of tracking over the 3-yr follow-up period varied according to age at baseline: correlation coefficients for Z-scores at baseline and yr 3 were highest for the oldest and youngest children (6–7 and 14–16 yr) compared with children with intermediate ages (Fig. 2). The pattern by baseline age differed slightly between boys and girls, with boys generally showing an increase in correlation coefficients at older ages than girls. Likewise, the degree of tracking was less for children who had begun puberty compared with those who were either prepubertal (Tanner stage 1) or sexually mature (Tanner stage 5) at the beginning of the 3-yr interval (Fig. 2). Individually, baseline age explained 2–4% of the variation in deviation from tracking, and baseline Tanner stage explained 2–9% of the variation in deviation from tracking across skeletal sites.
Figure 2.
Correlation coefficients for Z-scores at baseline and yr 3 according to age, sexual maturation and skeletal site. The pattern in correlation coefficients according to age is shown on the left, and the pattern in correlation coefficients according to sexual maturation is shown on the right.
Change in height status (height-for-age Z-score) was more strongly correlated with deviation from tracking than was change in weight status (weight-for-age Z-score) for whole body BMC and lumbar spine BMD (r = 0.61 and 0.46 vs. 0.48 and 0.37), but they performed similarly for the total hip (both r = 0.41), femoral neck (r = 0.31 and 0.38), and 1/3 radius BMD (r = 0.31 and 0.32). Change in BMI Z-score was weakly correlated with deviation from tracking (r = 0.23–0.29).
Because age, sexual maturation, and changes in height and weight Z-scores are correlated with each other, we conducted multiple regression analyses to determine whether they were independently associated with deviation from tracking. Age and Tanner stage at baseline and change in height and weight Z-scores over the 3-yr follow-up period were significant predictors of deviation from tracking for all skeletal sites (Table 3). Changes in height Z-score had a stronger association (i.e. larger β-coefficient) than change in weight Z-score for predicting deviation in tracking for whole body and lumbar spine, whereas there were less distinct trends for predicting deviation in tracking for the hip and the 1/3 radius. Race was associated (P < 0.05) with deviation from tracking, which likely reflects the different characteristics of black and non-black participants who did not have a yr 3 visit.
Table 3.
Multiple regression models predicting deviation from tracking (Z-score yr 3 minus Z-score yr 0)
| Total body BMC | Lumbar spine BMD | Total hip BMD | Femoral neck BMD | 1/3 radius BMD | |
|---|---|---|---|---|---|
| Boys | |||||
| Intercept | 0.12 (0.04, 0.20) | 0.12 (−0.09, 0.10) | −0.02 (−0.11, 0.06) | 0.03 (−0.06, 0.12) | 0.11 (0.01, 0.22) |
| Z-score Y0 | −0.06 (−0.10, −0.02)a | −0.09 (−0.14, −0.04) | −0.09 (−0.13, −0.05) | −0.12 (−0.16, −0.08) | −0.18 (−0.23, −0.13) |
| Black | −0.11 (−0.15, −0.07) | −0.12 (−0.16, −0.07) | −0.12 (−0.17, −0.08) | −0.10 (−0.13, −0.06) | −0.06 (−0.11, −0.00) |
| Age Y0 | |||||
| 6–7 | 0 | 0 | 0 | 0 | 0 |
| 8–9 | −0.03 (−0.12, 0.06) | 0.00 (−0.16, 0.16) | −0.10 (−0.20, 0.00) | 0.02 (−0.08, 0.13) | −0.05 (−0.16, 0.07) |
| 10–11 | −0.20 (−0.31, −0.09) | −0.10 (−0.22, 0.03) | −0.16 (−0.28, −0.04) | −0.14 (−0.26, −0.01) | −0.20 (−0.34, −0.06) |
| 12–13 | −0.43 (−0.59, −0.26) | −0.46 (−0.57, −0.35) | −0.36 (−0.54, −0.18) | −0.42 (−0.60, −0.23) | −0.63 (−0.84, −0.42) |
| 14–15 | −0.61 (−0.82, −0.41) | −0.58 (−0.72, −0.45) | −0.43 (−0.65, −0.21) | −0.48 (−0.70, −0.25) | −0.81 (−1.07, −0.55) |
| 16 | −0.97 (−1.23, −0.71) | −0.79 (−1.02, −0.56) | −0.61 (−0.89, −0.33) | −0.69 (−0.98, −0.40) | −1.14 (−1.47, −0.80) |
| Tanner stage Y0 | |||||
| 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0.19 (0.07, 0.30) | 0.21 (0.08, 0.33) | 0.15 (−0.02, 0.32) | 0.14 (−0.04, 0.32) | 0.16 (−0.04, 0.36) |
| 3 | 0.56 (0.42, 0.70) | 0.61 (0.36, 0.86) | 0.47 (0.25, 0.69) | 0.39 (0.16, 0.62) | 0.72 (0.46, 0.98) |
| 4 | 0.70 (0.58, 0.83) | 0.69 (0.55, 0.85) | 0.63 (0.41,0.86) | 0.63 (0.39, 0.86) | 0.82 (0.56, 1.09) |
| 5 | 0.63 (0.50, 0.77) | 0.66 (0.51, 0.82) | 0.60 (0.35, 0.85) | 0.57 (0.31, 0.83) | 0.76 (0.47, 1.06) |
| Δ Height Z-score | 0.76 (0.62, 0.88) | 0.50 (0.39, 0.61) | 0.41 (0.30, 0.51) | 0.22 (0.12, 0.33) | 0.25 (0.13, 0.37) |
| Δ Weight Z-score | 0.20 (0.12, 0.27) | 0.13 (0.40, 0.22) | 0.22 (0.14, 0.30) | 0.26 (0.17, 0.35) | 0.27 (0.17, 0.37) |
| Model R2 | 0.54 | 0.32 | 0.31 | 0.24 | 0.29 |
| Girls | |||||
| Intercept | 0.00 (−0.6, 0.7) | −0.02 (−0.09, 0.05) | −0.11 (−0.18, −0.04) | −0.09 (−0.17, −0.01) | 0.16 (0.06, 0.25) |
| Z-score Y0 | −0.05 (−0.08, −0.02) | −0.06 (−0.10, −0.03) | −0.07 (−0.10, −0.04) | −0.10 (−0.14, −0.07) | −0.14 (−0.19, −0.10) |
| Black | −0.08 (−0.11, −0.4) | −0.09 (−0.13, −0.05) | −0.09 (−0.13, −0.05) | −0.02 (−0.07, 0.02) | −0.06 (−0.11, −0.01) |
| Age Y0 | |||||
| 6–7 | 0 | 0 | 0 | 0 | 0 |
| 8–9 | −0.22 (−0.30, −0.14) | −0.23 (−0.35, −0.11) | −0.20 (−0.29, −0.11) | −0.16 (−0.26, −0.06) | −0.16 (−0.28, −0.05) |
| 10–11 | −0.18 (−0.29, −0.06) | −0.32 (−0.41, −0.24) | −0.28 (−0.41, −0.16) | −0.36 (−0.50, −0.23) | −0.44 (−0.59, −0.28) |
| 12–13 | −0.26 (−0.42, −0.10) | −0.44 (−0.53, −0.35) | −0.18 (−0.35, −0.01) | −0.23 (−0.42, −0.05) | −0.46 (−0.68, −0.25) |
| 14–15 | −0.47 (−0.65, −0.28) | −0.46 (−0.58, −0.33) | −0.20 (−0.40, 0.01) | −0.31 (−0.53, −0.09) | −0.47 (−0.72, −0.22) |
| Tanner stage Y0 | |||||
| 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0.40 (0.26, 0.54) | 0.49 (0.36, 0.63) | 0.42 (0.27, 0.58) | 0.45 (0.28, 0.61) | 0.36 (0.17, 0.55) |
| 3 | 0.55 (0.40, 0.70) | 0.61 (0.49, 0.78) | 0.47 (0.31, 0.63) | 0.47 (0.30, 0.65) | 0.49 (0.21,0.70) |
| 4 | 0.49 (0.32, 0.66) | 0.48 (0.36, 0.61) | 0.39 (0.21, 0.58) | 0.45 (0.25, 0.65) | 0.35 (0.12, 0.58) |
| 5 | 0.44 (0.25, 0.62) | 0.51 (0.38, 0.63) | 0.32 (0.12, 0.52) | 0.44 (0.22, 0.66) | 0.32 (0.07, 0.57) |
| ΔHeight Z-score | 0.63 (0.55, 0.72) | 0.47 (0.38, 0.57) | 0.34 (0.25, 0.43) | 0.18 (0.09, 0.28) | 0.20 (0.09, 0.32) |
| ΔWeight Z-score | 0.29 (0.22, 0.36) | 0.29 (0.21, 0.37) | 0.40 (0.32, 0.47) | 0.45 (0.37, 0.54) | 0.23 (0.14, 0.33) |
| Model R2 | 0.52 | 0.39 | 0.39 | 0.32 | 0.22 |
β -Coefficient (95% confidence interval).
Baseline bone measurements were still highly predictive of bone measurements 3 yr later, even after adjusting for the effects of height and weight (Table 4). In regression models predicting bone Z-scores at yr 3, bone Z-scores at baseline remained statistically significant (all P < 0.0001) for all skeletal sites when including height and weight Z-scores at baseline and change in Z-scores over the 3-yr period. With the exception of whole body BMC, changes in the magnitude of the regression coefficients for baseline bone Z-scores and the overall variance explained (model R2) were small (<8%).
Table 4.
Regression coefficients (β) for baseline (Y0) bone Z-scores predicting bone Z-scores at yr 3 (Y3) with and without adjusting for height and weight Z-scores
| Model | Males
|
Females
|
||
|---|---|---|---|---|
| β-Coefficient (se)a for BMD ZY0 | Model R2 | β-Coefficient (se)a for BMD ZY0 | Model R2 | |
| Whole body BMC Z-score | ||||
| Race, age, Tanner stage | 0.831 (0.023) | 0.71 | 0.849 (0.020) | 0.76 |
| + Ht ZY0, Wt ZY0 | 0.765 (0.033) | 0.71 | 0.820 (0.029) | 0.76 |
| + Ht ZY0, Wt ZY0, ΔHt Z, ΔWt Z | 0.835 (0.026) | 0.83 | 0.851 (0.022) | 0.86 |
| Lumbar spine BMD Z-score | ||||
| Race, age, Tanner stage | 0.827 (0.023) | 0.68 | 0.842 (0.020) | 0.75 |
| + Ht ZY0, Wt ZY0 | 0.808 (0.025) | 0.68 | 0.838 (0.022) | 0.75 |
| + Ht ZY0, Wt ZY0, ΔHt Z, ΔWt Z | 0.842 (0.024) | 0.73 | 0.896 (0.020) | 0.82 |
| Total hip Z-score | ||||
| Race, age, Tanner stage | 0.864 (0.022) | 0.72 | 0.875 (0.020) | 0.75 |
| + Ht ZY0, Wt ZY0 | 0.855 (0.024) | 0.72 | 0.878 (0.022) | 0.75 |
| + Ht ZY0, Wt ZY0, ΔHt Z, ΔWt Z | 0.884 (0.022) | 0.78 | 0.900 (0.019) | 0.82 |
| Femoral neck Z-score | ||||
| Race, age, Tanner stage | 0.846 (0.022) | 0.70 | 0.863 (0.021) | 0.73 |
| + Ht ZY0, Wt ZY0 | 0.844 (0.024) | 0.70 | 0.873 (0.024) | 0.73 |
| + Ht ZY0, Wt ZY0, ΔHt Z, ΔWt Z | 0.856 (0.023) | 0.74 | 0.867 (0.022) | 0.79 |
| 1/3 radius Z-score | ||||
| Race, age, Tanner stage | 0.776 (0.025) | 0.63 | 0.826 (0.022) | 0.71 |
| + Ht ZY0, Wt ZY0 | 0.760 (0.026) | 0.63 | 0.823 (0.023) | 0.71 |
| + Ht ZY0, Wt ZY0, ΔHt Z, ΔWt Z | 0.804 (0.025) | 0.67 | 0.844 (0.023) | 0.73 |
All P < 0.0001.
Discussion
The clinical utility of bone density measures in childhood depends largely on the potential of those measures to predict bone mineral status in the future, especially peak adult BMD. Tracking is a concept that is important for understanding normal physiological bone mineral accrual and is critical for use of BMD measurements for identifying abnormalities. Indeed, clinicians have relied upon the characteristic of tracking as justification for routine monitoring of height and weight, which typically remain at relatively constant percentiles in growing children, to guide their clinical decisions. We found that BMC and BMD showed a high degree of tracking over 3 yr of growth. The degree of tracking of bone mineral accrual was comparable to height and weight measures in our sample. It is noteworthy that the degree of tracking of bone was higher than the degree of tracking of serial changes in blood pressure (r = 0.39) (21) and serum cholesterol (r = 0.48–0.58) measured in children in other studies (22). Importantly, we found that children with a low bone mineral status tended to have low bone mineral status 3 yr later. This finding reinforces the conceptual foundation for use of BMC and BMD measurements as predictors of short-term fracture risk during childhood and adolescence. Additional longitudinal measures are necessary to determine whether these measures adequately predict peak bone mass and thereby possible risk of osteoporosis later in life.
That the degree of tracking was slightly less for children who were at ages of rapid growth and were transitioning through puberty was not surprising. Although the correlation coefficients between baseline and yr 3 measurements were slightly smaller at ages associated with rapid linear growth and sexual maturation, the correlation coefficients were still strong (r > 0.7) for all skeletal sites. This provides some reassurance to clinicians who must consider the utility of a bone density assessment when their patient may soon undergo a period of rapid growth and maturation. Other smaller studies also have demonstrated that bone mass and density track across puberty (9,10,11,12).
We addressed the effect of growth on bone density tracking in two ways. First, we showed that changes in height and weight Z-scores predicted deviation from tracking. Interestingly, change in height Z-score over the 3-yr follow-up period was more strongly related to deviation from tracking of the whole body and spinea than was change in weight Z-score, whereas they performed similarly for other skeletal sites. This information may be of use when clinicians evaluate the clinical importance of a change in bone mineral Z-score determined from a follow-up DXA scan because such changes would be expected in a child who is growing at a different pace relative to his or her peers. Secondly, like Foley et al. (11), we showed that baseline bone Z-score was highly predictive of bone Z-scores 3 yr later, independent of the expected effects of height and weight and changes in height and weight.
Despite the strong correlations between measurements of bone mineral status at baseline and 3 yr later, an individual’s bone mineral status was not perfectly constant over time. Nonetheless, 72–87% of children who had a bone Z-score of less than −1.5 at baseline had a Z-score of less than −1.0 3 yr later. Importantly, only 3–4% of children with a Z-score of more than −1.5 at baseline had a Z-score less than −1.5 3 yr later.
The diminution of tracking over time, albeit small, illustrates regression toward the mean. Regression toward the mean is the phenomenon whereby extreme values measured at one point in time are closer to the sample mean when measured in the future. This occurs as a result of variation in measurement or when there is variation within the individual. In this study, measurement error was introduced by imprecision in DXA measurements and in calculation of Z-scores. If the diminution in tracking was just due to error in assigning bone Z-scores at baseline, then regression toward the mean would have been greatest between baseline and yr 1 (Fig. 1). This was not the case. The small continued change in mean Z-scores over time likely reflects true changes in bone mineral status as well.
From a biological perspective, the presence of tracking in bone mineral status implies several possible mechanisms: 1) bone status is under strong genetic regulation, and the phenotype is manifest by childhood; 2) nongenetic programming events (e.g. fetal exposures) set a trajectory for bone mineral status; or 3) environmental determinants of bone density (e.g. dietary intake, physical activity) and general health track as well. Several studies have documented a genetic component to bone mass and density (23,24), and heritability studies have demonstrated that mother-daughter resemblance in bone density is present before puberty (25). As reviewed by Cooper et al. (26), length and weight at birth, reflecting intrauterine growth, are positively associated with bone mass in adulthood. Some evidence shows a low to moderate degree of tracking of dietary intake and physical activity during childhood and adolescence (27,28,29). Future studies are needed to determine the influence of changes in diet and physical activity on tracking of bone mineral status. These are particularly important because interventions to alter trajectories are needed to reduce fracture risk in those individuals with low bone mineral status.
Our findings are limited by a relatively short follow-up period and lack of peak bone mass measures. It would be desirable to look at the predictiveness of bone measures obtained serially from early childhood until peak bone mass is obtained. Nonetheless, demonstration of tracking over a 3-yr period is an important first step in demonstrating tracking over a longer period. Our findings over 3 yr of follow-up are consistent with smaller studies of pre- and early pubertal children that were followed for 7 to 8 yr (9,10,11). Also, demonstration of tracking over a 3-yr period during childhood and adolescence is important for clinicians and researchers who are concerned about fractures in childhood and adolescence.
Our findings reflect the natural history of bone mineral accrual in healthy children. Tracking studies are needed for children who have chronic medical conditions that may alter bone mineral accrual and cause fluctuations in tracking. Our findings show that tracking and the predictiveness of a bone mineral status measurement may diminish over time, requiring follow-up DXA scans to reevaluate bone mineral status.
This study has important strengths. The large sample size allowed us to examine the tails of the distribution, which are of clinical concern. We had highly standardized measurements of bone density measured by DXA, which currently is recommended by the International Society for Clinical Densitometry as an appropriate method for bone density assessment in children and adolescents (30). We used Z-scores to characterize bone mineral status, making our findings applicable to pediatricians who need this measure to evaluate their patients of different ages.
The strong degree of tracking found in this study provides support for use of bone mineral status measurements in growing children and adolescents. For many children, low bone mineral status will persist unless interventions are instituted. Early identification of these children provides a greater opportunity for therapeutic intervention.
Footnotes
This work was supported by the National Institute of Child Health and Human Development contracts NO1-HD-1-3228, -3329, -3330, -3331, -3332, and - 3333 and the National Center for Research Resources Grants M01-RR-08084 and -000240.
Disclosure Summary: J.A.S. has received a research grant from Hologic, Inc. H.J.K., V.G., J.M.L., S.O., T.N.H., X.H., M.M.F., K.K.W., and B.S.Z. have nothing to disclose.
First Published Online March 1, 2010
Abbreviations: BMC, Bone mineral content; BMD, bone mineral density; BMI, body mass index; DXA, dual-energy x-ray absorptiometry.
Whole body and spine are the sites recommended for clinical assessment of bone density in children by the International Society for Clinical Densitometry.
References
- National Institutes of Health Fact sheet osteoporosis. http://www.nih.gov/about/researchresultsforthepublic/Osteoporosis.pdf. Accessed February 15, 2010 [Google Scholar]
- National Institutes of Health 2000 Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement Online 2000 March 27–29;17:1–36. http://consensus.nih.gov/2000/2000Osteoporosis111html.htm. Accessed October 29, 2009 [PubMed] [Google Scholar]
- Seeman E 1994 Reduced bone density in women with fractures: Contribution of low peak bone density and rapid bone loss. Osteoporos Int 4(Suppl 1):S15–S25 [DOI] [PubMed] [Google Scholar]
- Hernandez CJ, Beaupré GS, Carter DR 2003 A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int 14:843–847 [DOI] [PubMed] [Google Scholar]
- Schönau E 2004 The peak bone mass concept: is it still relevant? Pediatr Nephrol 19:825–831 [DOI] [PubMed] [Google Scholar]
- Gafni RI, Baron J 2007 Childhood bone mass acquisition and peak bone mass may not be important determinants of bone mass in late adulthood. Pediatrics 119:S131–S136 [DOI] [PubMed] [Google Scholar]
- Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA 1999 A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children. The University of Saskatchewan Bone Mineral Accrual Study. J Bone Miner Res 14:1672–1679 [DOI] [PubMed] [Google Scholar]
- Boulton TJC 1996 The notion of tracking. In: Boulton J, Laron Z, Rey J, eds. Long-term consequences of early feeding. Philadelphia: Lippincott-Raven; 99–111 [Google Scholar]
- Cheng S, Völgyi E, Tylavsky FA, Lyytikäinen A, Törmäkangas T, Xu L, Cheng SM, Kröger H, Alèn M, Kujala UM 2009 Trait-specific tracking and determinants of body composition: a 7-year follow-up study of pubertal growth in girls. BMC Med 7:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari SL, Chevalley T, Bonjour JP, Rizzoli R 2006 Childhood fractures are associated with decreased bone mass gain during puberty: an early marker of persistent bone fragility? J Bone Miner Res 21:501–507 [DOI] [PubMed] [Google Scholar]
- Foley S, Quinn S, Jones G 2009 Tracking of bone mass from childhood to adolescence and factors that predict deviation from tracking. Bone 44:752–757 [DOI] [PubMed] [Google Scholar]
- Loro ML, Sayre J, Roe TF, Goran MI, Kaufman FR, Gilsanz V 2000 Early identification of children predisposed to low peak bone mass and osteoporosis later in life. J Clin Endocrinol Metab 85:3908–3918 [DOI] [PubMed] [Google Scholar]
- Ruff C 2005 Growth tracking of femoral and humeral strength from infancy through late adolescence. Acta Paediatr 94:1030–1037 [DOI] [PubMed] [Google Scholar]
- Van Coeverden SC, De Ridder CM, Roos JC, Van't Hof MA, Netelenbos JC, Delemarre-Van de Waal HA 2001 Pubertal maturation characteristics and the rate of bone mass development longitudinally toward menarche. J Bone Miner Res 16:774–781 [DOI] [PubMed] [Google Scholar]
- Magarey AM, Boulton TJ, Chatterton BE, Schultz C, Nordin BE, Cockington RA 1999 Bone growth from 11 to 17 years: relationship to growth, gender and changes with pubertal status including timing of menarche. Acta Paediatr 88:139–146 [DOI] [PubMed] [Google Scholar]
- Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, Shepherd JA 2007 The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab 92:2087–2099 [DOI] [PubMed] [Google Scholar]
- Cole TJ, Green PJ 1992 Smoothing reference centile curves: the lms method and penalized likelihood. Stat Med 11:1305–1319 [DOI] [PubMed] [Google Scholar]
- van Buuren S, Fredriks M 2001 Worm plot: a simple diagnostic device for modelling growth reference curves. Stat Med 20:1259–1277 [DOI] [PubMed] [Google Scholar]
- Tanner JM 1962 Growth at adolescence. 2nd ed. Oxford, UK: Blackwell Scientific [Google Scholar]
- Cummings SR, Palermo L, Browner W, Marcus R, Wallace R, Pearson J, Blackwell T, Eckert S, Black D 2000 Monitoring osteoporosis therapy with bone densitometry: misleading changes and regression to the mean. JAMA 283:1318–1321 [DOI] [PubMed] [Google Scholar]
- Beckett LA, Rosner B, Roche AF, Guo S 1992 Serial changes in blood pressure from adolescence into adulthood. Am J Epidemiol 135:1166–1177 [DOI] [PubMed] [Google Scholar]
- Porkka KV, Viikari JS, Taimela S, Dahl M, Akerblom HK 1994 Tracking and predictiveness of serum lipid and lipoprotein measurements in childhood: a 12-year follow-up. The Cardiovascular Risk in Young Finns Study. Am J Epidemiol 140:1096–1110 [DOI] [PubMed] [Google Scholar]
- Hopper JL, Green RM, Nowson CA, Young D, Sherwin AJ, Kaymakci B, Larkins RG, Wark JD 1998 Genetic, common environment, and individual specific components of variance for bone mineral density in 10- to 26-year-old females: a twin study. Am J Epidemiol 147:17–29 [DOI] [PubMed] [Google Scholar]
- Krall EA, Dawson-Hughes B 1993 Heritable and life-style determinants of bone mineral density. J Bone Miner Res 8:1–9 [DOI] [PubMed] [Google Scholar]
- Ferrari S, Rizzoli R, Slosman D, Bonjour JP 1998 Familial resemblance for bone mineral mass is expressed before puberty. J Clin Endocrinol Metab 83:358–361 [DOI] [PubMed] [Google Scholar]
- Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M 2006 Review: developmental origins of osteoporotic fracture. Osteoporos Int 17:337–347 [DOI] [PubMed] [Google Scholar]
- Singer MR, Moore LL, Garrahie EJ, Ellison RC 1995 The tracking of nutrient intake in young children. The Framingham Children’s Study. Am J Public Health 85:1673–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelder SH, Perry CL, Klepp KI, Lytle LL 1994 Longitudinal tracking of adolescent smoking, physical activity, and food choice behaviors. Am J Public Health 84:1121–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twisk JW, Kemper HC, van Mechelen W, Post GB 1997 Tracking of risk factors for coronary heart disease over a 14-year period: A comparison between lifestyle and biologic risk factors with data from the Amsterdam Growth and Health Study. Am J Epidemiol 145:888–898 [DOI] [PubMed] [Google Scholar]
- Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, Lorenc RS, Tosi LL, Ward KA, Ward LM, Kalkwarf HJ 2008 Dual energy x-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD pediatric official positions. J Clin Densitom 11:43–58 [DOI] [PubMed] [Google Scholar]