Abstract
Context: Studies in humans and animals indicate that estrogen negative feedback occurs at the level of the hypothalamus, but it is unclear whether estrogen also exerts an inhibitory effect directly at the pituitary.
Objectives: The aim of the study was to determine whether estrogen has a direct negative feedback effect at the pituitary and whether this varies with aging.
Design and Setting: A GnRH antagonist and graded doses of GnRH were used to isolate pituitary responsiveness before and after estrogen administration in Clinical Research Center studies at an academic medical center.
Subjects: Subjects were healthy postmenopausal women aged 48–56 yr (n = 8) or 70–75 yr (n= 8).
Interventions: A suppressive dose of the NAL-GLU GnRH antagonist was administered, followed by graded doses of GnRH before and after 1 month of estrogen administration.
Results: LH and FSH responses to GnRH decreased after estrogen administration (P = 0.01 and P = 0.0001, respectively). The ratio of FSH to LH amplitudes decreased in response to estrogen (P = 0.04) indicating a greater sensitivity of FSH than LH to inhibition by estrogen. The inhibitory effect of estrogen on FSH was attenuated with aging (P = 0.02), but was maintained for LH (P = 0.4).
Conclusions: Studies that control for endogenous GnRH and estradiol demonstrate a direct pituitary site of estrogen negative feedback on LH and FSH responsiveness to GnRH in women. The effect of estrogen on FSH responsiveness is greater than on LH and is attenuated with aging. These studies indicate that estrogen negative feedback occurs directly at the pituitary and contributes to the differential regulation of FSH and LH secretion.
Blockade of endogenous GnRH stimulation of the pituitary reveals a direct inhibitory effect of estradiol on pituitary secretion of LH and FSH in women.
The human menstrual cycle represents a delicate balance of coordinated signals between the hypothalamus, pituitary, and ovaries. The pituitary secretion of gonadotropins is influenced by hypothalamic GnRH pulse frequency and amplitude (1), GnRH receptor number (2), and postreceptor signaling (3). The final neuroendocrine output is also modulated by negative and positive feedback from ovarian steroids (estrogen and progesterone) (4) and peptides (inhibin) (5,6), such that LH and FSH pulses of specific amplitude and frequency are secreted as follicle growth and luteinization alter the ovarian hormonal milieu.
It is well known from animal and human models that low-dose estrogen helps to define the gonadotropin secretory profile in women by inhibiting hypothalamic secretion of GnRH (7,8,9), but the presence of a direct pituitary site of estrogen negative feedback has been more difficult to prove. Physiological studies in gonadectomized animals whose pituitaries were isolated from hypothalamic input (10,11,12,13,14), in addition to studies in pituitary cells in culture (15,16,17,18,19), have shown a direct, although often transient, inhibitory effect of estrogen on gonadotropin secretion. Recent characterization of the gonadotrope-specific estrogen receptor 1 (ESR1; also known as ERα) female knockout mouse provides definitive evidence of a direct inhibitory effect of estrogen at the gonadotrope in this species (20). Few studies in the human have attempted to specifically isolate the pituitary response to estrogen negative feedback from the known inhibitory effects of estrogen on the hypothalamus, and the results have been inconsistent (9,21,22). The question of whether low-dose estrogen exerts a negative feedback effect directly at the pituitary in women remains open.
There is now significant evidence to indicate that the neuroendocrine components of the reproductive system undergo significant changes with aging both in animal models (23,24) and in women (25), including our recent finding of a decrease in pituitary responsiveness to GnRH with aging in women (26). Some, but not all of the studies have suggested that the aging female brain retains its sensitivity to steroid feedback (8,27,28,29,30,31,32,33). However, none have isolated the pituitary from endogenous hypothalamic input to study the interaction of estrogen and aging on gonadotrope function.
To test the hypothesis that estrogen has a direct inhibitory effect at the pituitary, we determined the effect of 1 month of estrogen administration on the gonadotropin response to graded doses of GnRH using a model in which the pituitary is isolated from hypothalamic and ovarian input, as previously described (26). Isolation of the pituitary from endogenous hypothalamic GnRH stimulation was achieved through blockade of the GnRH receptor using a competitive GnRH antagonist, permitting control of the dose and interval of GnRH administration, both of which may be impacted by both aging and gonadal steroids (25). To control for endogenous steroid feedback and to investigate the impact of aging on estrogen negative feedback, we studied young and old postmenopausal women, who lack the variable ovarian hormonal milieu characteristic of normally cycling and perimenopausal women. The results of these studies indicate that estrogen has a direct inhibitory effect on pituitary responsiveness to GnRH and that this effect on FSH response is attenuated with aging.
Subjects and Methods
Subjects
Young (48–56 yr old; n = 8) and old (70–75 yr old; n = 8) postmenopausal women were studied. All subjects were healthy and had experienced their last menstrual period a minimum of 18 months previously, thereby fulfilling the definition of postmenopausal according to the STRAW criteria (34). Three older subjects had undergone bilateral oophorectomy in the past, whereas the majority had undergone natural menopause. Eighty-eight percent of the younger women and 63% of the older women had never taken hormone replacement therapy (HRT). Subjects were not on any medication known to interact with the neuroendocrine reproductive axis. Prolactin, TSH, complete blood count, and renal function tests were normal. Electrocardiograms and mammograms were normal in all subjects, and none had any contraindications to HRT, including a Factor V Leiden mutation. Subjects took ferrous gluconate 324 mg/d beginning 1 month before the inpatient protocol until 1 month after the study.
The study was approved by the Human Research Committee of the Massachusetts General Hospital, and signed informed consent was obtained from each subject before participation.
Experimental protocol
Subjects were admitted to the Clinical Research Center of the Massachusetts General Hospital at the same time of day at baseline and after 1 month of transdermal estrogen replacement (Climara; Bayer Healthcare Pharmaceuticals, Pine Brook, NJ; 0.05 mg/d, changing the patch every 86 h) designed to achieve estrogen levels of at least 50 pg/ml. As previously described (26) and as detailed in Fig. 1, blood was sampled every 30 min through an antecubital iv catheter for 4 h to assess baseline gonadotropin secretion. After 4 h of sampling, subjects received a maximally suppressive dose of a competitive GnRH antagonist (NAL-GLU GnRH antagonist 150 μg/kg) (35) administered sc. Blood sampling continued every 30 min until 7 h after antagonist injection when blood sampling increased in frequency to every 10 min. Beginning 8 h after antagonist administration, GnRH doses of 25, 75, 250, or 750 ng/kg were given iv every 4 h in random order, with the exception that the highest dose was always given last. The same order of dosing was maintained for both studies in a given subject. Subjects with an intact uterus received medroxyprogesterone acetate (5 mg/d × 10 d) at the completion of their second study. Estradiol (E2) was measured at baseline, and all blood samples were assayed for LH and FSH.
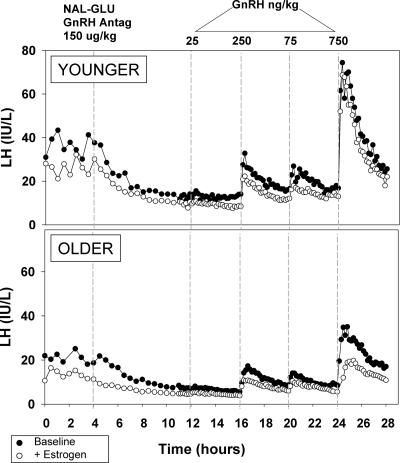
Figure 1.
Serum LH during the 28-h study before (closed circles) and after (open circles) 1 month of estrogen administration in representative younger and older subjects. Beginning at 0800 h, blood was sampled every 30 min for 4 h to assess baseline secretion. The subjects then received a sc injection of 150 μg/kg of the NAL-GLU GnRH antagonist. Seven hours after the injection, sampling frequency increased to every 10 min; 8 h after injection, GnRH doses of 25, 75, 250, and 750 ng/kg were administered iv in random order in 4-h intervals. Note the order of doses for both subjects as indicated. LH is expressed in international units per liter, as equivalents of the 2nd International Reference Preparation of human menopausal gonadotropins.
GnRH was obtained from Polypeptides (Torrance CA) and formulated in the Research Pharmacy of Massachusetts General Hospital. The NAL-GLU GnRH antagonist was obtained from the Contraceptive Development Branch of the National Institutes of Health and formulated as previously described (36). These peptides are used under investigator-initiated Investigational New Drug applications from the Food and Drug Administration. Preliminary studies in which GnRH was administered over the range of 15 to 1500 ng/kg established the range of GnRH doses that would compete with 150 μg/kg of the NAL-GLU GnRH antagonist and revealed the absence of a priming effect either with or without estrogen administration in this model.
Assays
Serum LH and FSH were measured using a two-site monoclonal nonisotopic system (Axsym; Abbott Laboratories, Abbott Park, IL), as previously described (9,37). LH and FSH were expressed in international units per liter, as equivalents of the Second International Reference Preparation 71/223 of human menopausal gonadotropins. The assay sensitivity for both LH and FSH is 1.6 IU/liter. The intraassay coefficients of variation (CVs) for LH and FSH are less than 7% and less than 6%, respectively, with interassay CVs for both hormones of less than 7.4%. E2 was measured using a direct immunoassay (Architect i2000; Abbott Laboratories), which has a sensitivity of 5 pg/ml (18.4 pmol/liter) and a functional sensitivity of 15 pg/ml (55.1 pmol/liter) (38). The intraassay CV for E2 is 6.4%, and the interassay CV is 10.6%.
Data analysis
Baseline LH and FSH were calculated as the arithmetic mean of levels measured every 30 min for 4 h before administration of the GnRH antagonist. The peak of the LH or FSH response to each dose of GnRH was taken as the highest level measured in the 4-h period after each dose of GnRH administered. The nadir of the LH or FSH response to each dose of GnRH was taken as the lowest level measured at t = −10 or 0 min in relation to GnRH administration. LH and FSH amplitudes were calculated as the difference between the peak and nadir. Data that were not normally distributed were log-transformed before analysis. Independent samples t tests were used for comparison of baseline values between young and old postmenopausal women, and paired samples t tests were used for comparison of E2 levels before and after estrogen administration. The LH and FSH responses to GnRH and the FSH/LH ratio before and after estrogen administration were compared between groups using ANOVA for repeated measures, followed by Student-Newman-Keuls post hoc testing. Results are expressed as the mean ± sem. A P value of <0.05 was considered to be statistically significant.
Results
Baseline characteristics
Characteristics of the subjects are presented in Table 1. Older and younger women were clearly separated by both age and years from menopause or HRT but did not differ in body weight or body mass index. Menopausal status was confirmed in all subjects at baseline by low E2 and elevated gonadotropins. Gonadotropins were lower in older compared with younger postmenopausal women at baseline as seen previously, but the difference was not statistically significant in the current study. E2 levels were not significantly different between young and old postmenopausal women at baseline or after estrogen but increased in both groups after 1 month of estrogen administration (P = 0.004). As indicated in Table 1, unstimulated gonadotropin levels, measured before administration of the GnRH antagonist or GnRH, were lower after estrogen administration for both FSH (P = 0.002) and LH (P = 0.03).
Table 1.
Characteristics of younger and older postmenopausal subjects
| Younger | Older | P value | |
|---|---|---|---|
| Age (yr) | 53.4 (0.9) | 72.2 (0.6) | <0.001 |
| Years postmenopause | 4.1 (1.3) | 28.3 (2.8) | <0.001 |
| Years postmenopause or HRT | 3.4 (0.5) | 19.7 (5.3) | 0.008 |
| Weight (kg) | 80.6 (2.8) | 72.2 (4.1) | 0.11 |
| BMI (kg/m2) | 29.1 (0.9) | 28.5 (1.6) | 0.74 |
| Baseline | |||
| Estradiol (pg/ml)a | 20.3 (4.6) | 18.9 (3.9) | 0.81 |
| LH (IU/liter) | 69.2 (5.0) | 65.5 (10.6) | 0.76 |
| FSH (IU/liter) | 133.7 (17.4) | 99.5 (13.6) | 0.14 |
| + Estrogen | |||
| Estradiol (pg/ml)a | 58.4 (12.6) | 79.6 (29.2) | 0.52 |
| LH (IU/liter) | 46.1 (8.0) | 49.6 (9.6) | 0.78 |
| FSH (IU/liter) | 57.1 (10.3) | 59.9 (10.0) | 0.85 |
Values are expressed as mean (se). Gonadotropins are expressed in IU/liter, as equivalents of the 2nd International Reference Preparation of human menopausal gonadotropins. P values represent younger vs. older comparisons. BMI, Body mass index.
To convert pg/ml to pmol/liter, multiply by 3.67.
Response to GnRH receptor blockade
Figure 1 shows the immediate decrease in LH levels after GnRH antagonist administration, consistent with blockade of the GnRH receptor in representative young and old postmenopausal women before and after E2 administration. The responses to exogenous GnRH that follow are then controlled for both GnRH dose and previous interpulse interval.
Effect of estrogen on LH and FSH responses to GnRH
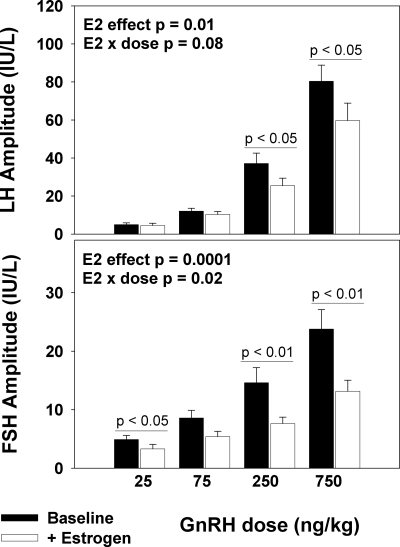
The amplitudes of both LH and FSH increased with increasing GnRH doses at baseline (P < 0.001 and P < 0.001, respectively) and after 1 month of low-dose estrogen exposure (P < 0.001 and P < 0.001, respectively; Fig. 2). The LH response to GnRH decreased in all subjects after estrogen administration (P = 0.01; Fig. 2). At the highest two doses of GnRH (75 and 250 ng/kg), LH response decreased by 26 and 23%, respectively, in the presence of E2. The FSH response to GnRH also decreased after estrogen administration (P = 0.0001). There was an interaction between estrogen and dose such that estrogen administration resulted in a greater decrement in FSH responses at higher doses of GnRH (P = 0.02; Fig. 2). The FSH response in the presence of E2 decreased by 11% at a GnRH dose of 25 ng/kg, 29% at 250 ng/kg, and 36% at 750 ng/kg.
Figure 2.
LH (upper panel) and FSH (lower panel) responsiveness to GnRH before and after 1 month of estrogen exposure, as indicated, in postmenopausal women (young and old combined), showing an inhibitory effect of estrogen on pituitary responsiveness to GnRH. Absolute LH and FSH amplitudes are shown in response to increasing doses of GnRH (25, 75, 250, and 750 ng/kg). LH and FSH amplitude are expressed in international units per liter, as equivalents of the 2nd International Reference Preparation of human menopausal gonadotropins.
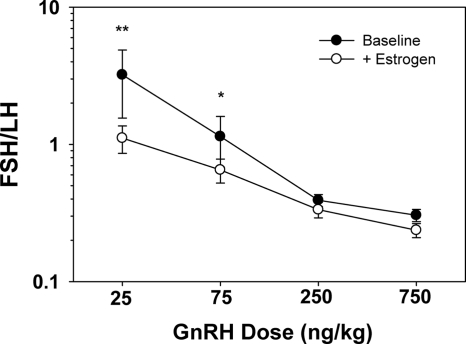
The amplitudes of FSH and LH were expressed as a ratio at each dose of GnRH to address the relative sensitivity of FSH and LH to estrogen negative feedback. As indicated in Fig. 3, the ratio of FSH to LH amplitudes in response to GnRH decreased with estrogen administration (P = 0.04), consistent with the interpretation that FSH is more sensitive to estrogen negative feedback at the pituitary than is LH.
Figure 3.
The ratio of FSH to LH amplitude decreased in response to estrogen administration in postmenopausal women (younger and older combined) (P = 0.04), indicating a greater sensitivity of FSH than LH to estrogen negative feedback at the pituitary. *, P = 0.05; **, P < 0.01.
Effect of age on estrogen inhibition of LH and FSH responses to GnRH
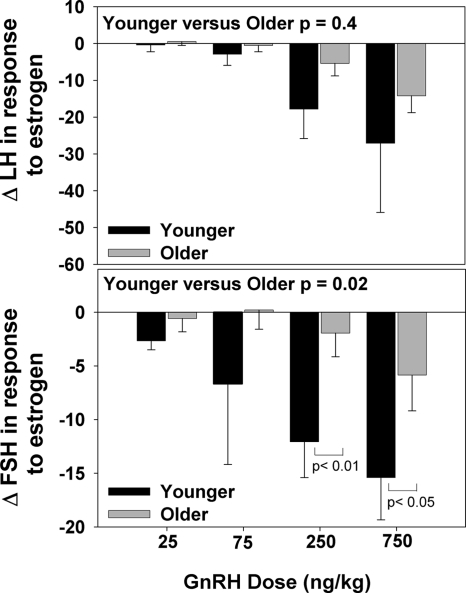
The LH and FSH responses to GnRH were attenuated in older compared with younger postmenopausal women at baseline (P = 0.03 and P = 0.02, respectively) as previously shown (26). The inhibitory effect of estrogen on FSH amplitude was significantly attenuated with aging (P = 0.02). Unlike FSH, the effect of estrogen on LH amplitude was not significantly affected by aging (P = 0.4) (Fig. 4).
Figure 4.
Change in LH (upper panel) and FSH (lower panel) responses to increasing doses of GnRH (25, 75, 250, and 750 ng/kg) after 1 month of estrogen administration in younger vs. older postmenopausal women indicates that aging attenuates the FSH (P < 0.02), but not the LH response (P = 0.4) to estrogen negative feedback at the pituitary.
Discussion
The present study demonstrates that estrogen levels within a physiological range have a direct negative feedback effect at the pituitary in women. Using a model in which the pituitary is isolated from endogenous GnRH and gonadal feedback is controlled (26), we found that 1 month of low-dose estrogen exposure in postmenopausal women resulted in inhibition of the pituitary response to GnRH with a more pronounced effect on FSH compared with LH secretion. Furthermore, we found that the potency of estrogen negative feedback at the pituitary on FSH responsiveness to GnRH diminishes with aging.
Our findings are consistent with the results of a number of animal studies that have demonstrated a direct pituitary site of estrogen negative feedback. Although both isoforms of the estrogen receptor (ERα [also known as ESR1] and ERβ [also known as ESR2]) are present in the pituitary, receptor knockout models suggest that ERα is the predominant receptor mediating estrogen negative feedback (39). The recent generation and characterization of the gonadotrope-specific Esr1 female knockout mouse has provided the most definitive evidence for direct pituitary estrogen signaling because these animals have LH β mRNA levels 3-fold higher than their wild-type counterparts (20). These knockout studies complement other in vivo models designed to study the pituitary in isolation. Clarke and Cummins (10) studied hypothalamus-pituitary disconnected ewes treated with pulsatile GnRH and found that treatment with estrogen led to a progressive decline in FSH and a transient reduction in LH. Similarly, estrogen administered to ovariectomized monkeys bearing hypothalamic lesions and treated with pulsatile GnRH led to a transient decrease in LH and FSH (13). When administered to hypophysectomized, ovariectomized rats with pituitary homografts, estrogen led to a blunted LH response to GnRH (12,14) but no change in the FSH response (14). Taken together, with a number of studies in pituitary cell cultures (15,16,17,18,19), these studies in animal models have demonstrated that estrogen can act directly at the pituitary to impart at least a transient inhibitory response to GnRH in a variety of animal species. These studies in animal and in vitro models also provide evidence that estrogen may differentially regulate FSH and LH at a pituitary level, although the direction of the difference is inconsistent between studies.
There are few studies that have attempted to isolate pituitary from hypothalamic effects of estrogen negative feedback in women, and results have been inconsistent in providing evidence that estrogen has a direct inhibitory effect at the pituitary. In the 1970s, Thompson reported that short-term estrogen administered to women with secondary amenorrhea produced a decreased FSH and LH response to a single pharmacological dose of GnRH (40). In two women with more complete deficiency of endogenous GnRH undergoing treatment with pulsatile GnRH, Marshall et al. (21) found that administration of 1 to 2 months of oral estrogen led to a greater decrease in FSH than LH. In postmenopausal women, the use of an ERα antagonist that does not cross the blood-brain barrier partially relieved the suppressive effect of 18 d of estrogen treatment on FSH, but not LH, consistent with an inhibitory effect of estrogen at the hypothalamus for both LH and FSH secretion and an additional inhibitory effect at the pituitary for FSH (22). In contrast, Welt et al. (9) demonstrated an increase in LH and FSH in normal women in response to estrogen receptor blockade using tamoxifen, but not in GnRH-deficient women in whom pulsatile GnRH was controlled. These data suggested a hypothalamic, but not a pituitary, site of estrogen negative feedback in the early follicular phase in women at a time when estrogen levels are just beginning to rise (9). A more recent study by our group, in postmenopausal women receiving a low-dose estrogen infusion, demonstrated that decreased LH levels at 24 h were temporally associated with decreased metabolic activity in the hypothalamus on positron emission tomography scan, but again there was no apparent effect of short-term estrogen at the pituitary (41). Taken together with the current results, there is now significant evidence that low-dose estrogen exerts a direct negative feedback effect at the pituitary. However, it is possible that the direct inhibitory effect at the pituitary that is apparent in the current studies after 1 month of estrogen administration may not occur with very short-term exposure to estrogen.
In the current study, FSH was more sensitive than LH to estrogen negative feedback, as previously seen in studies in women (21,22) and in some animal models (10,11,19). The regulation of FSH and LH secretion during the menstrual cycle is thought to occur via the differential effect of GnRH pulse frequency and amplitude on LH and FSH synthesis and secretion and by the inhibitory effect of circulating inhibin B on FSH, but not LH production (5). In the current study, there was a greater decrease in GnRH-stimulated FSH than LH after estrogen administration when controlling GnRH and estrogen exposure in women in whom endogenous circulating inhibin levels are very low. This suggests that an additional level of differential regulation of LH and FSH may occur via estrogen-stimulated intracellular signaling at the pituitary. The use of different GnRH agonists and antagonists has revealed ligand-induced selective signaling in tumor cell lines from different reproductive tract tissues (42). Although similar effects have not been observed to date when studies are confined to gonadotrope cell lines, it is possible that the differential effect that we have observed on LH and FSH is unique to competition between the NAL-GLU GnRH antagonist and natural sequence GnRH. An additional explanation for this differential effect might involve the intrapituitary antagonistic peptides, activin and follistatin (43). Activin acts in an autocrine/paracrine manner to increase FSHβ transcription, whereas follistatin binds to and neutralizes activin. Although some studies have suggested that activin may also increase LHβ transcription, the effect is trivial compared with the effect on FSH (44,45). When administered to ovariectomized rats, estrogen increases pituitary follistatin mRNA (46,47), whereas estrogen decreases activin mRNA in anterior pituitary cells from anestrous ewes (48). Both situations would favor decreased FSHβ transcription. Thus, it is possible that estrogen has a more pronounced inhibitory effect on FSH than LH, as observed in our study, because of an estrogen-induced alteration of the activin/follistatin ratio within the pituitary.
The effect of aging on estrogen negative feedback remains controversial. Some (27,28,29), but not all (30), animal studies have demonstrated diminished estrogen negative feedback on gonadotropin secretion with aging, but none have separated the effects of estrogen at the hypothalamus from those at the pituitary. Studies in women have also produced inconsistent results. Treatment with 1 wk of clomiphene citrate resulted in a decrease in gonadotropin levels in younger, but not older postmenopausal women (32). Because clomiphene citrate is thought to act as an estrogen agonist in postmenopausal women (49), these studies were interpreted as evidence of decreased sensitivity to estrogen negative feedback with aging. In contrast, 2 months of estrogen administration to naturally or prematurely menopausal women resulted in suppression of both LH and FSH, but no difference as a function of age (33). The lower LH pulse frequency after estrogen administration in postmenopausal women compared with prematurely menopausal women may suggest increased hypothalamic sensitivity to estrogen negative feedback with aging. We have previously shown that estrogen negative feedback on the frequency and overall quantity of hypothalamic GnRH secretion is maintained in older and younger postmenopausal women (8,31). However, in the current study, we have demonstrated that the inhibitory effect of estrogen on the pituitary is attenuated with aging in postmenopausal women. Although a similar trend was observed for LH, it did not reach statistical significance. These studies, therefore, suggest that whereas estrogen negative feedback at the hypothalamus is preserved with aging, negative feedback at the pituitary is attenuated.
In summary, we have established that estrogen has dual sites of negative feedback on gonadotropin secretion: the pituitary as well as the hypothalamus. The presence of more than one site of negative feedback has the potential advantage of allowing for more precise and differential control of FSH and LH levels. We have previously shown that aging attenuates pituitary responsiveness to GnRH (26). The current study suggests that estrogen negative feedback at the pituitary is also attenuated with aging, with a greater effect on FSH than LH. Future studies will need to assess the relative roles of ERα and ERβ in mediating this negative feedback effect at the pituitary.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants R01 AG13241 and M01 RR01066. N.D.S. received fellowship support from the NIH (5T32 HD007396). Product donation was received from Berlex Laboratories, Inc.
The study is registered with ClinicalTrials.gov, ID no. NCT 00386022.
Disclosure Summary: The authors have nothing to declare.
First Published Online February 4, 2010
Abbreviations: CV, Coefficient of variation; E2, estradiol; ERα, estrogen receptor α; HRT, hormone replacement therapy.
References
- Crowley Jr WF, Filicori M, Spratt DI, Santoro NF 1985 The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res 41:473–531 [DOI] [PubMed] [Google Scholar]
- Loumaye E, Catt KJ 1982 Homologous regulation of gonadotropin-releasing hormone receptors in cultured pituitary cells. Science 215:983–985 [DOI] [PubMed] [Google Scholar]
- Ando H, Hew CL, Urano A 2001 Signal transduction pathways and transcription factors involved in the gonadotropin-releasing hormone-stimulated gonadotropin subunit gene expression. Comp Biochem Physiol B Biochem Mol Biol 129:525–532 [DOI] [PubMed] [Google Scholar]
- Clarke IJ 2002 Multifarious effects of estrogen on the pituitary gonadotrope with special emphasis on studies in the ovine species. Arch Physiol Biochem 110:62–73 [DOI] [PubMed] [Google Scholar]
- Gregory SJ, Kaiser UB 2004 Regulation of gonadotropins by inhibin and activin. Semin Reprod Med 22:253–267 [DOI] [PubMed] [Google Scholar]
- Welt CK 2004 Regulation and function of inhibins in the normal menstrual cycle. Semin Reprod Med 22:187–193 [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS 1999 Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- Gill S, Sharpless JL, Rado K, Hall JE 2002 Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J Clin Endocrinol Metab 87:2290–2296 [DOI] [PubMed] [Google Scholar]
- Welt CK, Pagan YL, Smith PC, Rado KB, Hall JE 2003 Control of follicle-stimulating hormone by estradiol and the inhibins: critical role of estradiol at the hypothalamus during the luteal-follicular transition. J Clin Endocrinol Metab 88:1766–1771 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT 1984 Direct pituitary effects of estrogen and progesterone on gonadotropin secretion in the ovariectomized ewe. Neuroendocrinology 39:267–274 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT, Crowder ME, Nett TM 1989 Long-term negative feedback effects of oestrogen and progesterone on the pituitary gland of the long-term ovariectomized ewe. J Endocrinol 120:207–214 [DOI] [PubMed] [Google Scholar]
- McLean BK, Chang N, Nikitovitch-Winer MB 1975 Ovarian steroids directly alter luteinizing hormone (LH) release by pituitary homografts. Endocrinology 97:196–201 [DOI] [PubMed] [Google Scholar]
- Nakai Y, Plant TM, Hess DL, Keogh EJ, Knobil E 1978 On the sites of the negative and positive feedback actions of estradiol in the control of gonadotropin secretion in the rhesus monkey. Endocrinology 102:1008–1014 [DOI] [PubMed] [Google Scholar]
- Strobl FJ, Levine JE 1988 Estrogen inhibits luteinizing hormone (LH), but not follicle-stimulating hormone secretion in hypophysectomized pituitary-grafted rats receiving pulsatile LH-releasing hormone infusions. Endocrinology 123:622–630 [DOI] [PubMed] [Google Scholar]
- Phillips CL, Lin LW, Wu JC, Guzman K, Milsted A, Miller WL 1988 17β-estradiol and progesterone inhibit transcription of the genes encoding the subunits of ovine follicle-stimulating hormone. Mol Endocrinol 2:641–649 [DOI] [PubMed] [Google Scholar]
- Frawley LS, Neill JD 1984 Biphasic effects of estrogen on gonadotropin-releasing hormone-induced luteinizing hormone release in monolayer cultures of rat and monkey pituitary cells. Endocrinology 114:659–663 [DOI] [PubMed] [Google Scholar]
- Tang LK, Spies HG 1975 Effects of gonadal steroids on the basal and LRF-induced gonadotropin secretion by cultures of rat pituitary. Endocrinology 96:349–355 [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Waring DW 1981 Acute progesterone and 17 β-estradiol modulation of luteinizing hormone secretion by pituitaries of cycling rats superfused in vitro. Endocrinology 108:413–419 [DOI] [PubMed] [Google Scholar]
- Schally AV, Redding TW, Arimura A 1973 Effect of sex steroids on pituitary responses to LH- and FSH-releasing hormone in vitro. Endocrinology 93:893–902 [DOI] [PubMed] [Google Scholar]
- Singh SP, Wolfe A, Ng Y, DiVall SA, Buggs C, Levine JE, Wondisford FE, Radovick S 2009 Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor α (ESR1). Biol Reprod 81:488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JC, Case GD, Valk TW, Corley KP, Sauder SE, Kelch RP 1983 Selective inhibition of follicle-stimulating hormone secretion by estradiol. Mechanism for modulation of gonadotropin responses to low dose pulses of gonadotropin-releasing hormone. J Clin Invest 71:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma M, Bailey J, Miles JM, Bowers CY, Veldhuis JD 2008 Pituitary and/or peripheral estrogen-receptor α regulates follicle-stimulating hormone secretion, whereas central estrogenic pathways direct growth hormone and prolactin secretion in postmenopausal women. J Clin Endocrinol Metab 93:951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Kashon ML, Krajnak KM, Rosewell KL, Cai A, Scarbrough K, Harney JP, McShane T, Lloyd JM, Weiland NG 1997 Aging of the female reproductive system: a window into brain aging. Recent Prog Horm Res 52:279–303; discussion 303–305 [PubMed] [Google Scholar]
- Yin W, Gore AC 2006 Neuroendocrine control of reproductive aging: roles of GnRH neurons. Reproduction 131:403–414 [DOI] [PubMed] [Google Scholar]
- Hall JE 2007 Neuroendocrine changes with reproductive aging in women. Semin Reprod Med 25:344–351 [DOI] [PubMed] [Google Scholar]
- Shaw ND, Srouji SS, Histed SN, McCurnin KE, Hall JE 2009 Aging attenuates the pituitary response to gonadotropin-releasing hormone. J Clin Endocrinol Metab 94:3259–3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland BE, Preiss C 1975 Effects of aging on basal levels of serum gonadotropins, ovarian compensatory hypertrophy, and hypersecretion of gonadotropins after ovariectomy in female rats. Fertil Steril 26:271–276 [DOI] [PubMed] [Google Scholar]
- Huang HH, Marshall S, Meites J 1976 Capacity of old versus young female rats to secrete LH, FSH and prolactin. Biol Reprod 14:538–543 [DOI] [PubMed] [Google Scholar]
- Hwang C, Pu HF, Hwang CY, Liu JY, Yao HC, Tung YF, Wang PS 1990 Age-related differences in the release of luteinizing hormone and gonadotropin-releasing hormone in ovariectomized rats. Neuroendocrinology 52:127–132 [DOI] [PubMed] [Google Scholar]
- Shaar CJ, Euker JS, Riegle GD, Meites J 1975 Effects of castration and gonadal steroids on serum luteinizing hormone and prolactin in old and young rats. J Endocrinol 66:45–51 [DOI] [PubMed] [Google Scholar]
- Gill S, Lavoie HB, Bo-Abbas Y, Hall JE 2002 Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J Clin Endocrinol Metab 87:2297–2302 [DOI] [PubMed] [Google Scholar]
- Rossmanith WG, Reichelt C, Scherbaum WA 1994 Neuroendocrinology of aging in humans: attenuated sensitivity to sex steroid feedback in elderly postmenopausal women. Neuroendocrinology 59:355–362 [DOI] [PubMed] [Google Scholar]
- Santoro N, Banwell T, Tortoriello D, Lieman H, Adel T, Skurnick J 1998 Effects of aging and gonadal failure on the hypothalamic-pituitary axis in women. Am J Obstet Gynecol 178:732–741 [DOI] [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N 2001 Executive summary: Stages of Reproductive Aging Workshop (STRAW). Climacteric 4:267–272 [PubMed] [Google Scholar]
- Sharpless JL, Supko JG, Martin KA, Hall JE 1999 Disappearance of endogenous luteinizing hormone is prolonged in postmenopausal women. J Clin Endocrinol Metab 84:688–694 [DOI] [PubMed] [Google Scholar]
- Hall JE, Taylor AE, Martin KA, Rivier J, Schoenfeld DA, Crowley Jr WF 1994 Decreased release of gonadotropin-releasing hormone during the preovulatory midcycle luteinizing hormone surge in normal women. Proc Natl Acad Sci USA 91:6894–6898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welt CK, McNicholl DJ, Taylor AE, Hall JE 1999 Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab 84:105–111 [DOI] [PubMed] [Google Scholar]
- Sluss PM, Hayes FJ, Adams JM, Barnes W, Williams G, Frost S, Ramp J, Pacenti D, Lehotay DC, George S, Ramsay C, Doss RC, Crowley Jr WF 2008 Mass spectrometric and physiological validation of a sensitive, automated, direct immunoassay for serum estradiol using the Architect. Clin Chim Acta 388:99–105 [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS 1999 Reproductive phenotypes in the estrogen receptor-α knockout mouse. Ann Endocrinol (Paris) 60:143–148 [PubMed] [Google Scholar]
- Thompson IE, Arfania J, Taymor ML 1973 Effects of estrogen and progesterone on pituitary response to stimulation by luteinizing hormone-releasing factor. J Clin Endocrinol Metab 37:152–155 [DOI] [PubMed] [Google Scholar]
- Ottowitz WE, Dougherty DD, Fischman AJ, Hall JE 2008 [18F]2-fluoro-2-deoxy-D-glucose positron emission tomography demonstration of estrogen negative and positive feedback on luteinizing hormone secretion in women. J Clin Endocrinol Metab 93:3208–3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López de Maturana R, Pawson AJ, Lu ZL, Davidson L, Maudsley S, Morgan K, Langdon SP, Millar RP 2008 Gonadotropin-releasing hormone analog structural determinants of selectivity for inhibition of cell growth: support for the concept of ligand-induced selective signaling. Mol Endocrinol 22:1711–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muttukrishna S, Tannetta D, Groome N, Sargent I 2004 Activin and follistatin in female reproduction. Mol Cell Endocrinol 225:45–56 [DOI] [PubMed] [Google Scholar]
- Attardi B, Miklos J 1990 Rapid stimulatory effect of activin-A on messenger RNA encoding the follicle-stimulating hormone β-subunit in rat pituitary cell cultures. Mol Endocrinol 4:721–726 [DOI] [PubMed] [Google Scholar]
- McLachlan RI, Dahl KD, Bremner WJ, Schwall R, Schmelzer CH, Mason AJ, Steiner RA 1989 Recombinant human activin-A stimulates basal FSH and GnRH-stimulated FSH and LH release in the adult male macaque, Macaca fascicularis. Endocrinology 125:2787–2789 [DOI] [PubMed] [Google Scholar]
- Prendergast KA, Burger LL, Aylor KW, Haisenleder DJ, Dalkin AC, Marshall JC 2004 Pituitary follistatin gene expression in female rats: evidence that inhibin regulates transcription. Biol Reprod 70:364–370 [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Chin WW 1993 Regulation of follistatin messenger ribonucleic acid levels in the rat pituitary. J Clin Invest 91:2523–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta M, West LA, Turzillo AM, Nett TM 2001 Activin modulates differential effects of estradiol on synthesis and secretion of follicle-stimulating hormone in ovine pituitary cells. Biol Reprod 64:714–719 [DOI] [PubMed] [Google Scholar]
- Glasier AF 1990 Clomiphene citrate. Baillieres Clin Obstet Gynaecol 4:491–501 [DOI] [PubMed] [Google Scholar]