Abstract
Context: Sex and race differences in bone development are associated with differences in growth, maturation, and body composition.
Objective: The aim of the study was to determine the independent effects of sex, race, and puberty on cortical bone development and muscle-bone relations in children and young adults.
Design and Participants: We conducted a cross-sectional study of 665 healthy participants (310 male, 306 black) ages 5–35 yr.
Outcomes: Tibia peripheral quantitative computed tomography measures were made of cortical bone mineral content (BMC) and bone mineral density (BMD), periosteal (Peri) and endosteal circumferences, section modulus (Zp), and muscle area. Regression models were adjusted for tibia length, age, race, sex, and Tanner stage.
Results: All cortical measures were greater in blacks than whites (all P ≤ 0.001) in Tanner stages 1–4; however, differences in BMC, Peri, and Zp were negligible in Tanner stage 5 (all interactions, P < 0.01). Cortical BMC, Peri, and Zp were lower in females than males in all Tanner stages (all P < 0.001), and the sex differences in Peri and Zp were greater in Tanner stage 5 (interaction, P < 0.02). Cortical BMD was greater (P < 0.0001) and endosteal circumference was lower (P < 0.01) in Tanner 3–5 females, compared with males. Adjustment for muscle area attenuated but did not eliminate sex and race differences in cortical dimensions. Associations between muscle and bone outcomes did not differ according to sex or race.
Conclusion: Sex and race were associated with maturation-specific differences in cortical BMD and dimensions that were not fully explained by differences in bone length or muscle. No race or sex differences in the functional muscle bone unit were identified.
Sex and race are associated with maturation-specific differences in cortical bone mineral density and dimensions that are not fully explained by differences in muscle mass.
Skeletal growth is characterized by increases in cortical density and dimensions. The absolute and relative patterns of modeling on the periosteal and endosteal surfaces produce changes in cortical geometry that impact lifelong fracture risk (1,2). It is well-established that sex differences in cortical bone accrual during growth contribute to sex differences in skeletal fragility (3). Although fracture rates are significantly lower in black adults compared with whites (4), racial differences in cortical bone development have not been well-characterized.
Bone grows in response to the forces to which it is subjected (5). This capacity of bone to respond to mechanical loading with increased strength is greatest during growth (6). Hormones and nutrients affect mechanical loads by influencing linear growth and muscle mass and may alter the muscle-bone set point (7). Prior studies have suggested that females develop greater cortical bone relative to muscle mass during puberty, compared with males (7,8,9). To our knowledge, no studies have assessed race differences in the muscle-bone relations during development.
Sex and race differences in body proportions, body composition, and maturation relative to age may contribute to observed differences in cortical geometry and bone mineral density (BMD) and to differences in the relations between cortical bone and muscle mass. The objectives of this study were: 1) to determine the effects of sex and race on cortical volumetric BMD and structure in 665 healthy children and young adults, independent of body proportions, body composition, and maturation; and 2) to determine whether the relations between cortical bone and muscle (the functional muscle bone unit) differ according to race or sex.
Subjects and Methods
Study subjects
Children and young adults, ages 5 to 35 yr, were enrolled as healthy control subjects for bone studies at the Children’s Hospital of Philadelphia (CHOP). Participants were recruited from general pediatric and internal medicine clinics and the community using advertisements. Exclusion criteria included chronic diseases or medications known to affect growth, nutrition, or bone health. The protocols were approved by the Institutional Review Board at CHOP. Informed consent was obtained from young adult participants and the parents or guardians of those less than 18 yr of age.
Assessment of anthropometrics, pubertal development, and race
Weight and height were measured using a digital scale (Scaltronix, White Plains, NY) and stadiometer (Holtain Ltd., Crymych, UK), respectively. The same equipment was used in all participants. Age- and sex-specific Z-scores for height and body mass index (BMI, kg/m2) were calculated for subjects less than 20 yr of age (the upper bound of the reference data) (10). Pubertal development was determined using a validated self-assessment questionnaire (with parental assistance as necessary) in pediatric participants (11,12).
Participants self-identified race according to National Institute of Health categories. Among the 734 participants, 16 were Asian, seven were American Indian/Alaska Native, and 46 were of mixed race or declined to self-categorize. The data presented here are limited to the remaining 306 and 359 participants self-identified as black or white, respectively. Of these, five whites and two blacks identified their ethnicity as Hispanic.
Peripheral quantitative computed tomography (pQCT) assessment of bone and muscle
pQCT (Stratec XCT-2000; Orthometrix, Inc., White Plains, NY; software version 550) scans were obtained in the left tibia with slice thickness 2.3 mm and voxel size 0.4 mm. Cortical bone was assessed 38% proximal to the distal physis using cortmode 2 (threshold, 711 mg/cm3). Cortical measures included volumetric BMD (mg/cm3), periosteal and endosteal circumferences (mm), bone mineral content (BMC, mg/mm) and bone area (mm2) within the region defined by the endosteal and periosteal surfaces, and polar section modulus (Zp, mm3). Zp provides a composite measure of the effects of bone geometry on bone strength (13). Muscle and fat area (mm2) were assessed 66% proximal to the distal physis using threshold 40 mg/cm3 for fat-lean separation and 711 mg/cm3 for lean-bone separation. Quality control was monitored daily using a phantom. The coefficient of variation ranged from 0.5–1.6% for pQCT outcomes.
Statistical analysis
Analyses were conducted using STATA 10.0 (StataCorp, College Station, TX). Differences in means were assessed using Student’s t test or the Wilcoxon rank sum test as appropriate. Group differences in categorical variables were assessed using the χ2 test.
Natural log transformations resulted in linear relations between tibia length and pQCT measures of BMC, cortical geometry, and muscle area; therefore, these measures were log transformed in all analyses. The correlations (R) between log-transformed tibia length and pQCT outcomes were greater than 0.92 for cortical BMC, Zp, and periosteal circumference; 0.68 for endosteal circumference; and 0.87 for muscle area (all P < 0.001).
Log linear regression models examining pQCT outcomes included tibia length, race (black vs. white), sex (female vs. male), Tanner stage (stage 1 as the referent group), and age. Age and age2 were included in the models, as needed, to capture nonlinear relations. Multiplicative interaction terms were also included for combinations of sex, race, and Tanner stage. The sex effects did not differ according to race, and the race effects did not differ according to sex (tested using stratified models and sex-Tanner-race interaction terms); therefore, the sex and race effects are summarized in combined models. To determine whether race and sex differences in muscle or fat area explained observed differences in bone outcomes, the bone models were subsequently adjusted for muscle and fat area. To determine whether the relations between muscle and bone differed according to sex or race, multiplication interaction terms were included for sex-muscle, and race-muscle for cortical outcomes.
The magnitude of the effect of a covariate (e.g. race), on average, adjusting for other covariates in the model can be expressed as the exponentiated β-coefficient for that covariate. For example, the exponentiated β-coefficient for the race variable represents the ratio of the outcome value in blacks divided by the value in whites, adjusted for age, sex, tibia length, Tanner stage, and interaction terms. A β-coefficient of 0.095 would represent a ratio of 1.10 (= e0.095), indicating that the outcome is 10% greater in blacks, compared with whites, adjusted for all other covariates in the model.
Results
Study subject characteristics
The 665 participants are described in Table 1; 557 (84%) were 20 yr of age or less. Overall, the white subjects were older than the black subjects (P < 0.001) due to the greater proportion of whites in the adult group (76%) than the pediatric group (50%). Among peripubertal subjects (Tanner stages 2–4), blacks were younger than whites relative to Tanner stage (P < 0.0001 in males, P = 0.02 in females), consistent with more advanced maturation relative to age in blacks, compared with whites (14,15).
Table 1.
Demographic and anthropometric characteristics of subjects according to sex and race
| Males
|
Females
|
|||
|---|---|---|---|---|
| White | Black | White | Black | |
| n | 175 | 135 | 184 | 171 |
| Age (yr) | 15.3 ± 7.2 | 12.4 ± 5.1 | 15.0 ± 7.4 | 12.4 ± 5.9 |
| n per Tanner stage | ||||
| Stage 1 | 52 | 54 | 53 | 62 |
| Stage 2 | 18 | 14 | 18 | 16 |
| Stage 3 | 14 | 14 | 17 | 20 |
| Stage 4 | 20 | 27 | 37 | 33 |
| Stage 5 | 71 | 26 | 59 | 40 |
| Height (cm) | 157.7 ± 23.1 | 149.8 ± 19.0 | 151.1 ± 17.7 | 147.1 ± 17.7 |
| Height Z-scorea | 0.23 ± 0.82 | 0.25 ± 0.91 | 0.25 ± 0.80 | 0.52 ± 1.02 |
| BMI (kg/m2) | 20.0 ± 3.8 | 20.8 ± 5.4 | 19.7 ± 4.0 | 20.8 ± 5.1 |
| BMI Z-scorea | 0.13 ± 0.85 | 0.64 ± 1.1 | 0.15 ± 0.86 | 0.59 ± 1.1 |
| BMI (kg/m2)b | 23.8 ± 2.5 | 24.1 ± 2.9 | 22.7 ± 4.0 | 25.8 ± 5.5 |
All values are expressed as means ± sd, unless otherwise noted.
Z-scores are limited to subjects 20 yr of age or less because the national reference data for growth Z-scores are available through age 20.0 yr (10).
BMI limited to subjects greater than 20 yr of age.
Among subjects 20 yr of age or less, height Z-scores did not differ according to race within the males. Height Z-scores in the black females were significantly greater, compared with each of the other three sex–race categories in Table 1 (all P < 0.02). Among males and females 20 yr of age or less, BMI Z-scores were significantly greater in blacks vs. whites (P < 0.0001), consistent with recognized racial differences in BMI (16). Among subjects 20 yr of age or older, mean BMI was significantly greater in black females vs. white females (P < 0.02).
Sex and race differences in body proportions
Multivariate log linear regression models were used to assess sex and race differences in body proportions, indexed by tibia length relative to height, adjusted for Tanner stage and age. On average, tibia length relative to height was 4.7% greater [95% confidence interval (CI), 3.8, 5.5; P < 0.0001) in blacks than whites, and it was 1.2% greater (95% CI, 0.3, 2.0; P < 0.01) in females than males. These differences were comparable across Tanner stages. Subjects in Tanner stage 5 had 3.4% shorter tibia length relative to height (95% CI, 1.1, 5.8; P < 0.01) compared with earlier Tanner stages, consistent with greater axial growth in late puberty.
Sex and race differences in cortical BMC and geometry
The full multivariate regression model for Zp is provided to illustrate the independent effects of tibia length, age, sex, race, and Tanner stage (Table 2). Zp was positively associated with tibia length and demonstrated a nonlinear relation with age. Zp among participants in Tanner stages 2 or 3 was not significantly different than Tanner 1 but was significantly and progressively greater in Tanner stages 4 (P < 0.05) and 5 (P < 0.0001) compared with Tanner stage 1, adjusted for the other covariates.
Table 2.
Multivariate regression model for cortical Zp (R2 = 0.88)
| β (95% CI) | P | |
|---|---|---|
| Intercept | −5.89 (−6.96, −4.82) | <0.0001 |
| Ln (tibia length) | 2.17 (1.97, 2.37) | <0.0001 |
| Age | 0.032 (0.014, 0.051) | <0.001 |
| Age2 | −0.00067 (−0.001, −0.0003) | <0.001 |
| Tanner stage 2 | −0.107 (−0.261, 0.046) | 0.17 |
| Tanner stage 3 | 0.108 (−0.053, 0.269) | 0.19 |
| Tanner stage 4 | 0.185 (0.041, 0.331) | <0.05 |
| Tanner stage 5 | 0.307 (0.180, 0.433) | <0.0001 |
| Black race | 0.126 (0.080, 0.172) | <0.0001 |
| Black race by Tanner interaction | ||
| Black, Tanner 2 | 0.024 (−0.068, 0.115) | 0.62 |
| Black, Tanner 3 | −0.035 (−0.127, 0.057) | 0.46 |
| Black, Tanner 4 | 0.002 (−0.075, 0.078) | 0.94 |
| Black, Tanner 5 | −0.101 (−0.168, −0.033) | 0.003 |
| Female sex | −0.087 (−0.131, −0.042) | <0.0001 |
| Female sex by Tanner interaction | ||
| Female, Tanner 2 | 0.087 (−0.005, 0.178) | 0.06 |
| Female, Tanner 3 | −0.017 (−0.110, 0.076) | 0.71 |
| Female, Tanner 4 | −0.053 (−0.128, 0.023) | 0.17 |
| Female, Tanner 5 | −0.084 (−0.150, −0.018) | 0.012 |
Black race had a significant and independent effect on Zp (P < 0.001). Adjusted for the other covariates, Zp was 13.4% (β = 0.126) greater in blacks vs. whites in Tanner stage 1. This effect varied according to Tanner stage, as indicated by the significant black-Tanner 5 interaction term. The effect of black race was comparable across Tanner stages 1–4, but was attenuated in Tanner stage 5. The combined β-coefficients (0.126–0.101 = 0.025) for black race and the black-Tanner 5 interaction indicate that Zp was only 2.5% greater in blacks in Tanner 5, compared with whites.
Female sex had a significant and independent effect on Zp (P < 0.0001). Adjusted for the other covariates, Zp was 8.3% lower in females vs. males in Tanner stage 1. Zp was comparably lower in females vs. males in Tanner stages 1–4; however, in Tanner stage 5, the sex difference was larger. The combined β-coefficients (−0.087 − 0.084 = −0.171) for sex and the sex-Tanner 5 interaction demonstrate that Zp was 15.7% lower in females in Tanner 5, compared with males, adjusted for the other covariates.
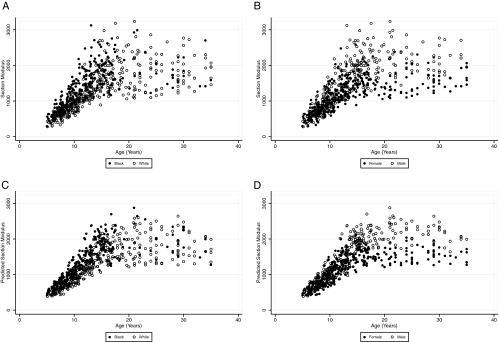
Figure 1 illustrates the sex and race differences in Zp according to age. Panels A and B represent the unadjusted results, illustrating the race and sex differences, respectively. Panels C and D represent the predicated values for Zp relative to age based on the model in Table 2, illustrating the race and sex effects, respectively.
Figure 1.
Sex and race differences in Zp according to age. A, Unadjusted results comparing whites (open circles) and blacks (filled circles). B, Unadjusted results comparing males (open circles) and females (filled circles). C, Race differences in the predicated values for Zp relative to age based on the model in Table 2, comparing whites (open circles) and blacks (closed circles). D, Sex differences in the predicated values for Zp relative to age based on the model in Table 2, comparing males (open circles) and females (closed circles). All 655 participants are included in each panel.
Comparable adjusted models were generated for cortical area, cortical BMC, and periosteal circumference, and the significant sex and race effects are summarized in Table 3. The sex, race, and interaction effects were similar to those observed for Zp, with one exception: the negative female-Tanner interaction term was not significant for cortical BMC (related to the significantly greater cortical BMD in pubertal females compared with males described below).
Table 3.
Summary of race and sex differences in cortical bone outcomes
| Outcome | Black race effect | Female sex effect | Black race effect adjusted for muscle | Female sex effect adjusted for muscle |
|---|---|---|---|---|
| Zp | R2 = 0.88 | R2 = 0.92 | ||
| β coefficient | 0.126, P < 0.0001 | −0.087, P < 0.0001 | 0.097, P < 0.0001 | −0.040, P = 0.029 |
| Tanner 5 interaction β | −0.101, P = 0.003 | −0.084, P = 0.012 | −0.072, P = 0.011 | −0.055, P = 0.045 |
| Cortical area | R2 = 0.88 | R2 = 0.92 | ||
| β coefficient | 0.076, P < 0.0001 | −0.076, P < 0.0001 | 0.054, P < 0.0001 | −0.042, P = 0.002 |
| Tanner 5 interaction β | −0.078, P = 0.002 | −0.050, P = 0.04 | −0.056, P = 0.005 | NS |
| Cortical BMC | R2 = 0.91 | R2 = 0.94 | ||
| β coefficient | 0.092, P < 0.0001 | −0.069, P < 0.0001 | 0.070, P < 0.0001 | −0.035, P = 0.006 |
| Tanner 5 interaction β | −0.089, P = 0.001 | NS | −0.067, P < 0.0001 | NS |
| Periosteal circumference | R2 = 0.88 | R2 = 0.92 | ||
| β coefficient | 0.038, P < 0.0001 | −0.027, P < 0.001 | 0.028, P < 0.0001 | −0.011, P = 0.08 |
| Tanner 5 interaction β | −0.033, P = 0.005 | −0.029, P = 0.014 | −0.024, P = 0.017 | NS |
| Endosteal circumference | R2 = 0.51 | R2 = 0.53 | ||
| β coefficient | 0.035, P = 0.001 | NS | 0.029, P = 0.006 | NS |
| Tanner 3 interaction β | NS | −0.062, P = 0.08 | NS | −0.070, P = 0.04 |
| Tanner 4 interaction β | NS | −0.081, P = 0.006 | NS | −0.079, P = 0.006 |
| Tanner 5 interaction β | NS | −0.046, P = 0.06 | NS | −0.036, P = 0.14 |
| Cortical volumetric BMD | R2 = 0.73 | |||
| β coefficient | 0.024, P < 0.0001 | NS | Not applicablea | |
| Tanner 3 interaction β | NS | 0.033, P < 0.0001 | ||
| Tanner 4 interaction β | NS | 0.039, P < 0.0001 | ||
| Tanner 5 interaction β | −0.012, P = 0.05 | 0.023, P < 0.0001 | ||
All results are adjusted for tibia length, age, Tanner stage, sex, and race; an example is shown in Table 2. NS, Not significant.
Cortical volumetric BMD was not associated with muscle cross-sectional area in the adjusted model.
The model for endosteal circumference revealed a different pattern. Blacks had a 3.6% greater endosteal circumference than whites (P = 0.001) across all Tanner stages, adjusted for tibia length, age, and sex. There was no evidence of a race-Tanner interaction. In contrast, endosteal circumference was comparable in males and females during Tanner stages 1 and 2. The sex-Tanner interaction was most pronounced in Tanner stage 4, where endosteal circumference was 7.8% lower in females vs. males (P = 0.006). Overall endosteal circumference was 6% lower in Tanner stages 3–5 females vs. males (P = 0.002).
Sex and race differences in cortical volumetric BMD
Cortical BMD was 2.4% greater (P < 0.0001) in blacks vs. whites, adjusted for the other covariates. This effect was marginally attenuated in Tanner stage 5 (P = 0.05). Cortical BMD was comparable in males and females in Tanner stages 1 and 2. However, cortical BMD was significantly greater in Tanner stages 3, 4, and 5 in females vs. males (P < 0.0001). Figure 2 illustrates the race and sex differences in cortical BMD according to age. Panels A and B represent the unadjusted results, and panels C and D represent the predicated values for cortical BMD relative to age, based on the model in Table 3.
Figure 2.
Sex and race differences in cortical BMD according to age. A, Unadjusted results comparing whites (open circles) and blacks (filled circles). B, Unadjusted results comparing males (open circles) and females (filled circles). C, Race differences in the predicated values for cortical BMD relative to age based on the model in Table 3, comparing whites (open circles) and blacks (closed circles). D, Sex differences in the predicated values for cortical BMD relative to age based on the model in Table 3, comparing males (open circles) and females (closed circles). All 655 participants are included in each panel.
Sex and race differences in muscle and fat area
Multivariate models were used to assess sex and race differences in muscle area relative to tibia length, adjusted for Tanner stage, sex, and age. Muscle area was 4.5% greater (95% CI, 0.46, 8.9; P < 0.01) in blacks vs. whites. Muscle area was 6.8% lower (95% CI, 3.1, 10.2; P < 0.0001) in females vs. males. Muscle area was significantly and progressively greater in Tanner stages 4 (P < 0.01) and 5 (P < 0.0001), adjusted for the other covariates in the model. Sex-Tanner and race-Tanner interaction terms were not significant.
Fat area was not significantly different in blacks and whites, adjusted for the other covariates (difference, 1.6% lower in blacks; 95% CI, −12.0, 10.0). Fat area was 30% (95% CI, 7.9, 56.8; P < 0.01) and 77% (95% CI, 51, 108; P < 0.0001) greater in females, compared with males in Tanner stages 4 and 5, respectively.
Impact of sex and race differences in muscle and fat area on cortical bone outcomes
Each model summarized in Table 3 was repeated, adjusted for muscle area. Muscle area was highly significantly (P < 0.0001) and independently associated with all cortical outcomes except BMD (P = 0.47). Table 3 summarizes the effects of adjustment for muscle area on the β-coefficients for the sex and race effects, including interaction terms. Adjustment for muscle attenuated the impact of black race on the cortical BMC and dimensions; however, the positive effect of black race remained significant, and the negative race-Tanner stage 5 interactions persisted. Adjustment for muscle also attenuated the impact of sex on cortical BMC and dimensions. The significant sex-Tanner 5 interaction terms for cortical area and periosteal circumference were not significant after adjustment for the relatively lower muscle in Tanner stage 5 females. Muscle was not associated with cortical BMD; therefore, the BMD models were not adjusted for muscle area.
The models summarized in Table 3 were also adjusted for fat area. Greater fat area was associated with greater muscle area (P < 0.0001) adjusted for tibia length, age, sex, Tanner stage, and race. Fat area was not significantly associated with any of the bone outcomes, independent of muscle area.
Lastly, evidence for sex or race differences in the functional muscle-bone unit was examined by including sex-muscle and race-muscle interaction terms in each model summarized in Table 3. None of these interaction terms was significant (all P > 0.3), indicating that the relations between muscle area and cortical BMC or geometry did not differ according to sex or race. Because prior studies have suggested that sex differences in the muscle-bone unit are evident after puberty (8,17), the tests for sex-muscle interactions were repeated and limited to the 313 Tanner 4–5 subjects; none of these interactions was significant (all P > 0.2).
Discussion
These data confirm well-recognized sex differences in cortical bone dimensions (3) and extend these observations to demonstrate the magnitude of the differences relative to tibia length and Tanner stage over a broad age range. These data are also consistent with a pQCT report that cortical BMD was greater in adolescent and adult females, compared with males (18). These data further demonstrate that cortical BMC, BMD, and dimensions were significantly greater in blacks compared with whites in Tanner stages 1 through 4; however, in Tanner stage 5, the differences in BMC and dimensions were negligible, and the differences in BMD were attenuated. Importantly, the significant sex and race differences in body proportions, body composition, and maturation observed here highlight the importance of adjusting for these factors in assessing the independent effects of sex and race on cortical bone.
Sex hormones play a vital role in bone development during puberty and in bone preservation throughout life. In a longitudinal pQCT study in peripubertal females, Wang et al. (19) reported that greater free estradiol levels were associated with smaller tibia endosteal but not periosteal circumferences. In contrast, testosterone levels were associated with greater periosteal and endosteal circumferences. The effects of sex hormones on cortical volumetric BMD during development are less clear. In the studies by Wang et al. (19,20), estradiol was not associated with cortical BMD. However, a cross-sectional pQCT study in healthy men, ages 25–45 yr, demonstrated that estradiol levels were positively associated with tibia cortical BMD (21).
Given the strong associations between muscle and bone, investigators advocate assessing bone outcomes relative to muscle (22). Schiessl et al. (7) hypothesized that estrogen lowers the bone remodeling threshold, such that females experience a greater gain in bone mass during puberty compared with males of similar muscle mass. Two studies support this hypothesis. First, Schoenau et al. (8) used pQCT to examine the influence of puberty on forearm muscle area and cortical bone area in 318 healthy children and their parents. The muscle-bone unit was quantified as the ratio of cortical area divided by muscle area. Before puberty, the ratios were comparable in males and females. However, the ratios were progressively and significantly greater in females compared with males in Tanner stages 4 and 5. Second, Hogler et al. (17) used magnetic resonance imaging and dual-energy x-ray absorptiometry (DXA) to estimate cortical volumetric BMD and dimensions in the femur in 83 Tanner 1 children and 44 young adults. When compared relative to muscle mass, the mature women had greater total and cortical bone area than the men.
In contrast, our analyses did not provide evidence of a sex-muscle interaction in the models for cortical BMC and geometry. That is, the highly significant positive association between cortical measures and muscle area did not differ significantly between males and females, adjusted for age, race, Tanner stage, and tibia length. Possible explanations for our different results include different measurement sites and techniques. In addition, differences in the statistical methods may have contributed to the observed differences. For example, the use of a ratio to assess cortical area-muscle area relations does not incorporate adjustments for sex differences in tibia lengths and potentially introduces statistical errors because muscle area and bone measures do not scale isometrically (23,24). Furthermore, the regression models described by Hogler et al. (17) do not include adjustment for bone length, and other findings in their study differed from ours: there were no sex differences in bone or muscle variables in children or in cortical BMD in adults. Finally, a recent 7-yr longitudinal pQCT study in pubertal females raised questions regarding the impact of muscle forces on bone growth: the peak velocity for bone area occurred 1 yr earlier than the peak velocity for muscle area (25).
To our knowledge, quantitative computed tomography (QCT) data on race differences in cortical bone in children are limited to two studies. Gilsanz et al. (26) obtained QCT scans in the femur midshaft in 80 black and 80 white children, matched on age, sex, height, weight, and Tanner stage. Cortical BMD did not differ according to sex, race, or Tanner stage. Overall, the length and the cross-sectional area of the femur were significantly greater in black children; however, the cortical area did not differ. The authors concluded that the greater cross-sectional area and similar cortical bone area would manifest as “the same amount of cortical bone placed further from the center of the bone, resulting in greater bone strength.” This pattern is consistent with our finding of greater periosteal circumference, endosteal circumference, and Zp in black children and adolescents in Tanner stages 1 through 4. The Gilsanz study (26) did not address whether the race effects differed according to Tanner stage or whether race differences in cortical geometry persisted after adjustment for the greater femur length in the black subjects. The second study assessed pQCT measures in 21 white and 23 black children, ages 9–12 yr (27). Tibia cortical BMD and total area were significantly greater in blacks compared with whites, adjusted for age, sex, tibia length, and muscle area. Given the limited age range, the study could not address whether the race differences were attenuated in mature participants. These two studies confirmed race differences in cortical geometry, provided conflicting reports for race differences in cortical BMD, and did not address race-Tanner interactions.
A recent QCT study provides additional insight into race differences in cortical bone in young adults (28), consistent with our observations. Saeed et al. (28) examined 60 white and 42 black adults, ages 35 to 45 yr, and reported that black males had greater cortical BMD in the femur compared with white males. Within males and females, there were no race differences in femoral neck cross-sectional area, adjusted for age, height, and weight. In a related study, Peacock et al. (29) reported similar results in premenopausal women ages 23 to 57 yr and in men ages 20 to 63 yr. Blacks had greater cortical BMD in the femoral shaft compared with whites, whereas femoral neck area did not differ. Men also had lower cortical BMD compared with women.
Taken together, the two studies in children and the two studies in non-elderly adults confirm our observation of race differences in bone geometry in pre- and peripubertal children and adolescents that are not present in young adults. These studies also support our observation of race differences in cortical volumetric BMD. None of these studies addressed whether the relations between muscle and bone differed according to race.
The data presented here provide insight into the structural basis for sex differences in DXA BMD in children (15) and young adults (30,31). However, the absence of significant race differences in young adults seen here is in variance with prior DXA studies (30,31), potentially due to inclusion of trabecular bone in the DXA scans and race difference in body proportions.
The greatest limitation of our study is the lack of longitudinal measures. Additional limitations include self-assessment of pubertal stage, lack of dietary intake and physical activity data, and lack of measures of vitamin D, PTH, growth factors, and cytokines to potentially explain the maturation-specific sex and race differences in cortical BMD and dimensions. However, the models explained at least 92% of the variability in cortical BMC and Zp, suggesting that a substantial proportion of the determinants of these measures is captured by these data. Furthermore, this is the largest study of race and sex differences in children and adolescents; the first comprehensive study to examine race and sex differences in children, adolescents, and young adults; and the first to investigate race differences in the relation between muscle and bone.
The data presented here may be useful for generating hypotheses about race and sex differences in fracture risk and highlight the importance of sex- and race-specific reference data for studies of disease effects on bone accrual and peak bone mass.
Footnotes
This work was supported by National Institutes of Health Grants R01-DK064966, R01-HD040714, R01-DK60030, and K24-DK076808; the University of Pennsylvania Clinical Translational Research Center (Grant UL1-RR024134); the Children’s Hospital of Philadelphia Research Institute; and the Nutrition Center at the Children’s Hospital of Philadelphia.
Disclosure Summary: The authors have no potential conflicts of interest.
First Published Online February 15, 2010
Abbreviations: BMC, Bone mineral content; BMD, bone mineral density; BMI, body mass index; CI, confidence interval; DXA, dual-energy x-ray absorptiometry; pQCT, peripheral QCT; QCT, quantitative computed tomography; Zp, section modulus.
References
- Duan Y, Beck TJ, Wang XF, Seeman E 2003 Structural and biomechanical basis of sexual dimorphism in femoral neck fragility has its origins in growth and aging. J Bone Miner Res 18:1766–1774 [DOI] [PubMed] [Google Scholar]
- Seeman E 2008 Structural basis of growth-related gain and age-related loss of bone strength. Rheumatology (Oxford) 47(Suppl 4):iv2–iv8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E 2002 Pathogenesis of bone fragility in women and men. Lancet 359:1841–1850 [DOI] [PubMed] [Google Scholar]
- Baron JA, Barrett J, Malenka D, Fisher E, Kniffin W, Bubolz T, Tosteson T 1994 Racial differences in fracture risk. Epidemiology 5:42–47 [DOI] [PubMed] [Google Scholar]
- Rauch F, Schoenau E 2001 The developing bone: slave or master of its cells and molecules? Pediatr Res 50:309–314 [DOI] [PubMed] [Google Scholar]
- Parfitt AM 1994 The two faces of growth: benefits and risks to bone integrity. Osteoporos Int 4:382–398 [DOI] [PubMed] [Google Scholar]
- Schiessl H, Frost HM, Jee WS 1998 Estrogen and bone-muscle strength and mass relationships. Bone 22:1–6 [DOI] [PubMed] [Google Scholar]
- Schoenau E, Neu CM, Mokov E, Wassmer G, Manz F 2000 Influence of puberty on muscle area and cortical bone area of the forearm in boys and girls. J Clin Endocrinol Metab 85:1095–1098 [DOI] [PubMed] [Google Scholar]
- Högler W, Blimkie CJ, Cowell CT, Kemp AF, Briody J, Wiebe P, Farpour-Lambert N, Duncan CS, Woodhead HJ 2003 A comparison of bone geometry and cortical density at the mid-femur between prepuberty and young adulthood using magnetic resonance imaging. Bone 33:771–778 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer- Strawn LM, Curtin LR, Roche AF, Johnson CL 2002 Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:45–60 [DOI] [PubMed] [Google Scholar]
- Tanner JM 1962 Growth at adolescence. 2nd ed. Oxford, UK: Blackwell Scientific Publication [Google Scholar]
- Morris NM, Udry JR 1980 Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adoles 9:271–280 [DOI] [PubMed] [Google Scholar]
- Liu D, Manske SL, Kontulainen SA, Tang C, Guy P, Oxland TR, McKay HA 2007 Tibial geometry is associated with failure load ex vivo: a MRI, pQCT and DXA study. Osteoporos Int 18:991–997 [DOI] [PubMed] [Google Scholar]
- Wu T, Mendola P, Buck GM 2002 Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988–1994. Pediatrics 110:752–757 [DOI] [PubMed] [Google Scholar]
- Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, Shepherd JA 2007 The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab 92:2087–2099 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Flegal KM, Carroll MD, Johnson CL 2002 Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 288:1728–1732 [DOI] [PubMed] [Google Scholar]
- Högler W, Blimkie CJ, Cowell CT, Inglis D, Rauch F, Kemp AF, Wiebe P, Duncan CS, Farpour-Lambert N, Woodhead HJ 2008 Sex-specific developmental changes in muscle size and bone geometry at the femoral shaft. Bone 42:982–989 [DOI] [PubMed] [Google Scholar]
- Schoenau E, Neu CM, Rauch F, Manz F 2002 Gender-specific pubertal changes in volumetric cortical bone mineral density at the proximal radius. Bone 31:110–113 [DOI] [PubMed] [Google Scholar]
- Wang Q, Alén M, Nicholson PH, Halleen JM, Alatalo SL, Ohlsson C, Suominen H, Cheng S 2006 Differential effects of sex hormones on peri- and endocortical bone surfaces in pubertal girls. J Clin Endocrinol Metab 91:277–282 [DOI] [PubMed] [Google Scholar]
- Wang Q, Nicholson PH, Suuriniemi M, Lyytikäinen A, Helkala E, Alen M, Suominen H, Cheng S 2004 Relationship of sex hormones to bone geometric properties and mineral density in early pubertal girls. J Clin Endocrinol Metab 89:1698–1703 [DOI] [PubMed] [Google Scholar]
- Lapauw BM, Taes Y, Bogaert V, Vanbillemont G, Goemaere S, Zmierczak HG, De Bacquer D, Kaufman JM 2009 Serum estradiol is associated with volumetric BMD and modulates the impact of physical activity on bone size at the age of peak bone mass: a study in healthy male siblings. J Bone Miner Res 24:1075–1085 [DOI] [PubMed] [Google Scholar]
- Schoenau E, Neu CM, Beck B, Manz F, Rauch F 2002 Bone mineral content per muscle cross-sectional area as an index of the functional muscle-bone unit. J Bone Miner Res 17:1095–1101 [DOI] [PubMed] [Google Scholar]
- Raubenheimer D 1995 Problems with ratio analysis in nutritional studies. Funct Ecol 9:21–29 [Google Scholar]
- Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB 1995 Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord 19:644–652 [PubMed] [Google Scholar]
- Xu L, Nicholson P, Wang Q, Alén M, Cheng S 2009 Bone and muscle development during puberty in girls: a 7-year longitudinal study. J Bone Miner Res 24:1693–1698 [DOI] [PubMed] [Google Scholar]
- Gilsanz V, Skaggs DL, Kovanlikaya A, Sayre J, Loro ML, Kaufman F, Korenman SG 1998 Differential effect of race on the axial and appendicular skeletons of children. J Clin Endocrinol Metab 83:1420–1427 [DOI] [PubMed] [Google Scholar]
- Wetzsteon RJ, Hughes JM, Kaufman BC, Vazquez G, Stoffregen TA, Stovitz SD, Petit MA 2009 Ethnic differences in bone geometry and strength are apparent in childhood. Bone 44:970–975 [DOI] [PubMed] [Google Scholar]
- Saeed I, Carpenter RD, Leblanc AD, Li J, Keyak JH, Sibonga JD, Lang TF 2009 Quantitative computed tomography reveals the effects of race and sex on bone size and trabecular and cortical bone density. J Clin Densitom 12:330–336 [DOI] [PubMed] [Google Scholar]
- Peacock M, Buckwalter KA, Persohn S, Hangartner TN, Econs MJ, Hui S 2009 Race and sex differences in bone mineral density and geometry at the femur. Bone 45:218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker AC, Melton 3rd LJ, Harris T, Borrud L, Shepherd J, McGowan J 2009 Age, gender, and race/ethnic differences in total body and subregional bone density. Osteoporos Int 20:1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston Jr CC, Lindsay R 1998 Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8:468–489 [DOI] [PubMed] [Google Scholar]