Abstract
The paraventricular nucleus of the hypothalamus (PVH) plays an important role in energy homeostasis, regulating neuroendocrine, behavioral, and autonomic functions. However, the role of the PVH in regulating thermogenesis and energy expenditure in brown adipose tissue (BAT) is unclear. The present study investigated the effect of activating neurons within the PVH on BAT thermogenesis. In urethane- and chloralose-anesthetized, artificially ventilated rats maintained at a core body temperature of 37.0–38.0°C, microinjection of N-methyl-d-aspartate (NMDA, 12 pmol in 60 nl) in the PVH did not increase BAT sympathetic nerve activity (SNA) or BAT thermogenesis. In contrast, the increase in BAT SNA evoked by body cooling was completely reversed by microinjection of NMDA in the PVH. Additionally, the increases in BAT SNA evoked by body cooling, by microinjection of prostaglandin E2 (170 pmol in 60 nl) in the medial preoptic area or by microinjection of bicuculline (30 pmol in 60 nl) in the dorsomedial hypothalamus were completely reversed by microinjection of bicuculline (30 pmol in 60 nl) in the PVH. Although the increases in BAT SNA and thermogenesis evoked by microinjection of NMDA (12 pmol in 60 nl) in the raphe pallidus (RPa) was markedly attenuated following microinjection of bicuculline (30 pmol) in the PVH, the increases in BAT SNA and thermogenesis evoked by microinjection of bicuculline (30 pmol in 60 nl) in the RPa were unaffected by microinjection of bicuculline in the PVH. These results demonstrate that disinhibition of neurons in the PVH inhibits BAT SNA likely via activation of a GABAergic input to BAT sympathetic premotor neurons in the RPa.
Keywords: thermoregulation, sympathetic premotor neurons, raphe, paraventricular nucleus, metabolism
brown adipose tissue (BAT) can contribute to energy homeostasis in rodents (22) and humans (32) through its mitochondrial uncoupling protein 1 (UCP-1)-mediated metabolism of free fatty acids to produce heat for thermoregulation and for metabolic energy expenditure. The paraventricular nucleus of the hypothalamus (PVH) plays an important role in energy homeostasis, regulating autonomic and neuroendocrine functions as well as behavior. The PVH contains a large number of neurons that are labeled following injection of transsynaptic viral tracer into BAT (3, 6, 34, 51), supporting the widely accepted role for PVH neurons in driving sympathetic outflow to BAT (29, 45, 47). However, the physiological evidence supporting a role of the PVH in regulating sympathetically mediated thermogenesis and energy expenditure in BAT remains unclear.
Increases in BAT and core body temperatures following microinjection of glutamate in the PVH has led to the suggestion that stimulation of neurons in the PVH activates thermogenesis in BAT (1). However, this conclusion is questionable since 1) high concentrations of glutamate (100–500 mM) were used, 2) there was no histological localization of the injection sites, 3) appropriate anatomical control injections were not performed, and 4) activation of neurons in the dorsomedial hypothalamus (DMH), located in close proximity to the PVH, increases the sympathetic outflow to BAT (7, 25, 52, 53).
A role for PVH neurons in driving the sympathetic outflow to BAT has also been suggested from the ability of PVH lesions to reduce the febrile-evoked increases in body temperature (4, 16, 23). Although BAT thermogenesis contributes to fever (5, 14), the effects of PVH lesions on BAT activation during fever are difficult to determine from these studies since no specific indexes of BAT thermogenesis were measured. The reductions in febrile increases in core body temperature following PVH lesions could have been due, for instance, to a reduction in cutaneous vasoconstriction or in adrenal catecholamine secretion. Thus, in the present study, we sought to provide a direct assessment of the influence of PVH neuron activation on BAT sympathetic nerve activity (SNA) and thermogenesis.
MATERIALS AND METHODS
All procedures conform to the regulations detailed in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Oregon Health and Science University. All efforts were made to minimize animal suffering and to limit the number of animals used. Male Sprague-Dawley rats (n = 55; Charles River, Indianapolis, IN) weighing 250–450 g were kept on a 12:12-h light-dark cycle and given ad libitum access to standard rat chow and water in a colony room maintained at 22–23°C. Rats were anesthetized with isoflurane (2–3% in oxygen), instrumented with femoral arterial and venous catheters, and transitioned to urethane and chloralose anesthesia (750 mg/kg and 60 mg/kg iv, respectively) over a 10-min period. All physiological variables were digitized [Micro 1401 MKII; Cambridge Electronic Design (CED), Cambridge, UK] and recorded on a computer hard drive for subsequent analysis (Spike 2; CED). Arterial blood pressure was recorded from the arterial catheter attached to a pressure transducer, and heart rate (HR) was derived from the arterial pressure signal. The trachea was cannulated, and the animals were ventilated (tidal volume: ∼1 ml/100 g body wt, ∼60 cycles/min) with 100% oxygen. End-expiratory CO2 was monitored using a capnometer (model 2200; Dynatech Electro-optics, Saline, MI), and ventilation rate was adjusted to maintain resting end-expiratory CO2 in the range of 4.0–5.5%. Neuromuscular blockade was achieved by administration of d-tubocurarine (0.5 mg iv, supplemented with 0.1 mg as needed to suppress spontaneous contractions of the diaphragm). Adequacy of anesthesia was verified before initial and supplemental neuromuscular blockade by absence of a withdrawal reflex or pressor response to foot pinch as well as by absence of a corneal reflex. Colonic (core) temperature (Tcore) was monitored using a copper-constantan thermocouple inserted 6 cm in the rectum and was maintained between 37 and 38°C with a water-perfused heating/cooling blanket and a heat lamp, except as noted. The trunk skin was shaved, and a copper-constantan thermocouple was taped to the abdominal skin to monitor skin temperature (Tskin) beneath the heating/cooling blanket. Animals were placed in a stereotaxic instrument with the incisor bar positioned 4 mm below the interaural line. To stabilize the spinal cord, a clamp was placed on the caudal thoracic spinal vertebrae.
Recording BAT SNA and Temperature
The temperature of BAT (TBAT) was monitored using a thermocouple meter (TC-1000; Sable Systems International, Las Vegas, NV) with a Type T needle style microprobe thermocouple (Physitemp, Clifton, NJ) inserted in the intact, left interscapular BAT fat pad. Postganglionic BAT SNA was recorded under mineral oil with a bipolar hook electrode from the central cut end of a small-diameter (∼100 μm) nerve bundle isolated from the ventral surface of the right interscapular fat pad after dividing it along the midline and reflecting it laterally. Nerve activity was filtered (1–300 Hz) and amplified (10,000–50,000×) with a Cyberamp 380 (Axon Instruments, Union City, CA). Spike 2 software (CED) was used to obtain a continuous measure (4 s bins) of BAT SNA amplitude by calculating the root mean square amplitude of the BAT SNA (square root of the total power in the 0.1 to 20 Hz band) from the autospectra of sequential 4-s segments of BAT SNA. Control values of BAT SNA were the averages of the BAT SNA amplitudes during the 32-s periods before all treatments, when Tcore was maintained between 37 and 38°C. Peak value for the BAT SNA response to a given treatment was defined as the average value during the 32-s period of maximal change in BAT SNA evoked by the treatment.
Microinjections
Glass micropipettes (outer tip diameter, 20–30 μm) were used for all microinjections, which were given over a 5- to 10-s period using a pressure injection system (model IIe; Toohey, Fairfield, NJ). The volume of the microinjection was determined using a reticule to measure the displacement of the meniscus in the micropipette. To make multiple microinjections at the same site, the micropipette was retracted vertically, emptied, rinsed with distilled water or saline, refilled, and then repositioned to the original coordinates.
Microinjection coordinates.
Coordinates were as follows: for the PVH, 1.5–1.8 mm caudal to bregma, 0.5–0.7 mm lateral to the midline with a 2–2.5° medial angle (just lateral to the sagital sinus with the tip angled toward the midline), and 7.7–8.0 mm ventral to dura; for the medial preoptic area (MPO), 0–0.3 mm caudal to bregma, 0.8 mm lateral to the midline, and 8.0 mm ventral to dura; for the DMH, 3.0–3.4 mm caudal to bregma, 0.5–0.7 mm lateral to the midline, and 8.2–8.5 mm ventral to dura; for the raphe pallidus (RPa), 3.0 mm caudal to lambda, on the midline, and 9.6–9.8 mm ventral to dura. Unless otherwise stated, microinjections were made unilaterally in the right PVH, the right DMH, or the right MPO, i.e., sites ipsilateral to the recorded BAT nerve.
The microinjection sites were marked by pressure microinjection of fluorescent polystyrene microspheres (FluoSpheres, F8797, F8801, or F8803; Molecular Probes, Eugene, OR) included in the injectate (1:200 dilution of FluoSpheres in the injectate). After the physiological recordings, rats were perfused (10% paraformaldehyde) transcardially, and brains were removed, postfixed (2–12 h), and sectioned (60-μm coronal sections) on a microtome. Sections were mounted on slides and counterstained with cresyl violet, and microinjection sites were localized and photographed, as described previously (26).
Drugs and Solutions
Drugs were obtained from Sigma (St. Louis, MO) and dissolved in saline. Isoflurane was obtained from Abbott Laboratories (North Chicago, IL).
Statistics
All statistics were performed using Systat software (version 10; Cranes Software International, Chicago, IL). Data are expressed as means ± SE. Statistical significance was assessed using an ANOVA for repeated measures comparing time 0 (just before a treatment) to the peak response within 5 min of the treatment for BAT SNA and within 10 min of the treatment for other variables. Statistical results with P < 0.05 were considered significant.
Protocols
Protocol 1.
To determine directly whether activation of neurons within the PVH increases sympathetic activation of BAT, N-methyl-d-aspartate (NMDA; 12 pmol in 60 nl) was microinjected in the PVH of rats (n = 5) whose Tcore was maintained between 37 and 38°C. A subset of these rats (n = 3) also underwent protocol 2 to determine whether activation of neurons within the PVH inhibits sympathetic activation of BAT. The other two rats received a subsequent microinjection of l-glutamate (6 nmol in 60 nl) in the PVH.
Protocol 2.
To determine whether activation of neurons within the PVH inhibits sympathetic activation of BAT, a stable level of BAT SNA was evoked by lowering the rats’ Tcore and Tskin and subsequently either NMDA (12 pmol in 60 nl; n = 7) or the GABAA antagonist bicuculline methiodide (Bic, 30 pmol in 60 nl; n = 13) was microinjected in the PVH. A subset of these animals (n = 2) received a subsequent microinjection of Bic targeting the PVH contralateral to the nerve recording; in these cases, sufficient time was allowed between microinjections for recovery of the cooling-evoked increase in BAT SNA and thermogenesis (typically 15–20 min). Because microinjection of Bic in the MPO can inhibit BAT SNA (31), we controlled for the possible diffusion of Bic to the MPO with another group of rats (n = 7, including three that had received a prior microinjection of Bic in the PVH) that received a microinjection of Bic in the rostral hypothalamus between the PVH and the MPO.
Protocol 3.
To determine whether disinhibition of neurons within the PVH inhibits sympathetic activation of BAT evoked under febrile conditions, rats (n = 11, including two that had undergone protocol 2) received a microinjection of prostaglandin E2 (PGE2; 60 ng in 60 nl) in the MPO followed 5–30 min later by a microinjection of Bic (30 pmol in 60 nl) in the PVH or in the area surrounding the PVH. Five animals also received a subsequent microinjection of Bic targeting the PVH contralateral to the nerve recording allowing 15–20 min between microinjections for recovery of the PGE2-evoked increases in BAT SNA and thermogenesis.
Protocol 4.
To determine whether disinhibition of neurons within the PVH inhibits sympathetic activation of BAT evoked by disinhibition of neurons within the DMH, rats (n = 9) received a microinjection of Bic (30 pmol in 60 nl) in the PVH or the area surrounding the PVH ∼5 min after receiving a microinjection of Bic (30 pmol in 60 nl) in the DMH. One rat received microinjections of Bic in the PVH both ipsilateral and contralateral to the nerve recording. Microinjection of the saline vehicle in the PVH did not affect the DMH-evoked increase in BAT SNA.
Protocol 5.
To determine whether disinhibition of neurons within the PVH inhibits sympathetic activation of BAT evoked by activation of neurons within the RPa, rats (n = 7) received a microinjection of NMDA (12 pmol in 60 nl) in the RPa before and after microinjection of Bic (30 pmol in 60 nl) in the PVH (in 5 of the 7 rats, the Bic microinjection was within the PVH; in the other 2 rats, the microinjection was outside of the PVH, thus serving as anatomical controls). This repeated-trial protocol was employed because of the short duration of the activation of BAT following microinjection of NMDA in the RPa. To control for this repeated-trial protocol, four rats also received a microinjection of NMDA in the RPa before and after microinjection (60 nl) of saline vehicle in the PVH. Additionally, we have previously demonstrated that repeated microinjections of NMDA in the RPa produce responses of consistent magnitude (24).
Protocol 6.
To determine whether disinhibition of neurons within the PVH inhibits sympathetic activation of BAT evoked by blockade of GABAA receptors within the RPa, rats (n = 6) received a microinjection of Bic (30 pmol in 60 nl) in the RPa followed within 5 min by a microinjection of Bic (30 pmol in 60 nl) in the PVH. Two of these six rats also underwent protocol 5 (i.e., received a microinjection of Bic in the PVH before and after injection of NMDA in the RPa).
RESULTS
Microinjection of NMDA in the PVH Does Not Activate BAT
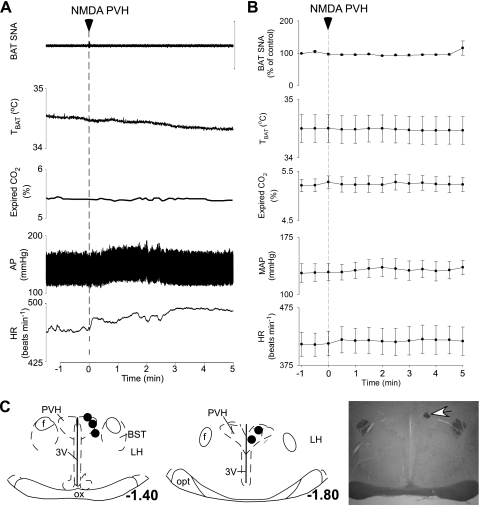
To test the hypothesis that activation of neurons within the PVH increase BAT SNA and thermogenesis, NMDA was microinjected in the PVH of rats whose Tcore was maintained between 37 and 38°C. In naïve anesthetized rats with core temperatures between 37 and 38°C, BAT SNA was low and exhibited no bursting discharge (Fig. 1). Microinjection of NMDA in the PVH had no effect on BAT SNA (99 ± 1% of control following NMDA; n = 5; Fig. 1 A and B), TBAT (control: 34.5 ± 0.2°C vs. after NMDA: 34.5 ± 0.2°C), expired CO2 (5.2 ± 0.1 vs. 5.3 ± 0.2%), mean arterial blood pressure (129 ± 11 vs. 136 ± 9 mmHg), or HR (413 ± 21 vs. 420 ± 24 beats/min). Microinjection of glutamate in the PVH also had no effect on the measured variables. Figure 1C shows the locations of the microinjection sites in the PVH.
Fig. 1.
Microinjection of N-methyl-d-aspartate (NMDA) in the paraventricular nucleus of the hypothalamus (PVH) does not increase sympathetic nerve activity (SNA) to brown adipose tissue (BAT), BAT temperature (TBAT), expired CO2, arterial pressure (AP), or heart rate (HR). A and B: representative example (A) and group data (B) (mean ± SE, n = 5 rats) in which each point is the 30-s average of the variable value. C: left and center, locations of the injection sites plotted on atlas drawings adapted from Ref. 38 and labeled with the distances from bregma. Right, photomicrograph showing a representative microinjection site (white arrow). 3V, third ventricle; BST, bed nucleus of the stria terminalis; f, fornix; LH, lateral hypothalamus; opt, optic tract; ox, optic chiasm.
Microinjection of NMDA or Bic in the PVH Inhibits Cooling-Evoked Activation of BAT
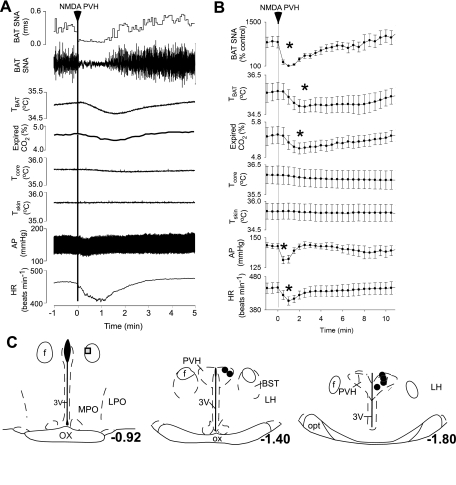
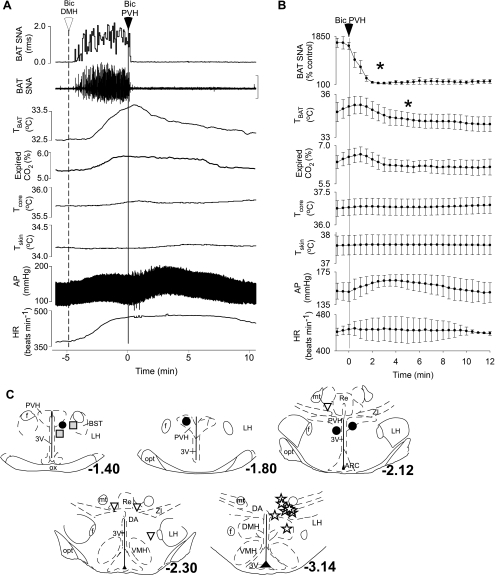
To test the hypothesis that activation of neurons within the PVH inhibit BAT SNA and thermogenesis, NMDA or Bic was microinjected in the PVH of rats whose skin and Tcore were lowered to elicit a stable level of cooling-evoked BAT SNA and thermogenesis. Figure 2 shows that microinjection of NMDA in the PVH rapidly and completely inhibited the cooling-evoked increase in BAT SNA (cooling-evoked: 861 ± 184% control vs. after NMDA: 108 ± 7% of control, representing a 99 ± 1% inhibition of BAT SNA at 1 min after NMDA microinjection; P < 0.001; n = 6). As expected from the short duration of action of NMDA, this inhibition of BAT SNA was transient, recovering within 3–10 min (Fig. 2, A and B). As shown in Fig. 2, A and B, microinjection of NMDA in the PVH also resulted in a transient decrease in TBAT (cooling-evoked: 35.7 ± 0.4°C vs. after NMDA: 34.9 ± 0.3°C; P < 0.02), expired CO2 (5.4 ± 0.2 vs. 5.0 ± 0.2%; P < 0.01), mean arterial blood pressure (143 ± 2 vs. 131 ± 3 mmHg; P < 0.01), and HR (447 ± 15 vs. 407 ± 13 beats/min; P < 0.02). Figure 2C shows that the six microinjection sites on which these results are based were centered within the PVH. The microinjection outside the PVH (Fig. 2C) was less effective in inhibiting cooling-evoked BAT SNA (54% inhibition).
Fig. 2.
Microinjection of NMDA in the PVH decreases SNA to BAT, TBAT, expired CO2, AP, and HR during whole body cooling. A and B: representative example (A) and group data (B) (mean ± SE, n = 6) in which each point is the 30-s average of the variable value. *P < 0.05 compared with just before microinjection in PVH. C: locations of the injection sites plotted on atlas drawings adapted from Ref. 38 and labeled with the distances from bregma. Symbols indicate sites at which microinjection of NMDA inhibited BAT SNA by >90% (filled circles) or by 54% (shaded square). LPO, lateral preoptic area; MPO, medial preoptic area.
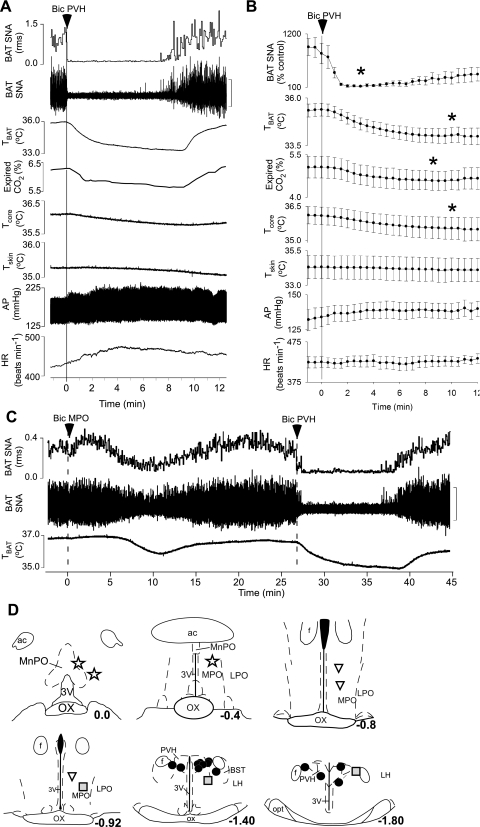
Microinjection of Bic in the PVH rapidly and completely inhibited the cooling-evoked increase in BAT SNA (Fig. 3, A and B; cooling-evoked: 795 ± 209% control vs. after Bic in the PVH: 119 ± 12% of control, representing a 99 ± 1% inhibition within 3 min of the microinjection of Bic; P < 0.001; n = 12). The Bic-evoked inhibition of BAT SNA lasted for ∼10–25 min (Fig. 3, A and B). Microinjection of Bic in the PVH (Fig. 3, A and B) also decreased TBAT (cooling-evoked: 35.4 ± 0.3°C vs. after Bic: 34.0 ± 0.4°C; P < 0.001), expired CO2 (5.1 ± 0.4 vs. 4.6 ± 0.3%; P < 0.001), and Tcore (36.1 ± 0.4 vs. 35.5 ± 0.5°C; P < 0.02). The 12 microinjection sites on which these data are based were located within the PVH (Fig. 3D).
Fig. 3.
During whole body cooling, microinjection of bicuculline (Bic) in the PVH decreases SNA to BAT, TBAT, expired CO2, and core body temperature (Tcore). A and B: representative example (A) and group data (B) (mean ± SE, n = 12) in which each point is the 30-s average of the variable values. *P < 0.05 compared with just before microinjection in PVH. C: inhibition of BAT thermogenic responses is smaller and of slower onset following Bic injections in the MPO compared with that following microinjection of Bic in the PVH. D: locations of the injection sites plotted on atlas drawings adapted from Ref. 38 and labeled with the distances from bregma. Symbols indicate sites at which microinjection of Bic inhibited BAT SNA by >90% (filled circles), by 50–70% (shaded squares), or by <50% (inverted triangles) or at which microinjection of Bic increased BAT SNA and thermogenesis (stars). ac, Anterior commissure.
To provide additional evidence that PVH is the locus of neurons whose activation elicits inhibition of BAT SNA, control microinjections of Bic were made in sites surrounding the PVH. As shown in Fig. 3C, Bic microinjections in sites immediately surrounding PVH (Fig. 3D) elicited only modest inhibitions of cooling-evoked BAT SNA. These inhibitions of BAT SNA and reductions in BAT thermogenesis were never complete and had longer onsets and slower times to peak effect than did those produced by Bic injections centered within PVH (Fig. 3D). Such responses are consistent with the possible diffusion of Bic to the nearby PVH. Unilateral microinjections of Bic in the caudal MPO, from ∼0.8 to 1.0 mm caudal to bregma (Fig. 3D), typically had little effect on BAT SNA and thermogenesis. Consistent with an earlier report (31), Bic injections in sites located in close proximity to the median preoptic nucleus (MnPO) (Fig. 3D) increased BAT SNA and BAT thermogenesis (data not shown).
Bic in the PVH Inhibits the Activation of BAT Evoked by Microinjection of PGE2 in the MPO
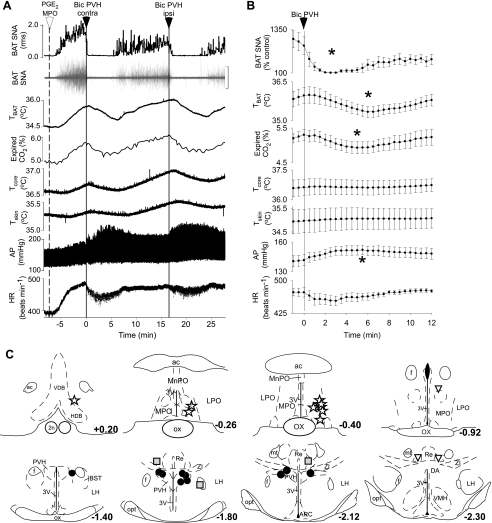
PGE2 in the MPO increased BAT SNA by 1,087 ± 251% of control (n = 6). Microinjection of Bic in the PVH rapidly and completely reversed the PGE2-evoked increase in BAT SNA (Fig. 4; 100 ± 1% inhibition within 3 min of the microinjection of Bic; P < 0.001; n = 6) and also decreased TBAT (PGE2 evoked: 36.0 ± 0.3°C vs. after Bic: 35.3 ± 0.2°C; P < 0.01) and expired CO2 (5.3 ± 0.2 vs. 4.9 ± 0.2%; P < 0.02). Microinjection of Bic in the PVH increased arterial pressure (Fig. 4, A and B; PGE2 evoked: 142 ± 5 mmHg vs. after Bic: 152 ± 5 mmHg; P < 0.05). The locations of the PGE2 microinjection sites (Fig. 4C) were in the MPO between +0.2 and −0.4 mm from bregma. The locations of the microinjection sites (Fig. 4C) at which Bic was effective in reversing the PGE2 in MPO-evoked increases in BAT SNA were within the PVH. As described above, for the inhibitions of cooling-evoked increases in BAT SNA, anatomical control microinjections of Bic in sites outside the PVH (Fig. 4C) produced reduced, slow-onset inhibitions of PGE2-evoked increases in BAT SNA.
Fig. 4.
Unilateral microinjection of Bic in the PVH reversed the increases in SNA to BAT, TBAT, and expired CO2, evoked by microinjection of prostaglandin E2 (PGE2) in the MPO. A and B: representative example (A) and group data (B) (mean ± SE, n = 6) in which each point is the 30-s average of the variable values. *P < 0.05 compared with just before microinjection in PVH. C: locations of the injection sites plotted on atlas drawings adapted from Ref. 38 and labeled with the distances from bregma. Symbols indicate sites at which microinjection of Bic inhibited BAT SNA by >90% (filled circles), by 50–70% (shaded squares), or by <50% (inverted triangles) or at which microinjection of PGE2 increased BAT SNA and thermogenesis (stars). 2n, Optic nerve; ARC, arcuate nucleus; DA, dorsal area of the hypothalamus; f, fornix; HDB, horizontal diagonal band; MnPO, median preoptic nucleus; mt, mammillothalamic tract; Re, nucleus reunions; VMH, ventromedial hypothalamic nucleus; VDB, vertical diagonal band; ZI, zona incerta.
Bic in the PVH Inhibits the Activation of BAT Evoked by Microinjection of Bic in the DMH/Dorsal Hypothalamic Area
Bic in the DMH/DA increased BAT SNA by 1,516 ± 166% of control (n = 4). Microinjection of Bic in the PVH rapidly and completely inhibited the increase in BAT SNA evoked by Bic in the DMH/dorsal hypothalamic area (DA; Fig. 5, A and B; DMH evoked: 1,504 ± 131% of control vs. after Bic in PVH: 149 ± 31% of control, representing a 96 ± 3% inhibition within 3 min of the microinjection in PVH; P < 0.01; n = 4) and also decreased TBAT (Fig. 5, A and B; DMH evoked: 35.1 ± 0.6 vs. 34.2 ± 0.5°C within 5 min of the microinjection of Bic in PVH; P < 0.02). The locations of the microinjection sites in which Bic increased BAT SNA and thermogenesis (Fig. 5C) were in the DMH or DA near −3.14 mm from bregma. The locations of the four microinjection sites (Fig. 5C) at which Bic was effective in reversing the Bic in DMH-evoked increases in BAT SNA were within the PVH. As described above for the inhibitions of cooling-evoked increases in BAT SNA, control microinjections of Bic in sites outside the PVH (Fig. 4C) produced markedly reduced, slow-onset inhibitions of the increases in BAT SNA evoked by Bic in DMH. Microinjections either dorsal or caudal to the PVH were not effective in inhibiting BAT SNA and thermogenesis, whereas microinjections just ventral or ventrolateral to the PVH (in the bed nucleus of the stria terminalis) were partially effective in inhibiting BAT SNA.
Fig. 5.
Unilateral microinjection of Bic in the PVH reversed the increase in SNA to BAT and TBAT evoked by microinjection of Bic in the dorsomedial nucleus of the hypothalamus (DMH)/dorsal hypothalamic area (DA). A and B: representative example (A) and group data (B) (mean ± SE, n = 4) in which each point is the 30-s average of the variable values. *P < 0.05 compared with just before microinjection in PVH. C: locations of the injection sites plotted on atlas drawings adapted from Ref. 38 and labeled with the distances from bregma. Symbols indicate sites at which microinjection of Bic inhibited BAT SNA by >90% (filled circles), by 50–70% (shaded squares), or by <50% (inverted triangles) or at which Bic increased BAT SNA and thermogenesis (stars). See Fig. 4 for abbreviations.
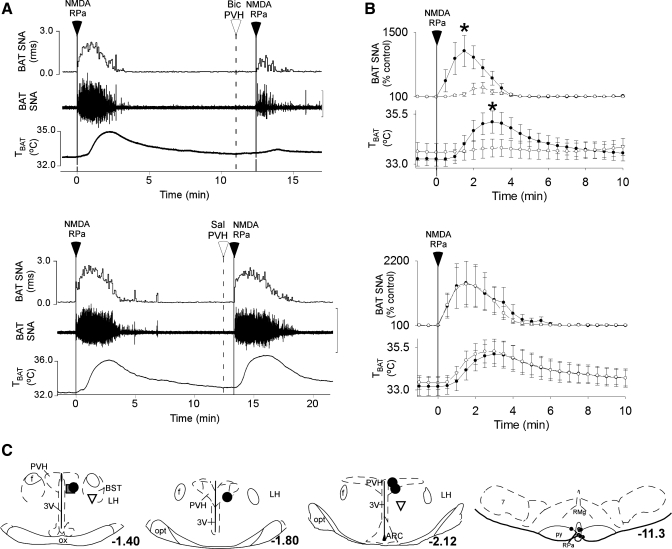
Bic in the PVH Attenuates the Activation of BAT Evoked by NMDA in the RPa
The increase in BAT SNA evoked by microinjection of NMDA in the RPa was greatly attenuated by prior microinjection of Bic in the PVH (Fig. 6, A and B; NMDA in RPa: 1,094 ± 338% of control vs. NMDA in RPa after Bic in PVH: 299 ± 119% of control, a 66 ± 8% reduction in the response amplitude; P < 0.01; n = 5), but not by prior microinjection of saline vehicle in the PVH (NMDA in RPa: 1,484 ± 706% of control vs. NMDA in RPa after saline in PVH: 1,447 ± 743% of control; P > 0.5). Similarly, the increase in TBAT evoked by microinjection of NMDA in the RPa was attenuated by prior microinjection of Bic in the PVH (Fig. 6, A and B; NMDA in the RPa: +1.8 ± 0.5°C vs. NMDA in the RPa after Bic in the PVH: +0.2 ± 0.1°C; P < 0.01) but was not attenuated by prior microinjection of saline vehicle in the PVH (NMDA in the RPa: +1.9 ± 0.7°C vs. NMDA in the RPa after saline in the PVH: +1.8 ± 0.6°C; P > 0.4). The locations of the medullary microinjection sites at which NMDA increased BAT SNA and thermogenesis (Fig. 6C, right) were in the RPa near −11.3 mm from bregma. The locations of the five microinjection sites (Fig. 6C) at which Bic was most effective in attenuating the increases in BAT SNA evoked by NMDA in RPa were within the PVH, whereas those outside the PVH (Fig. 6C) had little inhibitory effect on the BAT SNA and thermogenic responses to NMDA in the RPa.
Fig. 6.
The increases in SNA to BAT and in TBAT evoked by microinjection of NMDA in the raphe pallidus (RPa) is prevented by prior microinjection of Bic, but not saline vehicle, in the PVH. A and B: representative examples (A) and group data (B) (mean ± SE, n = 5) in which each point is the 30-s average of the variable values. Filled circles, data before microinjection in the PVH; open circles, data following microinjection of Bic or saline in the PVH. *P < 0.05, paired t-test between responses before and after microinjection of Bic in PVH. C: locations of the injection sites plotted on atlas drawings adapted from Ref. 38 and labeled with the distances from bregma. Symbols indicate sites at which microinjection of Bic inhibited BAT SNA by >75% (large filled circles), by 64% (shaded square), or by <50% (inverted triangles) or RPa sites at which NMDA increased BAT SNA and thermogenesis (small filled circles). 7, facial nucleus; ARC, arcuate nucleus; py, pyramidal tract; RMg, raphe magnus nucleus.
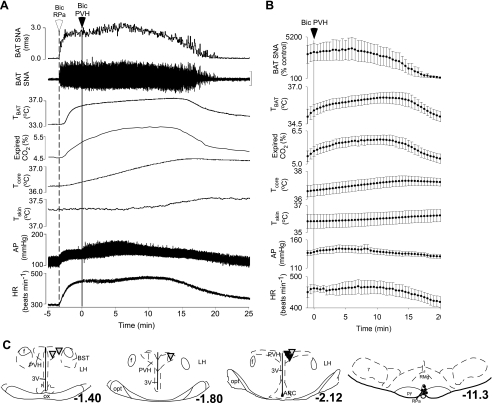
Bic in the PVH Does Not Attenuate the Activation of BAT Evoked by Bic in the RPa
To test the hypothesis that activation of neurons within the PVH inhibits BAT SNA and thermogenesis via a GABAergic input to the RPa, Bic was microinjected in the PVH subsequent to antagonism of GABAA receptors with Bic, into the RPa. As previously reported (26, 28), microinjection of Bic in the RPa produced an immediate and robust increase in BAT SNA and thermogenesis (Fig. 7A). In contrast to the inhibitory effect of microinjection of Bic in the PVH on BAT SNA and thermogenesis in all other protocols (Figs. 3–6), microinjection of Bic in the PVH had no effect on the increase in BAT SNA evoked by microinjection of Bic in the RPa (Fig. 7, A and B; plateau within 5 min of the microinjection of Bic in RPa: 3,337 ± 1,073% control vs. minimum within 10 min of microinjection of Bic in PVH: 3,053 ± 1,194% of control; P > 0.3; n = 6). Bic microinjection in the PVH did not reverse the increase in thermogenesis and metabolism evoked by microinjection of Bic in the RPa; in fact, TBAT, Tcore, Tskin, and expired CO2 continued to increase after microinjection of Bic in the PVH (Fig. 7, A and B). These results are consistent with a significant role for the activation of GABAA receptors in the RPa in mediating the inhibition of BAT SNA following activation of neurons in the PVH. The locations of the medullary microinjection sites at which Bic increased BAT SNA and thermogenesis (Fig. 7C, right) were in the RPa near −11.3 mm from bregma. The locations of the six microinjection sites (Fig. 7C) at which Bic had no effect on the increases in BAT SNA evoked by Bic in RPa were within the PVH. Of particular note are the two PVH sites (Fig. 7C) at which Bic inhibited (>75% inhibition) the increase in BAT SNA evoked by activation of NMDA receptors in the RPa but had no effect on the increase in BAT SNA evoked by blockade of GABAA receptors in the RPa.
Fig. 7.
The increases in SNA to BAT, TBAT, expired CO2, and Tcore evoked by microinjection of Bic in the RPa are not reversed by microinjection of Bic in the PVH. A and B: representative example (A) and group data (B) (mean ± SE, n = 6) in which each point is the 30-s average of the variable values. C: locations of the injection sites plotted on atlas drawings adapted from Ref. 38 and labeled with the distances from bregma. Symbols indicate PVH sites at which microinjection of Bic had no effect on the increase in BAT SNA evoked by microinjection of Bic in the RPa (inverted triangles), including two sites (solid inverted triangles) at which Bic in the PVH had inhibited the increase in BAT SNA evoked by microinjection of NMDA in the RPa (>75% inhibition, Fig. 6), or RPa sites at which microinjection of Bic increased BAT SNA, TBAT, expired CO2, Tcore, skin temperature (Tskin), AP, and HR (small filled circles).
DISCUSSION
These results provide the first direct evidence that neurons localized within the PVH can exert a potent inhibitory influence on the sympathetic outflow to BAT and thus on BAT energy expenditure and thermogenesis. Disinhibition (blockade of GABAA receptors) or excitatory amino acid activation of neurons within the PVH produced a complete inhibition of the BAT SNA and BAT thermogenesis evoked by both thermoregulatory and febrile stimuli. The activation of BAT inhibition by antagonism of GABAA receptors in the PVH indicates 1) that, at least under the conditions of our experiments, the BAT inhibitory mechanism in PVH is under a tonic GABAergic inhibition and 2) that PVH neurons mediating BAT inhibition have membrane mechanisms supporting spontaneous discharge or else they receive tonically active, receptor-mediated excitatory inputs capable of activating these PVH neurons when their tonic inhibition is relieved. The source of the tonically active GABAergic input to the PVH involved in the regulation of sympathetic outflow to BAT is unknown, although glutamic acid decarboxylase (GAD)-immunoreactive neurons with projections to the PVH have been reported in several areas surrounding the PVH, including the perisupraoptic area, the anterior perifornical area (including the bed nucleus of the stria terminalis), and the anterior hypothalamic area (44). It is also notable that the DMH has a dense projection to the PVH (20), although this projection is unlikely to be involved in the tonic GABAergic inhibition of PVH neurons involved in the regulation of BAT SNA, since few of these neurons are immunoreactive for GAD (44).
The finding that the increase in BAT SNA and thermogenesis evoked by NMDA receptor activation within the RPa is attenuated by activation of the PVH suggests that the PVH-neuronmediated inhibitory input to the neural circuits regulating BAT SNA must occur at, or downstream of, the BAT sympathetic premotor neurons (more specifically, in the RPa or in the spinal cord). This conclusion is further supported by the failure of PVH neural activation to inhibit the increases in BAT SNA and thermogenesis elicited by blockade of GABAA receptors in the RPa, consistent with a critical role for a GABAergic input to the BAT sympathetic premotor neurons in the RPa in mediating the PVH-evoked inhibition of BAT SNA and thermogenesis. Although the PVH does contain neurons that project to the RPa (15), the finding that PVH neurons are not GABAergic (49) argues against a direct GABAergic projection from the PVH to BAT sympathetic premotor neurons in the RPa in mediating the PVH-evoked inhibition of BAT SNA. The location of the GABAergic inputs to RPa that are driven by PVH neuronal activation and are responsible for the PVH-evoked inhibition of BAT SNA remains to be determined.
The present studies used the microinjection technique to activate local neurons selectively in several anatomical structures. Our finding that microinjections in the PVH were more effective than those in surrounding areas in eliciting rapid-onset, complete, and long-duration inhibitions of the increases in BAT SNA and thermogenesis evoked by natural as well as neurochemical stimuli strongly supports the PVH localization of the neurons critical for driving this inhibition of BAT energy expenditure. In addition, the locations of the PVH microinjection sites effective in inhibiting BAT SNA in the present studies correlate well with the locations of PVH neurons that are transsynaptically labeled by microinjection of pseudorabies virus in BAT, including the ventromedial parvocellular, the dorsal parvocellular, and the posterior parvocellular regions of the PVH (6, 34). We interpret the effectiveness of the occasional Bic microinjections made ventral or ventrolateral to the PVH as the result of reflux of the injectate dorsally along the pipette track in the PVH, although we cannot conclusively exclude a role for cells in this region in the BAT inhibitory effect.
The present results demonstrate that microinjection of Bic in the caudal MPO (0.8–1.0 mm caudal to bregma) had little effect on either the cooling-evoked or the PGE2-evoked increase in BAT SNA and thermogenesis. In contrast, microinjection of Bic in the rostral MPO (∼0.12 mm rostral-0.6 mm caudal to bregma) has been demonstrated to completely reverse the increase in BAT thermogenesis evoked by skin cooling (30, 36) or by PGE2 (35). The lack of effect of microinjections of Bic in sites between the PVH and the rostral MPO excludes the possibility that the present results arose from diffusion of Bic from the PVH to neurons within the rostral MPO.
The present results are in conflict with the suggestion that activation of neurons within the PVH activates BAT thermogenesis via the sympathetic system (1). Given the high concentrations and larger injection volumes of glutamate used in the previous study, the most likely explanation for this discrepancy is that the previously reported increase in sympathetically mediated BAT thermogenesis arose, not from an effect of glutamate to increase the discharge of PVH neurons, but rather from its effect on BAT sympathoexcitatory neurons in neighboring areas, such as the DMH or the MnPO, after diffusion of glutamate from the PVH. In this regard, both the DMH (7, 25, 52, 53) and the MnPO (31) contain neurons exerting an excitatory influence on the sympathetic outflow to BAT. In contrast to the absence of histological evidence of injection sites and appropriate anatomical control injections in the Amir study (1), the present study used careful mapping of all microinjection sites, with smaller injection volumes and anatomical control microinjections in areas surrounding the PVH to support the localization of a BAT sympathoinhibitory mechanism in the PVH. Microinjection of excitatory amino acids or Bic in the PVH did not increase BAT SNA or BAT thermogenesis, but rather they elicited decreases in BAT SNA, energy metabolism, and BAT thermogenesis, whereas microinjections of Bic in the DMH or near the MnPO resulted in robust increases in BAT SNA and thermogenesis.
The present results demonstrate that activation of neurons within the PVH inhibits the increase in BAT SNA and thermogenesis evoked by the febrile intermediary PGE2. In earlier studies, however, lesions of the PVH resulted in a partial attenuation of the increases in core temperature evoked by administration of lipopolysaccharide (LPS) (4, 16, 23), a pyrogen for which production of preoptic PGE2 is an important step in fever production (48). This apparent discrepancy may reflect a contribution of the PVH to the LPS-evoked pyrogenic mechanisms that are independent of the sympathetic activation of BAT thermogenesis, such as secretion of adrenal catecholamines, activation of the hypothalamic-pituitary-adrenal axis, or increased cutaneous vasoconstriction. These different results may also simply reflect the use of different pyrogens (PGE2 compared with LPS), since large lesions of the PVH had no effect on the increase in core temperature in rats given a large intracerebroventricular dose of PGE2 (16). A third possibility that remains untested is that the PVH may contain a population of cells that is involved in activating BAT SNA following LPS administration and another tonically inhibited population whose activation leads to inhibition of BAT SNA and BAT thermogenesis. Understanding the role of the PVH in the febrile response will require studies to assess the specific contributions of PVH neurons to individual thermal effector responses during fever.
Activation of neurons within the PVH has been reported to increase (21, 27, 39, 41), decrease (18, 19, 50), or have no effect on arterial pressure (10, 40), and the variability in the responses to stimulation of the PVH has been attributed to the method of stimulation, the activation of different populations of neurons within the PVH, and/or the presence of anesthesia (27). Thus the variable changes in arterial pressure elicited in the present studies by microinjection of NMDA or Bic in the PVH are not surprising. Our finding that microinjection of Bic in the PVH did not affect arterial pressure during whole body cooling but elicited a pressor response following microinjection of PGE2 in the preoptic area suggests that the cardiovascular response to activation of neurons within the PVH may also be influenced by the specific thermal condition of the animal at the time of experimental intervention. Whether the mechanisms by which thermal conditions influence the cardiovascular responses to activation of the PVH include alterations in the tonic GABAergic tone on PVH neurons or local changes in the levels of other neurotransmitters, such as nitric oxide, remains to be determined.
The novel insight provided by the present studies, that activation of neurons within the PVH inhibits BAT SNA, BAT metabolism, and BAT thermogenesis, may provide a clue to the mechanism underlying the inhibition of BAT SNA by glucoprivation, a model of hypoglycemia (11, 12). Neurons in the PVH express Fos in response to glucoprivation (43), and these may play a role not only in the glucoprivic inhibition of BAT SNA but potentially in that induced by fasting. In this respect, neuropeptide Y (NPY), which decreases sympathetic activation of BAT when injected in the PVH (13), is released in the PVH during fasting (17). Furthermore, in contrast to wild-type mice, which decrease UCP-1 mRNA in BAT during fasting, NPY knockout mice fail to decrease UCP-1 mRNA in BAT during fasting (37). In light of these data, we speculate that NPY acting within the PVH may play a role in the fasting-induced inhibition of BAT SNA, a hypothesis supported by the demonstration that NPY presynaptically inhibits GABA release on PVH neurons (9, 42) and by the inhibition of BAT SNA elicited by Bic in the PVH in the present study.
Perspectives and Significance
In small mammals, including human infants, BAT is the principal effector system for adaptive thermogenesis (5). Although the role of BAT in adaptive thermogenesis in adult humans remains controversial, it is now clear that adult humans have functional BAT (32). Interestingly, a correlation has been described between the level of BAT activation and low body weight (32, 46), thus supporting previous studies that have suggested that genetic variations in BAT effector systems (8) or decreased expression of UCP-1 in BAT (33) is correlated with weight gain in humans. Considering the importance of PVH neurons in mediating feeding responses (2) and in view of the potential role for decreased expression or reduced activation of BAT in the development or maintenance of obesity in humans, the present demonstration of the significant inhibitory regulation of BAT energy expenditure by neurons in the PVH provides continued support for a critical role for the PVH in overall energy homeostasis, including energy substrate acquisition, energy expenditure, including that necessary for thermoregulation, and energy storage. Because adult humans possess a sympathetically activated BAT that consumes energy, understanding the neural circuits that control BAT metabolism, including the roles of neurons in the PVH, and their neurochemical regulation could suggest novel therapeutic approaches to increase energy expenditure and combat obesity.
GRANTS
This work was supported by National Institutes of Health Grants NS-40987 (S. F. Morrison), DK-57838 (S. F. Morrison), and DK-065401 (C. J. Madden) and by an American Heart Association scientist development grant (C. J. Madden).
Acknowledgments
We are grateful to Kazuhiro Nakamura for comments on this manuscript and to Brad Sugden for histological assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Amir S Stimulation of the paraventricular nucleus with glutamate activates interscapular brown adipose tissue thermogenesis in rats. Brain Res 508: 152–155, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493–505, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 276: R1569–R1578, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Caldeira JC, Franci CR, Pela IR. Bilateral lesion of hypothalamic paraventricular nucleus abolishes fever induced by endotoxin and bradykinin in rats. Ann NY Acad Scis 856: 294–297, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 460: 303–326, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience 126: 229–240, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Clement K, Ruiz J, Cassard-Doulcier AM, Bouillaud F, Ricquier D, Basdevant A, Guy-Grand B, Froguel P. Additive effect of A–>G (−3826) variant of the uncoupling protein gene and the Trp64Arg mutation of the beta 3-adrenergic receptor gene on weight gain in morbid obesity. I J Obesity Related Metab Dis J Int Ass Study Obesity 20: 1062–1066, 1996. [PubMed] [Google Scholar]
- 9.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24: 155–163, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Darlington DN, Miyamoto M, Keil LC, Dallman MF. Paraventricular stimulation with glutamate elicits bradycardia and pituitary responses. Am J Physiol Regul Integr Comp Physiol 256: R112–R119, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Egawa M, Yoshimatsu H, Bray GA. Effects of 2-deoxy-D-glucose on sympathetic nerve activity to interscapular brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 257: R1377–R1385, 1989. [DOI] [PubMed] [Google Scholar]
- 12.Egawa M, Yoshimatsu H, Bray GA. Lateral hypothalamic injection of 2-deoxy-d-glucose suppresses sympathetic activity. Am J Physiol Regul Integr Comp Physiol 257: R1386–R1392, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Egawa M, Yoshimatsu H, Bray GA. Neuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in rats. Am J Physiol Regul Integr Comp Physiol 260: R328–R334, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Fyda DM, Cooper KE, Veale WL. Contribution of brown adipose tissue to central PGE1-evoked hyperthermia in rats. Am J Physiol Regul Integr Comp Physiol 260: R59–R66, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b). J Chem Neuroanat 13: 1–21, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Horn T, Wilkinson MF, Landgraf R, Pittman QJ. Reduced febrile responses to pyrogens after lesions of the hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 267: R323–R328, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Kalra SP, Dube MG, Sahu A, Phelps CP, Kalra PS. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc Natl Acad Sci USA 88: 10931–10935, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kannan H, Niijima A, Yamashita H. Inhibition of renal sympathetic nerve activity by electrical stimulation of the hypothalamic paraventricular nucleus in anesthetized rats. J Auton Nerv Sys 21: 83–86, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Katafuchi T, Oomura Y, Kurosawa M. Effects of chemical stimulation of paraventricular nucleus on adrenal and renal nerve activity in rats. Neurosci Lett 86: 195–200, 1988. [DOI] [PubMed] [Google Scholar]
- 20.Levin MC, Sawchenko PE, Howe PR, Bloom SR, Polak JM. Organization of galaninimmunoreactive inputs to the paraventricular nucleus with special reference to their relationship to catecholaminergic afferents. J Comp Neurol 261: 562–582, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Li YF, Mayhan WG, Patel KP. NMDA-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Heart Circ Physiol 281: H2328–H2336, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Lowell BB, VSS, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366: 740–742, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci 21: 4864–4874, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience 122: 5–15, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 286: R320–R325, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J Physiol 566: 559–573, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin DS, Haywood JR. Sympathetic nervous system activation by glutamate injections into the paraventricular nucleus. Brain Res 577: 261–267, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Morrison SF RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 276: R962–R973, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci Basic Clin 85: 18–25, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 292: R127–R136, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura K, Morrison SF. Preoptic mechanism for cold-defensive responses to skin cooling. J Physiol 586: 2611–2620, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Oberkofler H, Dallinger G, Liu YM, Hell E, Krempler F, Patsch W. Uncoupling protein gene: quantification of expression levels in adipose tissues of obese and non-obese humans. J Lipid Res 38: 2125–2133, 1997. [PubMed] [Google Scholar]
- 34.Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience 110: 515–526, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Osaka T Blockade of prostaglandin E2-induced thermogenesis by unilateral microinjection of GABAA receptor antagonist into the preoptic area. Brain Res 1230: 107–114, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Osaka T Cold-induced thermogenesis mediated by GABA in the preoptic area of anesthetized rats. Am J Physiol Regul Integr Comp Physiol 287: R306–R313, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Patel HR, Qi Y, Hawkins EJ, Hileman SM, Elmquist JK, Imai Y, Ahima RS. Neuropeptide Y deficiency attenuates responses to fasting and high-fat diet in obesity-prone mice. Diabetes 55: 3091–3098, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney, Australia: Academic, 1986.
- 39.Pittman QJ, Franklin LG. Vasopressin antagonist in nucleus tractus solitarius/vagal area reduces pressor and tachycardia responses to paraventricular nucleus stimulation in rats. Neurosci Lett 56: 155–160, 1985. [DOI] [PubMed] [Google Scholar]
- 40.Porter JP, Brody MJ. A comparison of the hemodynamic effects produced by electrical stimulation of subnuclei of the paraventricular nucleus. Brain Res 375: 20–29, 1986. [DOI] [PubMed] [Google Scholar]
- 41.Porter JP, Brody MJ. Neural projections from paraventricular nucleus that subserve vasomotor functions. Am J Physiol Regul Integr Comp Physiol 248: R271–R281, 1985. [DOI] [PubMed] [Google Scholar]
- 42.Pronchuk N, Beck-Sickinger AG, Colmers WF. Multiple NPY receptors Inhibit GABA(A) synaptic responses of rat medial parvocellular effector neurons in the hypothalamic paraventricular nucleus. Endocrinology 143: 535–543, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Ritter S, Dinh TT. 2-Mercaptoacetate and 2-deoxy-d-glucose induce Fos-like immunoreactivity in rat brain. Brain Res 641: 111–120, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol 332: 123–143, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Romanovsky AA Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 292: R37–R46, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Rousseau C, Bourbouloux E, Campion L, Fleury N, Bridji B, Chatal JF, Resche I, Campone M. Brown fat in breast cancer patients: analysis of serial (18)F-FDG PET/CT scans. Eur J Nuclear Med Mol Imaging 33: 785–791, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Saper Biomedicine CB Life, the universe, and body temperature. Science 314: 773–774, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Scammell TE, Griffin JD, Elmquist JK, Saper CB. Microinjection of a cyclooxygenase inhibitor into the anteroventral preoptic region attenuates LPS fever. Am J Physiol Regul Integr Comp Physiol 274: R783–R789, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol 494: 673–685, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamashita H, Kannan H, Kasai M, Osaka T. Decrease in blood pressure by stimulation of the rat hypothalamic paraventricular nucleus with l-glutamate or weak current. J Auton Nerv Sys 19: 229–234, 1987. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida K, Nakamura K, Matsumura K, Kanosue K, Konig M, Thiel HJ, Boldogkoi Z, Toth I, Roth J, Gerstberger R, Hubschle T. Neurons of the rat preoptic area and the raphe pallidus nucleus innervating the brown adipose tissue express the prostaglandin E receptor subtype EP3. Eur J Neurosci 18: 1848–1860, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Zaretskaia MV, Zaretsky DV, DiMicco JA. Role of the dorsomedial hypothalamus in thermogenesis and tachycardia caused by microinjection of prostaglandin E2 into the preoptic area in anesthetized rats. Neurosci Lett 340: 1–4, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res 928: 113–125, 2002. [DOI] [PubMed] [Google Scholar]