Abstract
Introduction
Work return is an indicator of recovery and functional status for cancer survivors. We investigated whether demographic, medical and functional factors predicted full-time work return following hematopoietic cell transplantation (HCT).
Methods
Adults (N=197), most with hematologic malignancy, completed assessments before their HCT and at intervals over 5 years. Assessments included treatment and demographic factors, and date of return to full-time work. We created binary variables, indicative of major impairment, from the Short Form 36 Health Survey (SF-36) mental (MCS) and physical (PCS) function component scores, dichotomized at 1 SD below population norms (≤40 vs. >40). PCS and MCS were imputed for 16% of the sample. Predictors of work return were analyzed using Cox proportional hazards regression.
Results
Of the 130 patients working full-time at pre-HCT, 88 (68%) were alive and relapse-free at 5 years. Of these, 53 (60%) had returned to full-time and 28 (32%) to part-time work. For the primary analyses at 6 month post-HCT, 14 patients had already died or relapsed and 10 had returned to work. Among the remaining 106 patients, those with PCS >40 returned to work faster (Hazard Ratio (HR) 2.38, 95% Confidence Interval (CI) 1.26–4.49). Female survivors were less likely to return to work than males (HR 0.54, 95% CI 0.29–0.99).
Conclusion
Return to work is a lengthy process for many survivors. Predictors of slower return include physical dysfunction and female gender.
Implications for cancer survivors
Realistic preparation for time off work is essential to long-term health and finances of cancer survivors.
Keywords: Hematopoietic cell transplantation, Return to work, Physical functioning, Mental functioning
Introduction
Return to work is a primary indicator of recovery and functional status for hematopoietic cell transplantation (HCT) and other cancer survivors. Although survivors are less likely to be employed when compared to healthy controls [1], the majority (approximately 60%–85%) of cancer survivors do return to work during the first several years following diagnosis [2-4] and this is generally true for HCT patients [5-11]. Although estimates vary, approximately 50–70% of allogeneic transplant survivors report working at one-year after transplant [7, 12, 13]. Long-term survivors of both allogeneic and autologous transplants, when compared to healthy case-matched controls, exhibit similar levels of working full-time (72% vs. 74%) at 10 years post-transplant [14].
A clear understanding of which HCT patients return to full-time work and their timing of work return, however, has been restricted by the predominance of cross-sectional samples rather than longitudinal analyses that consider competing risks of mortality and death or missing cases. For both HCT and other cancer survivors, few intervention studies have directly targeted work return or work-related rehabilitation following cancer [15]; therefore, research is needed to identify survivors at risk for poor occupational outcomes. HCT survivors who have delayed return to work, are unable to return to work, or face health-related limitations in the workplace, may incur a loss of income or health insurance or face other financial difficulties in paying for needed medical care [16].
Ongoing physical and mental health problems after HCT may put some survivors at risk of not returning to work. Physical recovery typically occurs back to levels measured at pre-HCT within one year [5]. Similarly, deficits in mental health often attenuate within the first year [5]. Nonetheless, some dysfunction remains over time or can have a late onset for certain patients [17, 18]. For allogeneic patients who survive the acute transplant-related toxicities, approximately 60% are diagnosed with chronic graft-versus-host disease (GVHD) that can persist for years [19-22]. Chronic GHVD-related toxicities may impair both cognitive and physical capacity necessary to meet work requirements. The literature on long-term GVHD impact is mixed, with some studies suggesting a relationship between chronic GVHD and work return, loss of employment or non-return to normal activities [5, 8, 23], whereas others report no association [9, 13].
Younger age at transplant is associated with a higher likelihood of work return in HCT recipients [24]. Although some studies have found no difference in work return by gender [6, 24], others suggest female HCT patients may be less likely to return to work after transplant [8, 13]. HCT survivors with more aggressive or advanced stage disease typically show poorer outcomes after transplant [25, 26], but differences in work return by disease and stage have not been reported [8]. Total body irradiation (TBI) as preparation for HCT is associated with decreased survival and other side effects [27], such as cortical atrophy and ventricular enlargement [28], but has not been found to be associated with differences in work return compared to other conditioning regimens [29].
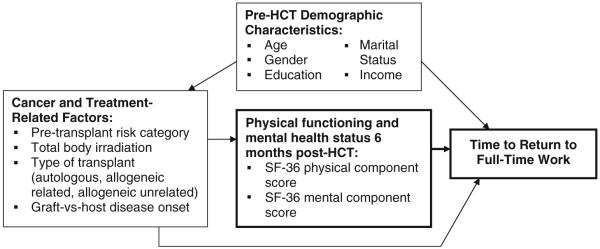
This study provides a longitudinal assessment of work return among HCT patients over the first 5 years after transplant. Using Steiner et al.’s earlier conceptual model of work return following cancer as a guide, we developed a conceptual model to investigate predictors of work return following HCT (Fig. 1) [15]. We were specifically interested in identifying predictors of return to work at 6 months post-HCT because return to work is neither expected nor recommended until this time-point by our institutional patient guidelines for post-transplant recovery during the period of immune compromise. Prior to 6 months post-HCT immune function is still impaired and many survivors face acute complications from treatment.
FIGURE 1.
Conceptual model of return to work following hematopoietic stem cell transplant. Adapted from Steiner et al. [15].
We hypothesized that survivors with better physical and mental health functioning by 6 months after transplant would return to work faster. First, we examined the demographic, physical and mental functioning, and treatment-related characteristics among HCT patients who were working full-time before transplant compared to those not working. Then, we tested our hypothesis that physical and mental functioning at 6 months post-HCT, female gender, and chronic GVHD would predict longer time to return to full time work, controlling for other demographic and treatment factors. Finally, we examined the self-reported work ability of survivors who returned to full-time work compared to their pre-transplant ability.
Methods
Participants and procedures
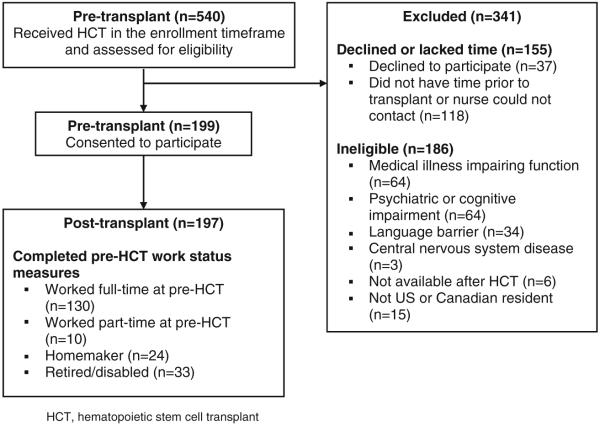
All procedures and materials were approved by the Fred Hutchinson Cancer Research Center Institutional Review Board. Eligible participants were ages 22 or older, were preparing for a first myeloablative autologous or allogeneic HCT, had sufficient English language proficiency to complete the assessments, and were available to complete the baseline assessment in the ambulatory clinic before transplantation conditioning began. The study sample included a total of 197 adults who consented to participate and completed the primary assessments at baseline (Fig. 2). Participants reporting full-time employment during the pre-transplant assessment period were included in the analyses of return to work (N=130). Full-time work included participants who reported school and work equaling full-time work (N=3) or full-time student status (N=2). We did not include survivors who had no previous history of work outside their home or who had not worked full-time before diagnosis and treatment, because of the potentially different factors influencing their employment.
FIGURE 2.
Flow of participants into the study.
We conducted pre-transplantation assessment in the ambulatory clinic and six month, 1-, 2-, 3- and 5-year assessments by mail. We followed participants until 5 years, death, or the development of recurrent malignancy, which-ever occurred first.
Measures
Assessments included demographic and treatment characteristics and date of part- or full-time work return. Patients completed the Short Form 36 Health Survey (SF-36), which measures 8 dimensions of mental and physical health, with a physical and a mental component summary score calculated relative to a standardized population-based T score with a mean of 50 and standard deviation (SD) of 10 [30]. For work ability, survivors working full-time were asked if they were able to accomplish as much work as they did before becoming sick and to indicate their ability to accomplish work compared to before diagnosis as a percent ranging from 0–100%.
Time to full-time work return was calculated as the difference between date of work return and date of transplant. The primary variables of interest for the hypothesis-driven analyses were the SF-36 physical (PCS) and mental (MCS) component scores at 6 months post-transplant, calculated as instructed by the instrument developers, by aggregating the eight scales of the SF-36 (higher scores indicate better health) [31, 32]. We created binary variables (>40=normal; ≤40=low) from the PCS and MCS to indicate patients with T-scores 1 SD below the US population norm of 50 [30].
Other variables included cancer and treatment-related factors. We generated a 3-level pre-transplant prognosis variable based on disease type and stage to indicate patients at low, moderate and high risk: Low=chronic myeloid leukemia (CML), chronic phase; myelodysplastic syndromes (MDS) with refractory anemia <5% blasts; Moderate=CML, accelerated phase; lymphoma, remission; acute leukemia, remission; chronic lymphocytic leukemia; follicular lymphoma in remission or relapse; MDS with refractory anemia >5% blasts; breast and ovarian adenocarcinoma in remission; High=CML, blast crisis; diffuse lymphoma in relapse; acute leukemia in relapse; multiple myeloma; breast and ovarian adenocarcinoma in relapse. We assessed whether the patient had total body irradiation in preparation for transplant; type of transplant (autologous, allogeneic related, allogeneic unrelated); and for allogeneic patients, whether they developed GVHD at any point during the study follow-up. Demographic variables considered were a categorized age variable (<35; 35–44; ≥45 years), gender, education (dichotomized at 4 years of college or more), current marital status (yes/no), and income >$45,000.
Statistical analyses
We compared the demographic characteristics for the survivors working full-time at pre-transplant to the survivors not working full-time. Because we were interested in examining how physical and mental health functioning at 6 months post-transplant predicted work return, our main regression analyses focused on the 106 survivors who worked at pre-transplant, but were still alive/relapse-free and had not returned to work by 6 months post-HCT. Our main research question was focused on the PCS and MCS measures; therefore, we calculated power for these key variables of interest and had approximately 85–90% power to detect a hazard ratio of 2.0 for both predictors.
Because PCS and MCS scores were missing at 6 months post-transplant for 17 patients, we used multiple imputation methodology to estimate plausible values for the missing PCS and MCS scores using SAS procedure PROC MI [33]. With multiple imputation, generally all available variables are included to increase the precision of the imputed datasets [34, 35]; therefore, we included all variables specified in the conceptual model (Fig. 1) as well as pre-transplant PCS and MCS scores. To fill in the missing values, we fit an imputation prediction model using these variables to create 10 complete datasets. Additional analyses utilizing only subjects with complete PCS and MCS scores (complete case analysis) were carried out to verify that similar results were obtained. As another check, we tested an ‘extreme’ imputed dataset where all missing PCS and MCS values had T-scores 1 SD below the US population norm (≤40).
Since we found no significant differences between the complete case and imputed datasets for the demographic characteristics and for competing risks for return to work (death or morphologic relapse), the descriptive statistics, figures and other analyses for the 106 survivors are based on the imputed datasets. For descriptive statistics, we calculated the average PCS and MCS values for each patient across the 10 imputed datasets and then dichotomized those at ≤40. P values were calculated using MICOMBINE in Stata. We calculated cumulative incidence curves of return to work by PCS and MCS, treating death or morphological relapse as competing risk events [36]. Hazard ratios [HR] and 95% confidence intervals [95% CI] of return to work within the first 5 years after transplant were calculated using multivariate Cox proportional hazard regression models [37] for PCS, MCS and other covariates hypothesized by our conceptual model to be important for return to work (described above under Measures).
Since some of the conceptual model variables are closely related, we did not include all variables in the conceptual model in our Cox proportional regression analyses. Because income and education are highly related, we examined income in models separate from education; however, income was not significantly related to work return and therefore, we only report education. We hypothesized that the onset of clinical extensive chronic GVHD would be more influential on work return than transplant type (only allogeneic patients are at risk for GVHD) and transplant type (autologous or allogeneic) was not included in the final model.
We entered GVHD onset as a time-dependent covariate and stratified by TBI due to proportional hazards violations in the main regression models. Female and male-specific models were generated because of evidence of differences in work return. In the female model, TBI was included as a main effect and allowed to vary over time because of its differential effect on work return over time in females. Due to the smaller sample sizes in these subgroups, the gender-stratified models were also limited to the variables associated with PCS in bivariate analyses or that were thought to be clinically important. Individuals who had not returned to full-time work during the 5 years of follow-up or died before returning to work were censored. The regression coefficients from the imputed datasets were combined with the Stata MIRA command to estimate a single set of summary coefficients using Rubin’s equation [33]. The proportional hazards assumption was tested for all covariates in the regression.
Finally, for ability to accomplish work, the mean and inter-quartile range were calculated at 6 months and 1, 2 and 3 years after transplant for the survivors working full-time at each time-point. This question was not asked at 5 years. All statistical analyses were performed using SAS version 9.0 (SAS Institute, Cary, NC) and Stata version 10.0 (Stata Corp, College Station, TX).
Results
Figure 2 displays participant enrollment. The 341 non-participants were more likely to be male (55% vs. 42%; P= 0.001) and to have an autologous transplant (28% vs. 19%) than the 197 participants, but were less likely to have an unrelated donor (28% vs. 38%; overall P=0.002 for transplant type). Age at transplant did not differ. Non-participants had more acute leukemia (31% vs. 18%) and less chronic myeloid leukemia (25% vs. 39%; overall P=0.003) cases than the non-participants. Of the 197 with completed baseline assessment, 130 reported working full-time before transplant. The 67 patients not employed full-time were working part-time (15%), were homemakers (36%), or were not working because of retirement, health or disability (49%).
Table 1 presents the demographic characteristics by pre-transplant work status. Survivors working full-time at pre-HCT (N=130) were less likely to be married than the survivors not working full-time (N=67) (69% vs. 85%, respectively, P=0.02). More working survivors had chronic myeloid leukemia in chronic phase (38% vs. 22%; overall P=0.02). Of the 130 survivors working at pre-transplant, at 6 months 10 (9%) had already returned to full-time work and 14 (12%) had died or relapsed (data not shown). We found no significant differences between the 10 early work returners and the 106 non-early returners by gender (male 60% vs. 44%; P=0.50), type of transplant (allogeneic related and unrelated 90% vs. 84%; P=0.77), or education (college degree or higher 70% vs. 52%; P=0.20). At 1 year, 29 (36%) of survivors were working full-time and 27 (25%) were working part-time. Of these part-time workers, 19 transitioned into full-time work by the end of follow-up. Another twenty-three survivors returned to part-time work at some point after HCT and continued to work part-time throughout the follow-up; these survivors did not differ demographically from survivors that returned to full-time work. By 5 years, of the 88 (68%) surviving participants, 53 (60%) reported working full-time and 28 (32%) part-time.
Table 1.
Pre-transplant demographics and treatment-related factors for those not working vs. working full-time before transplantation
| N(%) Demographics and treatment |
Not working full-time (N=67)a N(%) |
Working full-time (N=130) N(%) |
P value |
|---|---|---|---|
| Age at transplant, years | |||
| <35 | 14(21) | 33(25) | 0.10 |
| 35–49 | 29(43) | 69(53) | |
| ≥50 | 24(36) | 28(22) | |
| Sex, female | 44(66) | 71(55) | 0.14 |
| Education ≥4 years college | 26(39) | 66(51) | 0.11 |
| Married | 57(85) | 90(69) | 0.02 |
| Income ≥$45,000 | 37(55) | 84(65) | 0.20 |
| Stem cell donor | |||
| Autologous | 18(27) | 20(15) | |
| Allogeneic related | 25(37) | 59(45) | 0.15 |
| Allogeneic unrelated | 24(36) | 51(39) | |
| Diagnosis | |||
| Chronic myeloid leukemia | 15(22) | 49(38) | 0.02 |
| Chronic myeloid leukemia, accelerated or blast crisis | 2(3) | 11(8) | |
| Acute leukemia in remission | 5(8) | 17(13) | |
| Acute leukemia in relapse | 9(13) | 4(3) | |
| Lymphoma in remission | 0(0) | 3(2) | |
| Lymphoma in relapse | 5(8) | 8(6) | |
| Myleodysplasia | 7(10) | 13(10) | |
| Multiple myeloma | 4(6) | 4(3) | |
| Other | 20(30) | 21(16) | |
| Total body irradiation | 35(52) | 66(51) | 0.85 |
| Pre-transplant SF-36 PCS (≥40)b | 37(55) | 88(68) | 0.07 |
| Pre-transplant SF-36 MCS (≥40)b | 44(66) | 100(77) | 0.07 |
Includes individuals working part-time before HCT, who were homemakers, or not working because of retirement, health or disability-related reasons
SF-36 physical (PCS) and mental (MCS) component scores assessed before HCT; scores ≥40 indicate better functioning. Three subjects were not assessed
Our primary analyses focused on the hypothesized predictors of return to work for the 106 survivors who were alive, disease-free, and not working at 6 months post-HCT. We found few differences in demographic and treatment-related characteristics by PCS and MCS scores. Physical functioning limitations (68%) were more common than mental health limitations (25%) as indicated by PCS and MCS scores ≤40 (Table 2). Survivors with MCS scores within normal range (>40) were significantly less likely to have completed at least 4 years of college (69% vs. 41%, respectively, P=0.03). Because the direction of this relationship was in the opposite direction than hypothesized, we performed further analyses examining MCS and college education in both the complete case and imputed datasets. When examined in relationship to the other variables in the conceptual model, normal MCS scores continued to be related to lower education in both datasets. Further investigation is needed to determine whether this relationship can be replicated.
Table 2.
Demographics and treatment-related factors by SF-36 physical (PCS) and mental (MCS) component scores at 6 months post-transplant
| N=106a | SF-36 PCS at 6 months |
SF-36 MCS at 6 months |
||||
|---|---|---|---|---|---|---|
| Characteristic | PCS >40 34(32%) N(%) |
PCS ≤40 72(68%) N(%) |
P value | MCS >40 80(75%) N(%) |
MCS ≤40 26(25%) N(%) |
P value |
| Age at transplant, years | ||||||
| <35 | 12(35) | 16(22) | 0.65 | 22(28) | 6(23) | 0.49 |
| 35–49 | 14(41) | 40(56) | 42(53) | 12(46) | ||
| ≥50 | 8(24) | 16(22) | 16(20) | 8(31) | ||
| Female | 19(56) | 43(60) | 0.99 | 47(59) | 15(58) | 0.90 |
| Education ≥4 years college | 20(59) | 31(43) | 0.25 | 33(41) | 18(69) | 0.03 |
| Married | 21(59) | 51(71) | 0.23 | 53(66) | 18(69) | 0.86 |
| Pre-transplant risk group | ||||||
| Low | 19(56) | 26(36) | 0.12 | 34(43) | 11(42) | 0.65 |
| Moderate | 12(35) | 29(40) | 30(38) | 11(42) | ||
| High | 3(9) | 17(24) | 16(20) | 4(15) | ||
| Total body irradiation | 21(62) | 35(49) | 0.42 | 44(55) | 12(46) | 0.37 |
| Chronic graft-vs-host disease (GVHD)b | 18(53) | 48(67) | 0.22 | 48(60) | 18(69) | 0.51 |
Sample is comprised of the 106 HCT patients who had not returned to work and had not died or relapsed by 6 months post-HCT. An imputation prediction model generated SF-36 scores for 17 cases with missing PCS and MCS scores
Report of clinical extensive chronic GVHD onset at any point during the first 5 years after HCT
Figure 3 displays the cumulative incidence of return to work by MCS and PCS T-scores for the 106 disease-free patients who had not returned to work at 6 months. By the end of 5 year follow-up, 67% of survivors with normal range PCS scores at 6 months post-HCT had returned to work compared to 38% with low scores, whereas work return did not differ by MCS score. This trend was also apparent in the Cox regression models in Table 3. Survivors with PCS scores >40 at 6 months post-HCT were significantly more likely to return to work than those with scores ≤40 (HR 2.38, 95% CI 1.26, 4.49). Female survivors also were less likely to return to work after transplant than males (HR 0.54, 95% CI 0.29, 0.99). The extreme value imputation also yielded similar results to the main imputation analyses. The Hazard Ratios in the complete case analyses were similar in magnitude to the imputed regression, although gender was not significant. As a secondary analysis, these variables were examined in relation to return to part-time work, but none of the relationships were significant in multivariable Cox models.
FIGURE 3.
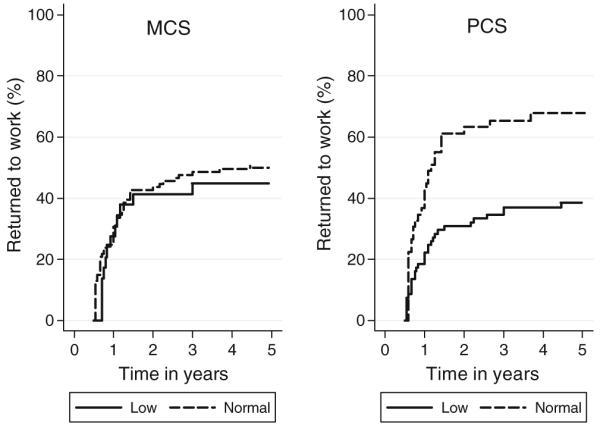
Cumulative incidence of return to full-time work by SF-36 mental (MCS) and physical (PCS) component scores (>40; ≤40) at 6 months post-transplant (N=106). Normal MCS and PCS scores (>40) indicate better physical or mental health functioning. Log-rank test of PCS survival curves significant (P value=0.01); MCS tests were non-significant.
Table 3.
Hazard ratios (HR) and 95% confidence intervals (95% CI) of return to full-time work from 6 to 60 months post-transplant
| Imputed analysis (N=106)a |
Complete case (N=89)b |
|||||
|---|---|---|---|---|---|---|
| HRc | 95% CI | P value | HRc | 95% CI | P value | |
| SF-36 PCS score 6 months post-HCT | ||||||
| ≤40 (referent) | 1 | 1 | ||||
| >40 | 2.38 | 1.26, 4.49 | 0.008 | 2.55 | 1.39, 4.70 | 0.003 |
| SF-36 MCS score 6 months post-HCT | ||||||
| ≤40 (referent) | 1 | 1 | ||||
| >40 | 1.22 | 0.58, 2.54 | 0.60 | 1.14 | 0.54, 2.43 | 0.73 |
| Age at transplant (years) | ||||||
| <35 (referent) | 1 | 1 | ||||
| 35–49 | 0.82 | 0.42, 1.58 | 0.55 | 0.92 | 0.46, 1.85 | 0.83 |
| 50 + | 0.60 | 0.25, 1.40 | 0.24 | 0.61 | 0.23, 1.58 | 0.31 |
| Gender | ||||||
| Male (referent) | 1 | 1 | ||||
| Female | 0.54 | 0.29, 0.99 | 0.05 | 0.58 | 0.29, 1.14 | 0.11 |
| Education ≥4 years college | ||||||
| No (referent) | 1 | 1 | ||||
| Yes | 0.85 | 0.46, 1.59 | 0.61 | 0.76 | 0.42, 1.44 | 0.40 |
| Married | ||||||
| Not married (referent) | 1 | 1 | ||||
| Married | 1.08 | 0.56, 2.07 | 0.81 | 1.22 | 0.61, 2.42 | 0.58 |
| Pre-transplant risk category | ||||||
| Low (referent) | 1 | 1 | ||||
| Moderate | 1.39 | 0.73, 2.71 | 0.34 | 1.19 | 0.60, 2.37 | 0.61 |
| High | 1.70 | 0.76, 3.79 | 0.20 | 1.36 | 0.57, 3.24 | 0.49 |
| Chronic graft-vs-host disease (GVHD)c | ||||||
| No (referent) | 1 | 1 | ||||
| Yes | 0.70 | 0.37, 1.31 | 0.24 | 0.62 | 0.31, 1.21 | 0.16 |
Sample is comprised of the 106 HCT patients who had not returned to work and had not died or relapsed by 6 months post-HCT. An imputation prediction model generated SF-36 scores for 17 cases with missing PCS and MCS scores
Complete case sample is comprised of the 89 HCT patients who had not returned to work by 6 months post-HCT, were alive at 6 months, and had responded to the SF-36 at 6 months post transplant (i.e., not missing MCS and PCS)
Models were stratified by total body irradiation (TBI). Chronic GVHD included as a time dependent covariate by onset of GVHD
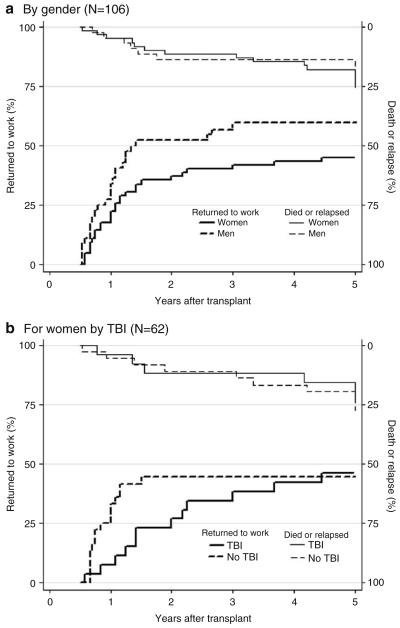
The gender-stratified analyses are presented in Fig. 4a and Table 4. Despite no difference in the risk of death/relapse between males and females, work return was significantly lower for women (Fig. 4a). All men who returned to full-time work did so by 3 years, while women continued to return to full-time work during the 5 years of follow-up. By 5 years, only 46% of women had returned to full-time work compared to 64% of men. As indicated in Table 4, normal range physical functioning continued to be the main predictor of work return for males (PCS score >40; HR 2.72, 95% CI 1.10, 6.70) and females (HR 2.80, 95% CI 1.09, 7.18).
FIGURE 4.
Cumulative incidence of return to full-time work. Figure 4a presents the cumulative incidence by gender, with competing incidence of death or relapse. Figure 4b presents the cumulative incidence of return to full-time work for women only by whether or not they received total body irradiation (TBI), with competing incidence of death or relapse.
Table 4.
Hazard ratios (HR) and 95% confidence intervals (95% CI) of return to full-time work by gender
| N=106 | Male N=44a |
Female N=62a |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| SF-36 PCS score 6 months post-HCT | ||||||
| ≤40 (referent) | 1 | 1 | ||||
| >40 | 2.72 | 1.10, 6.70 | 0.03 | 2.80 | 1.09, 7.18 | 0.03 |
| Age at transplant (years) | ||||||
| <35 (referent) | 1 | 1 | ||||
| 35–49 | 0.48 | 0.19, 1.13 | 0.09 | 1.36 | 0.52, 3.58 | 0.53 |
| 50+ | 0.87 | 0.26, 2.82 | 0.82 | 0.52 | 0.15, 1.78 | 0.30 |
| Total body irradiation | ||||||
| No (referent) | 1 | 1 | ||||
| Yes | 1.34 | 0.53, 3.38 | 0.53 | 0.53 | 0.22, 1.28 | 0.16 |
| Chronic graft-vs-host disease (GVHD)b | ||||||
| No (referent) | 1 | 1 | ||||
| Yes | 0.58 | 0.24, 1.37 | 0.22 | 0.92 | 0.41, 2.06 | 0.84 |
Sample is comprised of the patients who had not returned to work by 6 months post-HCT and had not died or relapsed by 6 months
Chronic GVHD included as a time dependent covariate by onset of GVHD
Additionally, we found that for women, TBI conditioning showed differential effects over time. TBI was entered as an average effect in the main female regression due to the small sample size and was not significant. In secondary analyses that allowed TBI to vary, the Hazard Ratio for TBI before 18 months was 0.29 (95% CI 1.20, 9.61). That is, women with TBI were 71% less likely to return to work during the first 18 months after transplant compared to women who did not receive TBI. After 18 months, only 1 female without TBI returned to work compared to 6 receiving this treatment, resulting in a Hazard Ratio after 18 months of 12.47 (95% CI 1.21, 128.94). Of the 26 women not receiving TBI (Fig. 4b), the 12 (46%) who returned to work in this group returned faster and did so by approximately 18 months after transplant. Women receiving TBI continued to return to full-time work throughout the follow-up, and by 5 years they were not different from the non-TBI group, with 36 (45%) working full-time. When cumulative incidence curves were examined for male survivors, no differences existed for TBI.
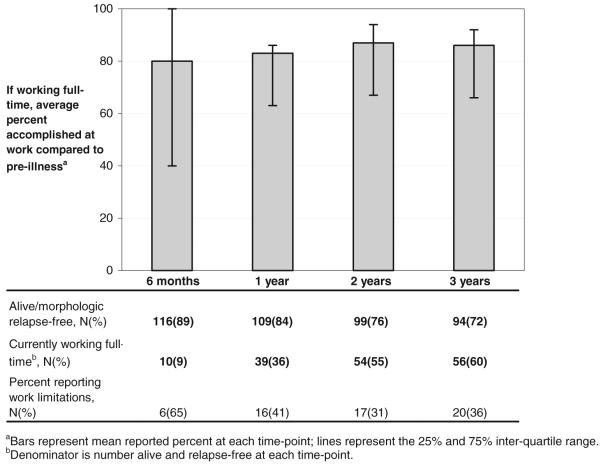
During the first three years of follow-up, all participants who had returned to full-time work were asked if their ability to accomplish work tasks had diminished since before they became sick. Across all time-points, survivors working full-time reported their ability to accomplish work, on average, as 80%–87% of their pre-illness ability (Fig. 5). Although the majority of working survivors reported high ability levels, between 3% to 11% of patients at each follow-up time reported their work accomplishments as 60% or less of their pre-illness ability. The percent of patients reporting work limitations decreased from 65% at 6 months to 36% at 3 years. Survivors with low PCS scores (≤40) at 6 months post-HCT were significantly more likely to report work related limitations at all 4 time-points (P<0.01 at each time); however, low MCS scores were only significant at 2 years (P=0.01). Because of the small number of survivors working full-time who reported work-related limitations, comprehensive assessment of potential underlying differences among those reporting limitations was not possible, although there were no gender, age or pre-transplant risk group differences at any of the time-points.
FIGURE 5.
Percent accomplished at work compared to pre-illness for patients working full-time.
Discussion
Results of this longitudinal study demonstrate that a majority of HCT survivors eventually return to the workforce but that a substantial minority face ongoing work-related limitations or do not return to full time work. By one year 36% of patients who were alive and worked full-time before transplant had returned to full-time work. By 5 years, this increased to 60%. As hypothesized, we found that those with better physical functioning by 6 months after transplant were 2 times more likely to return to work after HCT, although mental health did not predict their return to work. Of note, a majority of survivors had normal range mental health scores at the 6 month time point (75%). Socioeconomic characteristics, such as age, income and education, were not significantly associated with work return, nor were treatment factors such as chronic GVHD, although these comparisons may have been limited due to our relatively small sample size.
The proportion returning to work in the current study is lower than our earlier study done in a similar sample of 5 year survivors where 84% returned to full time work. In the previous study, 69% of survivors had died or relapsed by 5 years [5] whereas in the current study of patients transplanted about 10 years later, 36% had died or relapsed at this time. For the 130 survivors working full-time at pre-transplant in the current cohort, only 32% had died or relapsed by 5 years. The difference in rates of return to work for the current survivors may reflect improved survival rates and greater morbidity in some of those surviving, rather than declines in the overall percent returning to work.
Female survivors were 50% less likely to return to work than male patients, which is consistent with other findings [8, 13]. Although it is unclear from our study whether this finding was driven by differences in work-related intentions or job flexibility or because of health-related barriers, our secondary analyses suggest that the health impacts of HCT conditioning regimens may explain some of the gender work differences. Although other studies have not reported a relationship between TBI and work return [13, 29], we found that women receiving TBI were less likely to return to work than females without this conditioning treatment during the first 18 months after transplant. By 5 years after transplant, the proportions working were similar (46% vs. 45%, respectively). These results should be interpreted cautiously since they were not hypothesized and because of the small number of women returning to work after 18 months. Nonetheless, the findings suggest that subsequent studies investigating TBI and work return should consider gender differences in the analyses of predictors of return to work.
Over one-third of survivors working full-time at 3 years reported not being able to accomplish as much at work as they did before their illness. Although most patients working full-time reported a relatively high work capacity (on average 80%–87% of their pre-illness ability) over the 3 years of follow-up, the types of limitations faced by HCT survivors could not be assessed in our analyses. One study of working cancer survivors found that 18% reported difficulty performing physical tasks, 12% faced limitations with prolonged mental concentration and 22% had problems keeping pace with others at work [2]. For the HCT population, that often faces severe complications and slow recovery, more precise investigation of the specific type and severity of work limitations, the clinical characteristics that predispose certain patients to work limitations, and how these limitations differ over time is required to identify appropriate rehabilitation strategies.
The Institute of Medicine report, From Cancer Patient to Cancer Survivor: Lost in Transition, identified the need to expand employment-related services [38]. Addressing cancer survivors’ needs as they transition back to work is challenging because rehabilitation services are not commonly integrated with cancer care delivery systems [16]. Since HCT patients often travel long distances for their transplants, they may have added rehabilitation difficulties. Survivors who are unable to return to work may face barriers in accessing needed rehabilitation services if they lose their health insurance coverage or face financial difficulties. Only one intervention has specifically targeted work-related rehabilitation for HCT patients and found no improvements in employment [6]. Our results suggest that examining physical and cognitive difficulties after the acute HCT recovery phase may be important for identifying high-risk patients and that the effects of treatment may differ for males and females.
Our study has certain limitations. Our sample size was relatively small, which may have limited the power to detect other meaningful differences in our regression analysis. The exclusion of patients not working before HCT limits generalizability to those working full-time before transplant. We would assume there are added barriers for those not working full time before transplant who wish to enter the work force after HCT. Exclusion criteria for study entry, such as cognitive impairment or medical conditions, allowed us to focus on HCT patients more likely to be in the labor force at pre-transplant, but do not represent those with major comorbidities that could further complicate their return to work return following HCT. It is possible with more sensitive measures of chronic GVHD treatment duration or severity we may have found a stronger effect of GVHD on return to work. Additionally, we did not find differences in work return by pre-transplant education, income or marital status, potentially because a large proportion of patients enrolled in the study were of a relatively high socioeconomic status. Studies in more socioeconomically diverse HCT samples may be more likely to find differences in these variables for work return prompted by the economic need of some patients.
Further, we were unable to assess work-related intentions, changes in occupations, work hours or content, or the economic impact of cancer on the patients and families; therefore, we can not consider whether some survivors attempted to return to work and were unable or if some survivors elected not to return. In particular, evaluation of these work-related variables may be needed to understand the gender differences in work return found in our analyses. Also, we were unable to investigate the influence of health insurance coverage because virtually all patients undergoing treatment at the study site were insured. Future research should develop comprehensive assessments that include types of tasks required at work, and how these factors may impact work return for HCT patients.
Both the model-based and ‘extreme’ value imputation analyses were similar to our complete case analyses, providing evidence that those missing SF-36 assessments at 6 months were not necessarily of poorer health. The one difference was the change in significance of gender in the multiply imputed analysis, for a hazard ratio of similar magnitude, illustrating the advantage of using imputation to gain power.
Our results provide important prospective, longitudinal information on return to work for HCT survivors. As more patients survive after HCT, with potentially higher rates of long-term functional deficits, comprehensive information about return to work will inform transplant recipients, clinicians and employers about appropriate expectations and identify patients in need of rehabilitation services. The prevalence of cross-sectional studies of HCT survivors has limited the examination of trajectories in work return and ability over time. We found that HCT patients with better physical functioning 6 months after transplant were more likely to return to work — and return to work faster — in the first five years after transplant. Clinical trials to retain physical capabilities during treatment, or improve them in the immediate post-transplant period, are needed to facilitate both the pace of return to work and work effectiveness for those returning. Interventions may need to be targeted by gender and to assist survivors with continued work-related difficulties to improve employment outcomes in this population.
Acknowledgments
Supported by grants from the National Cancer Institute (CA 63030, CA78990, CA112631). We thank Thomas Wickizer for his helpful comments on an earlier draft.
Footnotes
Conflict of interest We report no conflicts of interest.
Presented in part at the American Society of Preventive Oncology Annual Meeting; March 16–18, 2008; Bethesda, MD
Contributor Information
Anne C. Kirchhoff, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N. D5-220, Seattle, WA 98109, USA; Department of Health Services, University of Washington School of Public Health and Community Medicine, Seattle, WA, USA, akirchh@u.washington.edu
Wendy Leisenring, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N. D5-220, Seattle, WA 98109, USA, wleisenr@fhcrc.org; Department of Biostatistics, University of Washington School of Public Health, Seattle, WA, USA.
Karen L. Syrjala, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N. D5-220, Seattle, WA 98109, USA, ksyrjala@fhcrc.org; Department of Psychiatry and Behavioral Sciences, University of Washington School of Medicine, Seattle, WA, USA
References
- 1.de Boer AG, Taskila T, Ojajarvi A, van Dijk FJ, Verbeek JH. Cancer survivors and unemployment: a meta-analysis and meta-regression. JAMA. 2009;301(7):753–62. doi: 10.1001/jama.2009.187. [DOI] [PubMed] [Google Scholar]
- 2.Bradley CJ, Bednarek HL. Employment patterns of long-term cancer survivors. Psycho-Oncology. 2002;11(3):188–98. doi: 10.1002/pon.544. [DOI] [PubMed] [Google Scholar]
- 3.Short PF, Vasey JJ, Tunceli K. Employment pathways in a large cohort of adult cancer survivors. Cancer. 2005;103(6):1292–301. doi: 10.1002/cncr.20912. [DOI] [PubMed] [Google Scholar]
- 4.Spelten ER, Sprangers MA, Verbeek JH. Factors reported to influence the return to work of cancer survivors: a literature review. Psycho-Oncology. 2002;11(2):124–31. doi: 10.1002/pon.585. [DOI] [PubMed] [Google Scholar]
- 5.Syrjala KL, Langer SL, Abrams JR, Storer B, Sanders JE, Flowers ME, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291(19):2335–43. doi: 10.1001/jama.291.19.2335. [DOI] [PubMed] [Google Scholar]
- 6.Hensel M, Egerer G, Schneeweiss A, Goldschmidt H, Ho AD. Quality of life and rehabilitation in social and professional life after autologous stem cell transplantation. Ann Oncol. 2002;13(2):209–17. doi: 10.1093/annonc/mdf031. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Fairclough D, Parsons SK, Soiffer RJ, Fisher DC, Schlossman RL, et al. Recovery after stem-cell transplantation for hematologic diseases. J Clin Oncol. 2001;19(1):242–52. doi: 10.1200/JCO.2001.19.1.242. [DOI] [PubMed] [Google Scholar]
- 8.Duell T, van Lint MT, Ljungman P, Tichelli A, Socie G, Apperley JF, et al. EBMT Working Party on Late Effects. EULEP Study Group on Late Effects. European Group for Blood and Marrow Transplantation Health and functional status of long-term survivors of bone marrow transplantation. Ann Intern Med. 1997;126(3):184–92. doi: 10.7326/0003-4819-126-3-199702010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Deeg HJ, Leisenring W, Storb R, Nims J, Flowers ME, Witherspoon RP, et al. Long-term outcome after marrow transplantation for severe aplastic anemia. Blood. 1998;91(10):3637–45. [PubMed] [Google Scholar]
- 10.Edman L, Larsen J, Hagglund H, Gardulf A. Health-related quality of life, symptom distress and sense of coherence in adult survivors of allogeneic stem-cell transplantation. Eur J Cancer Care (Engl) 2001;10(2):124–30. doi: 10.1046/j.1365-2354.2001.00251.x. [DOI] [PubMed] [Google Scholar]
- 11.Molassiotis A, Boughton BJ, Burgoyne T, van den Akker OB. Comparison of the overall quality of life in 50 long-term survivors of autologous and allogeneic bone marrow transplantation. J Adv Nurs. 1995;22(3):509–16. doi: 10.1046/j.1365-2648.1995.22030509.x. [DOI] [PubMed] [Google Scholar]
- 12.Heinonen H, Volin L, Uutela A, Zevon M, Barrick C, Ruutu T. Quality of life and factors related to perceived satisfaction with quality of life after allogeneic bone marrow transplantation. Ann Hematol. 2001;80(3):137–43. doi: 10.1007/s002770000249. [DOI] [PubMed] [Google Scholar]
- 13.Socie G, Mary JY, Esperou H, Robert DV, Aractingi S, Ribaud P, et al. Health and functional status of adult recipients 1 year after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2001;113(1):194–201. doi: 10.1046/j.1365-2141.2001.02678.x. [DOI] [PubMed] [Google Scholar]
- 14.Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol. 2005;23(27):6596–606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- 15.Steiner JF, Cavender TA, Main DS, Bradley CJ. Assessing the impact of cancer on work outcomes: what are the research needs? Cancer. 2004;101(8):1703–11. doi: 10.1002/cncr.20564. [DOI] [PubMed] [Google Scholar]
- 16.Short PF, Vargo MM. Responding to employment concerns of cancer survivors. J Clin Oncol. 2006;24(32):5138–41. doi: 10.1200/JCO.2006.06.6316. [DOI] [PubMed] [Google Scholar]
- 17.Syrjala KL, Chapko MK, Vitaliano PP, Cummings C, Sullivan KM. Recovery after allogeneic marrow transplantation: prospective study of predictors of long-term physical and psychosocial functioning. Bone Marrow Transplant. 1993;11(4):319–27. [PubMed] [Google Scholar]
- 18.Broers S, Kaptein AA, Le Cessie S, Fibbe W, Hengeveld MW. Psychological functioning and quality of life following bone marrow transplantation: a 3-year follow-up study. J Psychosom Res. 2000;48(1):11–21. doi: 10.1016/s0022-3999(99)00059-8. [DOI] [PubMed] [Google Scholar]
- 19.Blume KG, Forman S, Appelbaum FR, editors. Thomas’ hematopoietic cell transplantation. 3rd ed. Blackwell; Malden: 2004. [Google Scholar]
- 20.Flowers ME, Lee S, Vogelsang G. An update on how to treat chronic GVHD. Blood. 2003;102(6):2312. doi: 10.1182/blood-2003-06-2064. [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–33. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 22.Vogelsang GB. How I treat chronic graft-versus-host disease. Blood. 2001;97(5):1196–201. doi: 10.1182/blood.v97.5.1196. [DOI] [PubMed] [Google Scholar]
- 23.Wingard JR, Curbow B, Baker F, Piantadosi S. Health, functional status, and employment of adult survivors of bone marrow transplantation. Ann Intern Med. 1991;114(2):113–8. doi: 10.7326/0003-4819-114-2-113. [DOI] [PubMed] [Google Scholar]
- 24.Mosher CE, Redd WH, Rini CM, Burkhalter JE, DuHamel KN. Physical, psychological, and social sequelae following hematopoietic stem cell transplantation: a review of the literature. Psycho-Oncology. 2009;18(2):113–27. doi: 10.1002/pon.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell JA, Larratt L, Brown C, Turner AR, Chaudhry A, Booth K, et al. Allogeneic blood stem cell and bone marrow transplantation for acute myelogenous leukemia and myelodysplasia: influence of stem cell source on outcome. Bone Marrow Transplant. 1999;24(11):1177–83. doi: 10.1038/sj.bmt.1702051. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Loberiza FR, Rizzo JD, Soiffer RJ, Antin JH, Weeks JC. Optimistic expectations and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2003;9(6):389–96. doi: 10.1016/s1083-8791(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 27.Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med. 2006;144(6):407–14. doi: 10.7326/0003-4819-144-6-200603210-00007. [DOI] [PubMed] [Google Scholar]
- 28.Jager HR, Williams EJ, Savage DG, Rule SA, Hajnal JV, Sikora K, et al. Assessment of brain changes with registered MR before and after bone marrow transplantation for chronic myeloid leukemia. AJNR Am J Neuroradiol. 1996;17(7):1275–82. [PMC free article] [PubMed] [Google Scholar]
- 29.Socie G, Clift RA, Blaise D, Devergie A, Ringden O, Martin PJ, et al. Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia: long-term follow-up of 4 randomized studies. Blood. 2001;98(13):3569–74. doi: 10.1182/blood.v98.13.3569. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 31.Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264–79. [PubMed] [Google Scholar]
- 32.Ware JE, Jr, Kosinski M, Keller SD. SF-36 physical and mental health summary scales: A user’s manual. The Health Institute; Boston: 1994. [Google Scholar]
- 33.Rubin DB. Multiple imputation for non-response in surveys. John Wiley and Sons, Inc.; New York: 1987. [Google Scholar]
- 34.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681–94. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 35.Wood AM, White IR, Royston P. How should variable selection be performed with multiply imputed data? Stat Med. 2008;27(17):3227–46. doi: 10.1002/sim.3177. [DOI] [PubMed] [Google Scholar]
- 36.Pintilie M. Dealing with competing risks: testing covariates and calculating sample size. Stat Med. 2002;21(22):3317–24. doi: 10.1002/sim.1271. [DOI] [PubMed] [Google Scholar]
- 37.Hosmer DW, Lemeshow S. Applied survival analysis. John Wiley and Sons, Inc.; New York: 1999. [Google Scholar]
- 38.Institute of Medicine . From Cancer Patient to Cancer Survivor: Lost in Transition. 2005. [Google Scholar]