Abstract
Background
Minimally invasive surgical techniques, specifically the thoracoscopic approach, have been applied to congenital diaphragmatic hernia (CDH) with varying outcomes from selected centers. The aim of our study was to examine the rate of successful completion and compare outcomes between open and thoracoscopic approaches in CDH repair.
Methods
We performed a retrospective analysis of infants with CDH repair (From February 2004 to January 2008). Patients were divided into thoracoscopic and open groups, based on operative approach. We analyzed demographic, clinical, and hospitalization characteristics to compare the completion rate and outcomes in these two groups.
Results
Analysis of 31 infants with CDH (14 thorascocopic and 17 open) demonstrated no differences in sex (P = 0.132), age (P = 0.807), birthweight (P = 0.256), weight at operation (P = 0.647), pulmonary hypertension (P = 0.067), preoperative intensive care unit (ICU) days (P = 0.673), ventilator days (P = 0.944), or use of a patch (P = 0.999) between the groups. Seventy-nine percent of thoracoscopic operative approaches were completed successfully. There was a significant difference between the open and thoracoscopic groups with respect to estimated gestational age (39 versus 36.5 weeks; P = 0.006) and operating room time (70 versus 145 minutes; P = 0.004). The total (P = 0.662), ICU (P = 0.889), and postoperative (P = 0.619) length of stay and days on ventilator (P = 0.705), as well as days until initial enteral feeds (P = 0.092), were not significantly different between groups. There were no deaths and no evidence of recurrence, with a mean follow-up of 346 days.
Conclusions
In our early experience, the thoracoscopic approach for congenital diaphragmatic hernia repair was completed in 80% of our patient population with minimal exclusion criteria. Further study, with larger sample sizes, is needed to ascertain differences in outcomes, such as length of stay and initiation of enteral feeding.
Introduction
Congenital diaphragmatic hernia (CDH) occurs in approximately 1 in 2500–4000 live births.1 Medical and surgical management of these patients has changed over the past several decades. “Gentle ventilation” strategies, high-frequency oscillatory ventilation (HFOV), extracorporeal membrane oxygenation (ECMO), and other methods of supportive care have changed the intensive care management of CDH.2,3 Parallel with these intensive care improvements has been a paradigm shift over the past 20 years from emergent operation to elective repair.4 The combination of innovations in intensive care and elective operation has resulted in an improvement in survival for these children.2,3,5
As the early mortality from this condition has decreased, opportunities to utilize minimally invasive surgery (MIS) have been applied to the correction of CDH. In other thoracic conditions, thoracoscopy has been suggested to have significant advantages over the traditional open approaches. These benefits may include a decrease in the pain and incisional morbidity of a thoracotomy (i.e., subsequent scoliosis, chest deformities, and shoulder muscle girdle weakness), reduced surgical stress and immunologic derangement, faster recovery, and shorter hospitalizations.6–19 Initial experience with minimally invasive repair of diaphragmatic hernia in adults and children20 eventually led to work on neonates and infants.21–24 Very little is known about the outcomes in selected patients receiving minimally invasive treatment, compared with that of open repair. We reviewed our early experience with MIS CDH repair to evaluate the rate of successful completion and to compare outcomes between the minimally invasive and open approaches.
Methods
Patients
A retrospective review of all CDH cases at Seattle Children's Hospital (Seattle, WA) was undertaken. Every infant having an International Classification of Diseases-9th Revision (ICD-9) procedure code for repair of CDH (756.6) between February 2004 and January 2008 was identified through our operating-room database. Patients were excluded from the study if they had a repair after 50 weeks postconception, if their hernia was recurrent, if they had congenital cardiac anomalies not including conditions such as patent ductus arteriosus or foramen ovale, if their hernia was preoperatively diagnosed as a Morgagni hernia, or if they had ECMO support during their hospitalization. Historically, patients requiring ECMO support tend to require patch repair for larger defects, and it is a relative contraindication, for some surgeons, to perform patch repair thoracoscopically. Therefore, we chose, a priori, to treat ECMO as a relative exclusion criteria. Infants were grouped into the MIS group if the approach for repair was thoracoscopic and grouped into the open group if the approach was laparotomy or thoracotomy. Follow-up and evaluation for complications in the intra- and perioperative period was through a review of the electronic medical record. This study was approved by the Institutional Review Board at Seattle Children's Hospital (#E-07-310-01).
Operative management
We have been performing minimally invasive CDH repairs since 2005. Prior to that, all CDH repairs were performed as open, either via laparotomy or thoracotomy. Operative repair was performed when the patient demonstrated both hemodynamic and pulmonary stability. The decision to perform MIS repair was made by the individual surgeon. Preoperative anatomic characteristics (i.e., presence of the stomach or liver in the thorax) and the presence of pulmonary hypertension were not contraindications to the thoracoscopic approach.
Operative technique
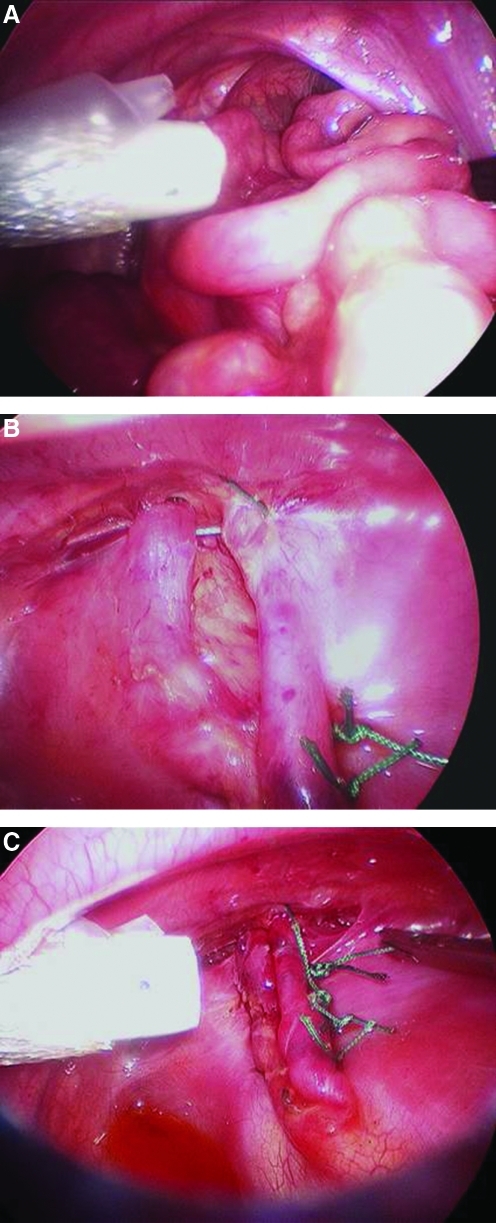
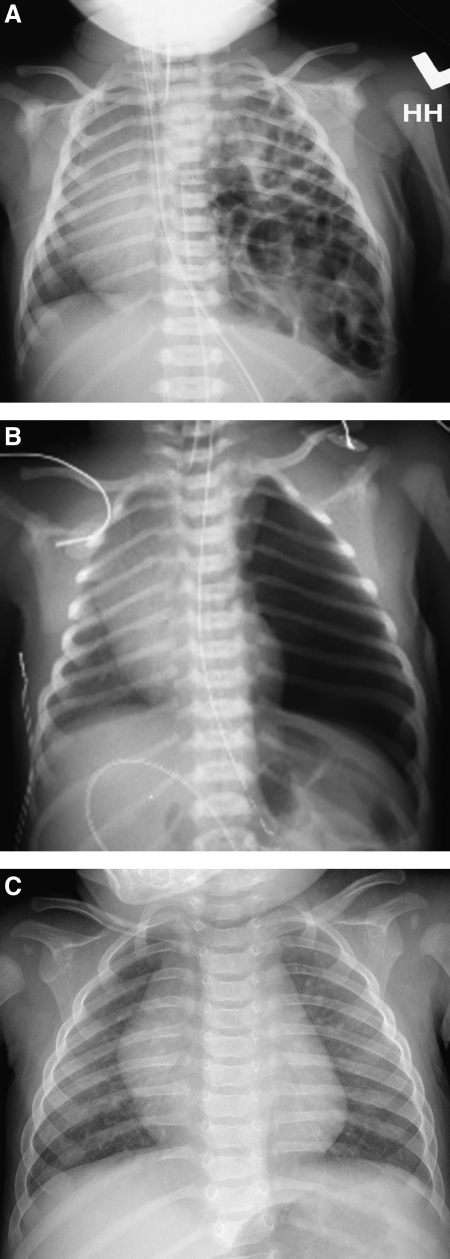
Thoracoscopic repair was performed with the patient in the lateral decubitus position, utilizing three to four 3- or 5-mm ports for access to the chest, similar to what has been described previously.21 Insufflation with carbon dioxide to 4 mm Hg was used in most cases. Herniated viscera, including left lobe of the liver, stomach, intestine, and spleen, were reduced into the abdomen, if necessary (Fig. 1). The posterior rim of the diaphragm was mobilized and the defect was repaired primarily with nonabsorbable sutures. A pericostal stitch was employed in cases where there was an absence of diaphragm at the posterolateral chest wall.23 Given our historic results with patch repair, in instances in which a patch repair was deemed necessary, the approach was converted to open.25 Thoracotomy was performed with the patient in the lateral decubitus position at the 9th–10th intercostal space. Laparotomy was performed in the supine position with a left subcostal incision. Reduction of the herniated viscera and repair of the diaphragmatic defect was performed in a fashion similar to the thoracoscopic approach. In all cases, thoracostomy tube use was based on surgeon preference. Chest radiographs were taken pre- and postoperatively in all patients (Fig. 2).
FIG. 1.
Thoracoscopic repair of congenital diaphragmatic hernia. (A) Reduction of herniated viscera. (B) Pericostal stitch used to approximate the posterolateral aspect of the defect. (C) Completed repair.
FIG. 2.
Representative pre- and postoperative chest radiograph (CXR). (A) Preoperative CXR. (B) Immediate postoperative CXR. (C) Two-month postoperative CXR.
Statistical analysis
Descriptive data included sex, age, and weight at operation, side of defect, estimated gestational age, birthweight, APGAR (Activity, Pulse, Grimace, Appearance, and Respiration) scores, presence of pulmonary hypertension, and ventilator days. Operative characteristics included operative time, type of repair, conversion to open procedure (thoracoscopic approach), and intraoperative complications. Postoperative variables included complications, deaths, ventilator days, days until initiation of enteral feedings, and length of intensive care unit (ICU) and hospital stay. Variables with skewed distributions are presented as medians with interquartile ranges. Comparisons between the two study groups were conducted by using the nonparametric two-sample Mann-Whitney test for continuous variables and Fisher's exact test for binomial variables. A two-sided P-value of <0.05 was considered significant. The analyses were conducted by using the STATA 10.0 software package (College Station, TX).
Results
All patients were born at an outside institution and transferred to our facility. Of the 58 children with CDH that underwent operative repair, 27 were excluded for reasons outlined above, leaving 31 eligible for this study. The primary reason for exclusion was ECMO use during hospitalization (10 infants), since thoracoscopic repair of CDH while on ECMO support was a relative contraindication for some surgeons. Seventeen patients were approached in an open fashion (group 1; 5 laparotomy and 12 thoracotomy) and 14 were approached thoracoscopically (group 2). Left-sided defects (29:2) and male gender (22:9) were more common (Table 1). Weights at birth and operation, age at operation, and APGAR scores were not significantly different between the groups. Children undergoing a thoracoscopic repair were characterized by a younger gestational age. Hospitalization characteristics, such as preoperative days spent in the ICU or on the ventilator, were not different between groups. The frequency of pulmonary hypertension, based on a preoperative echocardiogram, was not significantly different between groups.
Table 1.
Open and Thoracoscopic Repair of Congenital Diaphragmatic Hernia: Comparison of Patient and Hospitalization Characteristics
| Group 1: open repair | Group 2: thoracoscopic repair | P-valuea | |
|---|---|---|---|
| Patients | 17 | 14 | |
| Sex M:F | 10:7 | 12:2 | 0.132 |
| Side L:R | 16:1 | 13:1 | 0.999 |
| Birthweight (kg) | 3.2 (2.1–4) | 2.9 (1–3.9) | 0.256 |
| Weight at operation (kg) | 3.2 (2.1–4) | 3.2 (2.1–4.7) | 0.647 |
| Age at operation (days) | 3 (2–24) | 3 (2–150) | 0.807 |
| Estimated gestational Age (weeks) | 39 (37–41) | 36.5 (29–40) | 0.006 |
| APGARs, 1 minute | 7 (2–9) | 5 (2–8) | 0.063 |
| APGARs, 5 minutes | 8 (6–9) | 8 (2–9) | 0.385 |
| Hospitalization | |||
| Preoperative ICU LOS (days) | 2 (1–7) | 3 (0–9) | 0.673 |
| Ventilator time prior to operation (days) | 2 (0–7) | 3 (0–9) | 0.944 |
| Pulmonary hypertension (%) | 7 (41) | 11 (79) | 0.067 |
Results are in medians and ranges, unless otherwise indicated; binomial variables were compared by using Fisher's exact test, and continuous variables were analyzed by using the two-sample Mann-Whitney test.
APGARs, Activity, Pulse, Grimace, Appearance, and Respiration scores; ICU, intensive care unit; LOS, length of stay.
Intraoperatively, we found no difference between the proportions of primary repairs that were able to be performed via the different approaches (Table 2). Operating time was defined as time from skin incision to skin closure and was found to be significantly shorter with the open approach (70 versus 145 minutes). Eleven (79%) of the group 2 patients underwent successful thoracoscopic repair. The 3 remaining MIS patients were converted to an open approach once a large defect requiring a patch repair was identified. There were no intraoperative complications.
Table 2.
Open and Thoracoscopic Repair of Congenital Diaphragmatic Hernia: Comparison of Operative Characteristics
| Group 1: open repair | Group 2: thoracoscopic repair | P-valuea | |
|---|---|---|---|
| Patients | 17 | 14 | |
| Operation time (minute) | 70 (50–260) | 144.5 (83–288) | 0.004 |
| Primary repair (%) | 14 (82) | 11 (79) | 0.999 |
| Conversion (%) | — | 3 (21) | — |
| Complications | 0 | 0 | — |
| Hernia sac (%) | 3 (18) | 1 (7) | — |
Results are in medians and ranges, unless otherwise indicated; binomial variables were compared by using Fisher's exact test, and continuous variables were analyzed by using the two-sample Mann-Whitney test.
Though length of stay was lower in the MIS group (21 versus 24 days), this difference was not statistically significant (Table 3). Similarly, postoperative length of stay in the ICU (7 days), days spent on a ventilator (4 days), and median number of days before initiation of enteral feeding were not statistically significant. There were 2 postoperative complications in the MIS group and 4 complications in the open group (Table 3). Conversion of a thoracoscopic procedure was not considered a complication. One patient in the MIS group (that was converted to an open thoracotomy) developed scoliosis diagnosed 10 months postoperatively. This 45-degree apex right thoracic curve was treated with a Lycra® (TheraTogs Inc., Telluride, CO) body suit and was followed by orthopedic surgery. The other MIS patient was diagnosed with an inferior vena cava thrombus on routine postoperative echocardiogram, most likely due to an umbilical vein catheter. The patient was started on anticoagulation and had no adverse sequelae.
Table 3.
Open and Thoracoscopic Repair of Congenital Diaphragmatic Hernia: Comparison of Postoperative Characteristics and Complications
| Group 1: open repair | Group 2: thoracoscopic repair | P-valuea | |
|---|---|---|---|
| Number of Patients | 17 | 14 | |
| Total length of stay | 24 (9–46) | 21 (5–52) | 0.662 |
| Postoperative length of stay (days) | 22 (8–43) | 17.5 (3–51) | 0.619 |
| Postoperative ICU Length of stay (days) | 7 (1–21) | 7 (0–28) | 0.889 |
| Postoperative vent days (days) | 4 (1–18) | 4 (0–25) | 0.705 |
| Postoperative initiation of feeds (days) | 5 (2–19) | 4 (1–18) | 0.092 |
| Complications (%) | 4 (24) | 2 (14) | |
| SVT (3) | Scoliosis | ||
| Pectus | IVC thrombus |
Two-sample Mann-Whitney test.
SVT, supraventricular tachycardia; IVC, inferior vena cava.
In the open group, postoperative supraventricular tachycardia (SVT) occurred in 3 patients. Cardiology was consulted in each instance, and the SVT either resolved or is well-managed on medication at follow-up. A fourth patient was diagnosed with pectus excavatum during a follow-up pulmonary clinic visit 16 months after his thoracotomy that was not present at birth. There has been no recurrence of the diaphragmatic hernia in either group during our follow-up period (mean, 346 days; 261 for thoracoscopic and 416 for open), and there were no in-hospital deaths.
Discussion
Thoracoscopic CDH repair is safe and feasible in a variety of children. Eighty percent of our infants undergoing a primary MIS approach were successfully treated. Every patient that was converted to a traditional approach had a large defect requiring patch repair. We did not have any in-hospital deaths. Additionally, we did not find any statistically significant difference in postoperative ventilator requirements, ICU and hospital length of stay (LOS), or time to enteral nutrition.
There has been steady progress in the management of children with CDH over the past several decades. We have transitioned from an era of immediate and emergent repair to one in which elective repair is performed after physiologic stabilization. Even though the overall survival of neonates with CDH approaches 80%,3,26 there is still room for improvement, including identifying the appropriate role of MIS management. Although several studies have reviewed the successful experience with the thoracoscopic repair,20–24,27 only one has directly compared minimally invasive and open repairs.28 While they focused on neonates with only Bochdalek repairs, our inclusion criteria was more broad.
Early reports on MIS repair of CDH noted a high conversion rate and varying outcomes, depending on the type of hernia and age of the patient.23 The initial Michigan experience advocated for the MIS approach in Morgagni defects and urged caution in children with a Bochdalek hernia due to a concern for elevated PCO2 levels, acidemia, and an associated 57% conversion rate. Other studies have proposed selection criteria for the thoracoscopic approach to CDH repair by utilizing strict ventilatory and radiographic criteria [i.e., intra-abdominal stomach, minimally ventilator support (peak inspiratory pressure <24 mm Hg), and absence of pulmonary hypertension] to exclude patients from MIS.21 We were able to perform MIS repair of CDH in infants with a median age of 3 days. Utilizing our criteria for repair, we were able to perform MIS repair in patients without evidence of intra-abdominal stomach and also in patients with evidence of pulmonary hypertension. As the medical and supportive care for CDH continues to improve, the ability to plan elective repair will increase and selecting the appropriate patients for minimally invasive repair will be paramount. Technically, the thoracoscopic repair was performed in an equivalent manner to the open procedure. Thoracoscopy provided excellent visualization and exposure of the herniated viscera and defect. We have utilized simple exclusion criteria to select patients for this procedure and safely demonstrated equivalent outcomes to the open procedure.
Conclusions
This study was limited by our small sample size. In order to demonstrate a 20% reduction in total LOS, postoperative LOS, and postoperative ICU LOS, we would need to study 90, 104, and 223 patients in each arm, respectively. A well-powered study demonstrating a 20% reduction in postoperative ventilator days or shorter return to initiation of enteral feeds would require 405 and 156 patients in each arm, respectively. The current prospective, multi-institutional, and multinational effort by the Congenital Diaphragmatic Hernia Study Group is a valuable resource in gathering observational data on this condition with a relatively low incidence. Studies utilizing their registry will hopefully help to address clinical questions, such as short- and long-term benefits, complications, and differences in approach for the repair with more statistical power.29
Acknowledgment
The principal investigator, Stephen Kim, takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Statement
No competing financial interests exist.
References
- 1.Langham MR., Jr Kays DW. Ledbetter DJ, et al. Congenital diaphragmatic hernia. Epidemiology and outcome. Clin Perinatol. 1996;23:671–688. [PubMed] [Google Scholar]
- 2.Weber TR. Kountzman B. Dillon PA. Silen ML. Improved survival in congenital diaphragmatic hernia with evolving therapeutic strategies. Arch Surg. 1998;133:498–502. doi: 10.1001/archsurg.133.5.498. discussion, 502–503. [DOI] [PubMed] [Google Scholar]
- 3.Downard CD. Jaksic T. Garza JJ, et al. Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg. 2003;38:729–732. doi: 10.1016/jpsu.2003.50194. [DOI] [PubMed] [Google Scholar]
- 4.Harting MT. Lally KP. Surgical management of neonates with congenital diaphragmatic hernia. Semin Pediatr Surg. 2007;16:109–114. doi: 10.1053/j.sempedsurg.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Chiu PP. Sauer C. Mihailovic A, et al. The price of success in the management of congenital diaphragmatic hernia: Is improved survival accompanied by an increase in long-term morbidity? J Pediatr Surg. 2006;41:888–892. doi: 10.1016/j.jpedsurg.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto T. Segawa O. Lane GJ, et al. Laparoscopic surgery in newborn infants. Surg Endosc. 1999;13:773–777. doi: 10.1007/s004649901096. [DOI] [PubMed] [Google Scholar]
- 7.Goldschlager T. Frawley G. Crameri J, et al. Comparison of thoracoscopic drainage with open thoracotomy for treatment of paediatric parapneumonic empyema. Pediatr Surg Int. 2005;21:599–603. doi: 10.1007/s00383-005-1423-8. [DOI] [PubMed] [Google Scholar]
- 8.Kishan S. Bastrom T. Betz RR, et al. Thoracoscopic scoliosis surgery affects pulmonary function less than thoracotomy at 2 years postsurgery. Spine. 2007;32:453–458. doi: 10.1097/01.brs.0000255025.78745.e6. [DOI] [PubMed] [Google Scholar]
- 9.Novitsky YW. Litwin DE. Callery MP. The net immunologic advantage of laparoscopic surgery. Surg Endosc. 2004;18:1411–1419. doi: 10.1007/s00464-003-8275-x. [DOI] [PubMed] [Google Scholar]
- 10.Sylla P. Kirman I. Whelan RL. Immunological advantages of advanced laparoscopy. Surg Clin North Am. 2005;85:1–18. doi: 10.1016/j.suc.2004.09.005. vii. [DOI] [PubMed] [Google Scholar]
- 11.Tolg C. Abelin K. Laudenbach V, et al. Open versus thorascopic surgical management of bronchogenic cysts. Surg Endosc. 2005;19:77–80. doi: 10.1007/s00464-003-9328-x. [DOI] [PubMed] [Google Scholar]
- 12.Winslow ER. Brunt LM. Perioperative outcomes of laparoscopic versus open splenectomy: A meta-analysis with an emphasis on complications. Surgery. 2003;134:647–653. doi: 10.1016/s0039-6060(03)00312-x. discussion 654–655. [DOI] [PubMed] [Google Scholar]
- 13.Rothenberg SS. Pokorny WJ. Experience with a total muscle-sparing approach for thoracotomies in neonates, infants, and children. J Pediatr Surg. 1992;27:1157–1159. doi: 10.1016/0022-3468(92)90579-v. discussion, 1159–1160. [DOI] [PubMed] [Google Scholar]
- 14.Bal S. Elshershari H. Celiker R. Celiker A. Thoracic sequels after thoracotomies in children with congenital cardiac disease. Cardiol Young. 2003;13:264–267. [PubMed] [Google Scholar]
- 15.Chetcuti P. Myers NA. Phelan PD, et al. Chest wall deformity in patients with repaired esophageal atresia. J Pediatr Surg. 1989;24:244–247. doi: 10.1016/s0022-3468(89)80003-x. [DOI] [PubMed] [Google Scholar]
- 16.Frola C. Serrano J. Cantoni S, et al. CT findings of atrophy of chest wall muscle after thoracotomy: Relationship between muscles involved and type of surgery. AJR Am J Roentgenol. 1995;164:599–601. doi: 10.2214/ajr.164.3.7863878. [DOI] [PubMed] [Google Scholar]
- 17.Kucukarslan N. Kirilmaz A. Arslan Y, et al. Muscle sparing thoracotomy in pediatric age: A comparative study with standard posterolateral thoracotomy. Pediatr Surg Int. 2006;22:779–783. doi: 10.1007/s00383-006-1776-7. [DOI] [PubMed] [Google Scholar]
- 18.Van Biezen FC. Bakx PA. De Villeneuve VH. Hop WC. Scoliosis in children after thoracotomy for aortic coarctation. J Bone Joint Surg Am. 1993;75:514–518. doi: 10.2106/00004623-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Westfelt JN. Nordwall A. Thoracotomy and scoliosis. Spine. 1991;16:1124–1125. doi: 10.1097/00007632-199109000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Silen ML. Canvasser DA. Kurkchubasche AG, et al. Video-assisted thoracic surgical repair of a foramen of Bochdalek hernia. Ann Thorac Surg. 1995;60:448–450. doi: 10.1016/0003-4975(95)00100-y. [DOI] [PubMed] [Google Scholar]
- 21.Yang EY. Allmendinger N. Johnson SM, et al. Neonatal thoracoscopic repair of congenital diaphragmatic hernia: Selection criteria for successful outcome. J Pediatr Surg. 2005;40:1369–1375. doi: 10.1016/j.jpedsurg.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Liem NT. Thoracoscopic surgery for congenital diaphragmatic hernia: A report of nine cases. Asian J Surg. 2003;26:210–212. doi: 10.1016/S1015-9584(09)60305-5. [DOI] [PubMed] [Google Scholar]
- 23.Arca MJ. Barnhart DC. Lelli JL, Jr, et al. Early experience with minimally invasive repair of congenital diaphragmatic hernias: Results and lessons learned. J Pediatr Surg. 2003;38:1563–1568. doi: 10.1016/s0022-3468(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 24.Becmeur F. Reinberg O. Dimitriu C, et al. Thoracoscopic repair of congenital diaphragmatic hernia in children. Semin Pediatr Surg. 2007;16:238–244. doi: 10.1053/j.sempedsurg.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Riehle KJ. Magnuson DK. Waldhausen JH. Low recurrence rate after Gore-Tex/Marlex composite patch repair for posterolateral congenital diaphragmatic hernia. J Pediatr Surg. 2007;42:1841–1844. doi: 10.1016/j.jpedsurg.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Bagolan P. Casaccia G. Nahom A, et al. Severe congenital diaphragmatic hernia (CDH): a critical analysis of eight years' experience. Eur J Pediatr Surg. 2002;12:95–100. doi: 10.1055/s-2002-30159. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen TL. Le AD. Thoracoscopic repair for congenital diaphragmatic hernia: Lessons from 45 cases. J Pediatr Surg. 2006;41:1713–1715. doi: 10.1016/j.jpedsurg.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 28.Cho SD. Krishnaswami S. McKee JC, et al. Analysis of 29 consecutive thoracoscopic repairs of congenital diaphragmatic hernia in neonates compared to historical controls. J Pediatr Surg. 2009;44:80–86. doi: 10.1016/j.jpedsurg.2008.10.013. discussion, 86. [DOI] [PubMed] [Google Scholar]
- 29.Tsao K. Lally KP. The Congenital Diaphragmatic Hernia Study Group: A voluntary international registry. Semin Pediatr Surg. 2008;17:90–97. doi: 10.1053/j.sempedsurg.2008.02.004. [DOI] [PubMed] [Google Scholar]