Abstract
Background
Several studies have demonstrated the importance of personality constructs on health behaviors and health status. Having a pessimistic outlook has been related to negative health behaviors and higher mortality. However, the construct has not been well explored in cancer populations.
Methods
Survival time of 534 adults, who were diagnosed with lung cancer and had a pessimistic explanatory style, was examined. The patients had completed the Minnesota Multiphasic Personality Inventory (MMPI) approximately 18.2 years prior to receiving their lung cancer diagnosis. MMPI Optimism-Pessimism (PSM) scores were divided into high (60 or more) and low scores (less than 60), and log-rank tests and Kaplan-Meier curves were used to determine survival differences. Multivariate Cox models were used for assessing prognostic values of pessimism along with other known predictors for lung cancer survival outcome. Booting strapping of the survival models was used as a sensitivity analysis.
Results
At the time of lung cancer diagnosis, patients were on average 67 years old; 48% were female; 85% had non-small cell lung cancer (NSCLC); 15% had small cell lung cancer (SCLC); 30% were stage I; 4% were stage II; 31% were stage III/limited; and 35% were stage IV/extensive. Patients who exhibited a non-pessimistic explanatory style survived approximately six months longer than patients classified as having a pessimistic explanatory style.
Conclusion
Among lung cancer patients, those having a pessimistic explanatory style experienced less favorable survival outcome, which may be related to cancer treatment decisions. Further research in this area is warranted.
Keywords: Explanatory Style, Optimism, Pessimism, Lung Cancer, MMPI, Survival
INTRODUCTION
“Mind-body” relationships have been revered since the time of Socrates. Personality or emotional factors may have a direct impact on physiological states or mind, which are evident in the current emphasis on stress-reduction, relaxation, meditation, and activities related to disease prevention and wellness promotion. Indeed, psychosocial factors may be predictive of poor disease outcome, including cancer survivorship. Pessimism and optimism are personality constructs that have been shown to be important in the general population and in some medical populations.1, 2 Having a pessimistic explanatory style means the individual attributes bad events to internal, stable, and global causes; he/she tends toward self-blame, fatalism, and catastrophic thinking.3 Recently, the role of having a pessimistic explanatory style has been explored in several cancer populations. For example, Kung and colleagues found that having a pessimistic explanatory style was associated with poor quality of life in head and neck and thyroid cancer survivors;4 Petersen and colleagues found that a pessimistic explanatory style was predictive of poor quality of life in 268 breast cancer survivors.5 In contrast, optimism has also been associated with positive health outcomes in a number of studies; for instance, higher levels of optimism have been associated with lower blood pressure during daily life6, better recovery from coronary artery bypass surgery7, and longer survival in head and neck cancer patients.8 However, such factors may be of little importance when the body experiences serious or fatal medical conditions, or potentially lethal diseases such as lung cancer. Using Seligman’s theory of causal attribution,3 the current retrospective study examined the relationship between a pessimistic explanatory style and survival in a group of patients diagnosed with lung cancer.
METHODS AND MATERIALS
Study Design and Patients
The present study is a retrospective, observational cohort design aimed at examining the relationship between explanatory style, as measured by scores on the Optimism-Pessimism (PSM) scale of the Minnesota Multiphasic Personality Inventory (MMPI), and survival in patients diagnosed with lung cancer. Participants in this study were primary lung cancer patients who were enrolled into the Epidemiology and Genetics of Lung Cancer Research Program at Mayo Clinic Rochester since 1997, in which all patients at Mayo Clinic who were diagnosed with lung cancer have been offered enrollment in a prospective cohort study.9–12 All patients enrolled provided informed consent, and the study has been approved by the Institutional Review Board. Trained study personnel reviewed the medical records; all patients completed health-related surveys when they entered the cohort study and again at 6 and 12 months and were mailed similar surveys on an annual basis. Information on demographics, previous or concurrent illnesses, tobacco usage and exposure, tumor staging, nutritional habits, and cancer therapy were abstracted and entered into the database. Comorbidities were combined into three variables for having any other lung disease, any other cancer, and any other disease. Having another lung disease included asthma, chronic bronchitis, emphysema, COPD, pneumonia, tuberculosis, or cystic fibrosis. Any other cancer was defined as a cancer diagnosis except lung cancer. Any other disease was defined as any disease except cancer or a lung disease; this category included diabetes, heart disease, arthritis, anemia, migraines, drug addiction, and any other medical problem. For a more detailed discussion of variable definitions, see Visbal, et al 2004.13 The Revised TNM Staging System of non- small cell lung cancer was utilized.14 All cancer treatment decisions were deferred to the individual patient’s healthcare providers, and enrollment into the research program did not in any way influence clinical decision-making. Patients in our current study may have been referred for routine evaluation or as a result of physician concern about psychological functioning. The study used retrospective data from 534 individuals diagnosed with lung cancer who had completed the MMPI prior to receiving their lung cancer diagnosis. Medical records included information on age, sex, disease stage, smoking status, and survival status. If patients completed more than one MMPI, the PSM score from the earliest MMPI completed prior to lung cancer diagnosis was used to assess PSM. The MMPI was completed at the request of the patient’s physicians as part of the patient’s medical care or as a participant in a large research study. Patients who were asked to complete an MMPI were more likely among the most complex of referrals at our tertiary care medical center, since the physician making the request believed there were psychological factors intertwined with the presenting symptoms.
Measures
Smoking Status
Patients who reported smoking fewer than 100 cigarettes in their lifetime were classified as “never smokers.” Patients who had not smoked a cigarette in the past 30 days were classified as “former smokers”, and patients who reported smoking any cigarette in the past 30 days were classified as “current smokers.”15
The Minnesota Multiphasic Personality Inventory
The original MMPI consisted of 550 unique true/false items about thoughts, feelings, attitudes, physical symptoms, emotional symptoms, and previous life experiences.16 The original MMPI and its current revision, the MMPI-2,17 have been the most widely used and thoroughly researched of the self-reported measures of personality functioning.18 All of the analyses in the manuscript are based on the original MMPI.
The PSM scale for the MMPI was derived from Martin E.P. Seligman’s theory of explanatory style19 and constructed from the items of the MMPI item pool.20 According to Seligman’s theory, the manner in which individuals explain the cause of significant life events (both good and bad events) exerts considerable influence over three aspects of their future physical and mental health, which include decreased quality of physical health, increased risk for depression, and reduced occupational or academic achievement.21 More specifically, Seligman’s theory postulates that people who (1) attribute the causes of adverse events in their lives to themselves (i.e., an interval explanation, “It’s me…”), (2) carry the expectation that the condition will persist (i.e., a stable explanation, “… happened again, as usual…”), and (3) that it will affect other aspects of their functioning (e.g., a global explanation, “… and now my life will be ruined; I’ll never get to…”) are at risk for undermining their subsequent physical health, emotional and mental functioning, and life achievement.
The PSM scale yields normalized T-scores (mean=50, SD=10). Lower PSM scores indicate having an optimistic explanatory style; whereas, higher scores indicate a pessimistic explanatory style. Similar to previous research, PSM scores were divided into two groups for analysis: PSM scores representing the non-pessimistic (e.g., optimistic) patients were in the first group (PSM scores of less than 60). Subjects with PSM scores of 60 or higher (one standard deviation above the mean) were considered to have a pessimistic explanatory style.4, 5 While this cut point has been used in other studies of pessimists,1, 2, 4 it is not based upon a clinical cutoff score. We used recursive partitioning22 to determine the appropriateness of the score as our cut point when modeling survival times; the optimal cut point for our study cohort fell at 58, but we used a normalized score of 60 as being nearly optimal for the purpose of comparability with published literature.
Analyses
PSM scores were divided into pessimistic explanatory style or non-pessimistic explanatory style, and t-tests were used to determine demographic differences between patients’ highest and lowest in PSM scores. Log-rank tests and Kaplan-Meier curves were used to determine survival differences. Stepwise Cox proportional hazards models were used to model survival adjusting for age, gender, smoking status, cancer type, stage, and comorbidities. Variables were entered into the model one at a time. Modeling stopped when no other variables met the 0.05 significance level for entry into the model. Backwards modeling (starting with a saturated model and dropping one variable at a time) was used to confirm the results of the stepwise model. Martingale residuals were explored to determine the functional form of the PSM variable, and diagnostic checks were conducted to verify the fit of the final models. Bootstrapping of the survival analysis models was carried out as a sensitivity test of our findings by generating 10,000 random samples from with replacement from our observed dataset. 23
RESULTS
A total of 534 subjects had completed a MMPI prior to receiving their lung cancer diagnosis between 1997 and 2006. Table 1 shows patients with and without a MMPI. There is no indication that patients with MMPI scores were clinically different on these characteristics than patients who did not complete an MMPI, and explanatory style is unlikely to have biased completion rates for the MMPIs among medical outpatients.24 Table 2 provides baseline demographics for the 534 patients who form the basis of this report. On average, patients had completed the MMPI 18.2 years prior to being diagnosed with lung cancer. Most patients had non small cell lung cancer [NSCLC] (85%), and 34% of patients with NSCLC had early stage (I or II) lung cancer. Additionally, most patients were current or former smokers (80%). None of the demographic variables were significantly different between patients with a pessimistic explanatory style and patients with a non-pessimistic explanatory style.
Table 1.
Selected characteristics of lung cancer patients who completed the MMPI prior to being diagnosed with lung cancer.
| Characteristics1 | MMPI2 (N=534) | No MMPI (N=8786) |
|---|---|---|
| Age at DX3 | ||
| Mean (SD) | 67.4 (10.39) | 65.7 (11.14) |
| Stage | ||
| I | 147 (30) | 1999 (23) |
| II | 21 (4) | 589 (7) |
| III/Limited | 152 (31) | 2659 (31) |
| IV/Extensive | 176 (36) | 3310 (39) |
| Cancer Type | ||
| SCLC | 72 (15) | 933 (11) |
| NSCLC | 424 (85) | 7624 (89) |
| Median Time From Diagnosis to Last Followup | 14.8 mos | 15.5 mos |
Unless specified, number and percentage in parentheses are presented.
Minnesota Multiphasic Personality Inventory.
Diagnosis.
Table 2.
Characteristics of 534 lung cancer patients who completed an MMPI
| Non-Pessimistic Explanatory Style PSM<60 (n=304) | Pessimistic Explanatory Style PSM>60 (n=230) | Total (n=534) | |
|---|---|---|---|
| Characteristics1 | |||
| Age at MMPI2 Mean (SD) | 48.4 (12.94) | 49.9 (12.49) | 49.0 (12.76) |
| Median | 49 | 51 | 50.0 |
| Range | 14 to 97 | 15 to 78 | 14 to 97 |
| Age at DX3 Mean (SD) | 67.6 (11.10) | 67.0 (9.40) | 67.4 (10.39) |
| Median | 68.5 | 68.0 | 68 |
| Range | 34 to 98 | 39 to 88 | 34 to 98 |
| Years From MMPI to DX Mean (SD) | 19.0 (10.08) | 17.1 (9.38) | 18.2 (9.82) |
| Median | 18.6 | 16.6 | 17.5 |
| Range | 0.01 to 43.5 | 0.02 to 43.4 | 0.01 to 43.5 |
| Smoking Status | |||
| Missing | 1 | 2 | 3 |
| Never Smoker | 48 (16) | 21 (9) | 69 (13) |
| Former Smoker | 117 (39) | 89 (39) | 206 (39) |
| Current Smoker | 117 (39) | 103 (45) | 220 (41) |
| Current or former smoker | 21 (7) | 15 (7) | 36 (7) |
| Gender | |||
| Female | 149 (49) | 108 (47) | 257 (48) |
| Male | 155 (51) | 122 (53) | 277 (52) |
| Cancer Type | |||
| SCLC | 37 (13) | 35 (16) | 72 (15) |
| NSCLC | 243 (87) | 181 (84) | 424 (85) |
| Stage | |||
| Missing | 24 | 14 | 38 |
| I | 82 (28) | 65 (30) | 147 (30) |
| II | 10 (4) | 11 (5) | 21 (4) |
| III/Limited | 94 (34) | 58 (27) | 152 (31) |
| IV/Extensive | 94 (34) | 82 (38) | 176 (36) |
| Any Surgery | |||
| Yes | 123 (40) | 75 (33) | 198 (37) |
| No | 181 (60) | 155 (67) | 336 (63) |
| Any Chemotherapy | |||
| Yes | 85 (28) | 62 (27) | 147 (28) |
| No | 219 (72) | 168 (73) | 387 (72) |
| Any Radiation Therapy | |||
| Yes | 69 (23) | 40 (17) | 109 (20) |
| No | 235 (77) | 190 (83) | 425 (80) |
| Any Other Cancer | |||
| Yes | 32 (11) | 34 (15) | 66 (12) |
| No | 272 (89) | 196 (85) | 468 (88) |
| Any Other Lung Disease | |||
| Yes | 61 (20) | 53 (23) | 114 (21) |
| No | 243 (80) | 177 (77) | 420 (79) |
Unless specified, number and percentage in parentheses are presented.
Minnesota Multiphasic Personality Inventory.
Diagnosis.
There were 110 patients who completed more than one MMPI before their lung cancer diagnosis. Of the 110 patients with multiple MMPI scores, 12 (11%) went from being classified as pessimists to non-pessimists and 13 (12%) changed from non-pessimists to pessimists. The median change for the patients was a decrease of one-half point on their PSM scores. We used the first PSM score reported prior to lung cancer diagnosis in order to achieve a greater temporal span between the PSM score and cancer diagnosis. This minimizes the likelihood that a PSM score was affected by proximity to the personal stresses of receiving a diagnosis of lung cancer and the significant symptoms of illness preceding the cancer diagnosis related to the cancer.
Pessimistic Explanatory Style and Survival
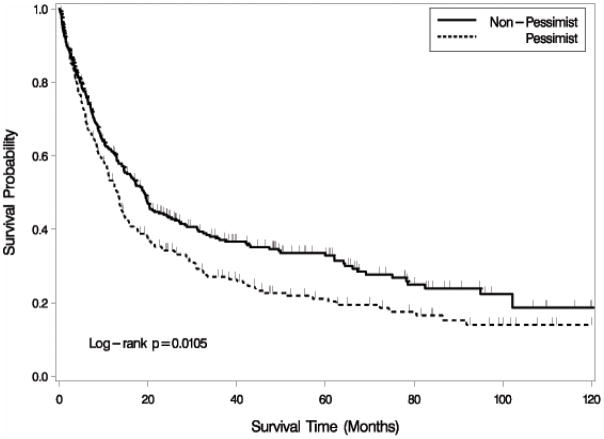
Table 3 shows median survival times (in months) and the five-year survival rate for all 534 patients and compares patients with low versus high scores on the PSM scale. Patients (both women and men) in the non-pessimistic category survived about six months longer compared to patients with a pessimistic explanatory style, as shown in the Kaplan-Meier curve in Figure 1. Five year survival rates for the two groups were 32.9% for non-pessimists and 21.1% for pessimists.
Table 3.
Median Survival (Months) by PSM Groups
| 95% Confidence Interval | 5 Year Survival Rate | ||||
|---|---|---|---|---|---|
| Median | Lower | Upper | Log-rank p-value | ||
| Overall population | 14.8 | 13.4 | 18.7 | 27.8% | |
| PSM<60 (Non-pessimistic) | 19.2 | 14.8 | 23.5 | 0.0105 | 32.9% |
| 60≤PSM (Pessimistic) | 13.1 | 10.9 | 14.8 | 21.1% | |
Figure 1.
Kaplan-Meier Curve of Survival Time by PSM Groups. Patients with a pessimistic explanatory style have shorter survival times than other patients.
The results from univariate Cox models are shown in Table 4. Stage of disease and treatment were highly correlated, i.e., 95% of patients with stage II cancer had surgery, 30% of patients with stage III had surgery, and only 6% of patients with stage IV had surgery. Since there was strong colinearity between stage and treatment, only treatment was used in the multivariate models along with other covariates. Results from the stepwise multivariate model using pessimists versus non-pessimists are shown in Table 5, confirming that patients with a pessimistic explanatory style have significantly worse survival rates even after adjusting for other known prognostic variables (hazard ratio=1.25, p=0.03). Having comorbidities seems associated with significantly better survival in (Table 4); however, the effects are confounded by age, smoking status, and treatment. In particular, patients with comorbidities were more likely to have surgery and more likely to be current smokers. Patients with other cancers were on average about five years older than patients without other cancers. After adjusting for these confounding effects as presented in Table 5, the effects of comorbidities are no longer significant.
Table 4.
Univariate Cox Models for Survival
| Variable | p-value | Hazard Ratio | 95% Hazard Ratio Confidence Limits | |
|---|---|---|---|---|
| Pessimist (60+) | 0.0108 | 1.305 | 1.064 | 1.601 |
| Age at Diagnosis | <0.0001 | 1.022 | 1.012 | 1.033 |
| Male | 0.0141 | 1.292 | 1.053 | 1.585 |
| Stage II | 0.1500 | 0.655 | 0.368 | 1.165 |
| Stage III/Limited | 0.2087 | 1.155 | 0.923 | 1.445 |
| Stage IV/Extensive | <0.0001 | 4.134 | 3.305 | 5.171 |
| Former Smoker | 0.0452 | 0.805 | 0.651 | 0.995 |
| Current Smoker | 0.0045 | 1.346 | 1.097 | 1.653 |
| Current or former smoker | 0.0034 | 1.706 | 1.193 | 2.441 |
| Chemotherapy | 0.0058 | 1.365 | 1.094 | 1.703 |
| Radiation | 0.0253 | 1.317 | 1.035 | 1.676 |
| Surgery | <0.0001 | 0.234 | 0.182 | 0.300 |
| Other Lung Treatment | 0.2965 | 1.601 | 0.662 | 3.870 |
| Any Cancer | 0.0675 | 0.748 | 0.549 | 1.021 |
| Any Lung Disease | 0.0012 | 0.655 | 0.506 | 0.846 |
| Any Other Disease | <0.0001 | 0.391 | 0.293 | 0.521 |
Table 5.
Multivariate Cox Model for Survival Including Pessimism, Treatment, and Comorbidities
| Variable | p-value | Hazard Ratio | 95% Hazard Ratio Confidence Limits | |
|---|---|---|---|---|
| Pessimist (60+) | 0.0339 | 1.25 | 1.02 | 1.55 |
| Age at Diagnosis | <0.0001 | 1.04 | 1.03 | 1.05 |
| Male | 0.02 | 1.28 | 1.04 | 1.58 |
| Any Surgery | <0.0001 | 0.23 | 0.17 | 0.31 |
| Any Radiation Therapy | 0.17 | 1.22 | 0.92 | 1.63 |
| Any Chemotherapy | 0.70 | 0.95 | 0.73 | 1.24 |
| Current Smoker | 0.0005 | 1.54 | 1.21 | 1.96 |
| Some Smoking History | 0.0005 | 2.02 | 1.36 | 3.01 |
| Any Other Cancer | 0.35 | 1.18 | 0.83 | 1.66 |
| Any Other Lung Disease | 0.66 | 1.07 | 0.80 | 1.42 |
A stratified analysis was used to assess the relationship between pessimism and stage. Results suggest that having a pessimistic explanatory style is prognostic for survival in patients with stage I or II cancer but is not prognostic for patients with stage III or IV cancer. The observation was supported by a multivariate Cox model using only patients with stage I or II lung cancer (Table 6). After adjusting for demographics, comorbidities, and treatment, having a pessimistic explanatory style was still significantly related to shorter survival times (log-rank test p=0.02, hazard ratio=1.91).
Table 6.
Multivariate Cox Model for Survival for Patients with Stage I or II Cancer
| Variable | p-value | Hazard Ratio | 95% Hazard Ratio Confidence Limits | |
|---|---|---|---|---|
| Pessimist (60+) | 0.02 | 1.91 | 1.10 | 3.29 |
| Age at Diagnosis | 0.001 | 1.06 | 1.02 | 1.10 |
| Male | 0.17 | 1.47 | 0.85 | 2.53 |
| Any Surgery | 0.01 | 0.39 | 0.19 | 0.81 |
| Any Radiation Therapy | 0.10 | 1.95 | 0.87 | 4.36 |
| Any Chemotherapy | 0.64 | 1.21 | 0.53 | 2.76 |
| Current Smoker | 0.03 | 1.87 | 1.06 | 3.31 |
| Some Smoking History | 0.10 | 2.52 | 0.84 | 7.57 |
| Any Other Cancer | 0.33 | 0.72 | 0.38 | 1.38 |
| Any Other Lung Disease | 0.25 | 1.42 | 0.79 | 2.56 |
Several different sensitivity analyses provided results that were similar to the original analyses. A simulation involving 10,000 bootstrapped samples showed the robustness of the results. Seventy-two percent of the bootstrapped samples resulted in significant p-values, with non-pessimists having longer survival times than pessimists. In the A small minority of samples (0.4% or 42 out of 10,000 samples) had results that showed that had results with pessimists having longer survival times, but the survival differences were not significant in all of those samples.
Another sensitivity analysis used the last MMPI score reported before diagnosis instead of the first reported MMPI. In this analysis, the log-rank p-value for differences in survival increased slightly and the difference in median survival decreased from 6.0 months to 5.3 months.
The relationship between depression (as measured by MMPI, Scale 2, Depression) and survival was also explored, but there was no indication that depression was related to survival time (data not shown).
DISCUSSION
This retrospective study examined the relationship between survival and explanatory style using scores from the PSM scale of the MMPI. The major finding in the study was that, explanatory style, obtained from a large sample of patients diagnosed with lung cancer and measured (on average) 18 years prior to receiving their lung cancer diagnosis, was statistically significant and clinically relevant for survival. More specifically, patients classified as having an optimistic or a non-pessimistic explanatory style survived an average of six months longer compared to the patients with a pessimistic explanatory style. Furthermore, the relationship was independent of smoking status, cancer stage, treatment, comorbidities, age, and gender.
In examining stage of cancer, the prolonged survival time was only sustained among patients with stage I or II lung cancer, which indicates that if a patient has advanced disease, then the potentially protective aspect of a non-pessimistic explanatory style can be overwhelmed by the severity of the disease process. Nevertheless, the results support the premise that if a patient is diagnosed with lung cancer, and if the patient has a pessimistic explanatory style, the patient may be less likely to have surgery, and may live six months less compared to patients with a non-pessimistic style. This may be due to the overall risk for comorbid medical problems (i.e., as predicted by Seligman’s theory of pessimistic causal attribution), which in turn decreases the likelihood of receiving a surgical intervention. However, if a patient’s lung cancer was identified in the early stage, and if the patient has a non-pessimistic explanatory style, then the patient’s estimated survival is significantly improved, in part because surgical resection of the tumor can be completed as a curative treatment. This six-month potential benefit related to an optimistic explanatory style is more impressive when one considers that the median survival time for this lung cancer patient population is less than one year.9
Seligman’s theory of explanatory style has been shown to be predictive of health status, mortality, health behaviors, and quality of life among general medical outpatients. The examination of a possible relationship between a pessimistic explanatory style and survivorship in oncology populations is a relatively new and provocative area of investigation. Such studies have yielded mixed results. Some suggest that having a pessimistic explanatory style prior to receiving a cancer diagnosis might be predictive of survival time and immune function, while others have not found such an association. Rausch and colleagues25 found that higher levels of optimism were associated with improved immune function in women newly diagnosed with early stage breast cancer. Even in healthy subjects, researchers have found optimism to be associated with higher immune parameters, including higher T-lymphocyte numbers and natural killer cell activity.26, 27 In a population of black women coinfected with human immunodeficiency virus and human papillomavirus (HPV), Byrnes and colleagues28 found that women who were more optimistic had better immunity. Specifically, greater optimism was related to higher natural killer cell cytotoxicity and cytotoxic/suppressor cell numbers after controlling for presence/absence of HPV, behavioral/lifestyle factors, and subjective impact of negative life events.
In a recent review article Coyne and colleagues,29 noted mixed results from studies (often flawed by small samples and numerous potential confounders) examining broad personality factors related to disease progression and mortality among cancer patients. In summarizing the literature, Coyne and colleagues decry the lack of substantial support for widely held beliefs about the impact of personality factors, using as a research model their own large-scale work in head and neck cancers. Contrary to Coyne and colleagues’ view, we deliberately chose not to use a broad measure of emotional well being. Rather, we used a theory-derived measure, characterizing an enduring psychological and cognitive construct known as causal attribution. A summary of our approach follows:
Originally, Mayo Clinic researchers collaborated with Seligman, and using Seligman’s procedures for analyzing expository text developed and published a bidirectional scale of Optimism-Pessimism (PSM) derived from the item pool of the original MMPI. Thus, Seligman’s theory of causal attribution was operationalized in the constructs of durable “pessimistic” and “optimistic” personality traits. According to Seligman’s theory, individuals with a pessimistic explanatory style are at significant risk for later problems in three important areas of life functioning: (a) greater likelihood of adverse medical conditions, (b) proneness toward mental health issues (particularly depression), and (c) reduced achievement (either occupational or academic).
Seligman’s theory and the PSM scale have been validated among general medical outpatients. For example, a pessimistic explanatory style was significantly associated with increased mortality among medical outpatients who completed the MMPI approximately 30 years prior to follow up.1 Overall, results from several previous studies30–34 have added considerable support to Seligman’s theory and the potentially adverse impact of having a pessimistic explanatory style. In general, we have found, as the theory predicts, patients classified as having a pessimistic explanatory style are at risk for poorer medical outcomes, while being in the non-pessimist range of scores appears to be a psychological protective factor. Seligman’s theory predicts that lung cancer patients who were classified as having a pessimistic explanatory style would be at risk for gradually accruing adverse medical conditions over time, which may be related to the decreased likelihood of surgical treatment for patients in this explanatory category. Note: we are not implying a causal relationship between a pessimistic explanatory style and the development of lung cancer.
Some limitations of the study should be considered when interpreting the results. First, the retrospective design of the present study does not ensure that the measures were given at consistent times or to all possible participants. Patients who completed a MMPI might have exhibited behaviors or coping styles different from individuals who did not complete the MMPI; thereby, introducing selection bias into the study sample. Another potential source of bias relates to the measurement of optimism-pessimism at only one point in time. There is uncertainty about whether a relationship existed between explanatory style and survival prior to diagnosis. A related limitation is that information was not available on cancer recurrence or co-morbid medical conditions that may have occurred since the time of cancer diagnosis and could impact survival. Therefore, longitudinal studies are recommended to further investigate these possibilities. A final limitation is the generalizability of the results; the sample was primarily Caucasian and only consisted of patients diagnosed with lung cancer, therefore, the results may not apply to more diverse populations or other types of cancer.
Despite the limitations, our study adds to the growing literature on explanatory style (e.g., optimism-pessimism) and survival in the general population, in medical populations, and specifically in lung cancer patients. Future investigations may benefit from designing and testing interventions, which address enhancing positive aspects of explanatory style and evaluating the potential physiological mechanisms responsible for increased survival. For example, patients diagnosed with cancer could learn cognitive behavioral techniques to challenge negative thinking patterns and engage in effective, accurate problem solving. This may ultimately aid in enhancing current approaches to patient care, such that clinicians may improve survival not only by developing new medical treatments but also by targeting patient’s psychosocial characteristics most likely to negatively affect cancer treatment decisions and ultimate outcomes.
Acknowledgments
Funding Source: This work was support by National Institutes of Health grants, R01 CA 115857 and R01 CA 84354, awarded to Ping Yang, M.D., Ph.D.
We would like to thank Susan Ernst, M.A., for her technical assistance with the manuscript.
Footnotes
CONFLICTS OF INTEREST
None.
References
- 1.Maruta T, Colligan RC, Malinchoc M, et al. Optimists vs pessimists: survival rate among medical patients over a 30-year period. Mayo Clin Proc. 2000;75:140–143. doi: 10.4065/75.2.140. [DOI] [PubMed] [Google Scholar]
- 2.Maruta T, Colligan RC, Malinchoc M, et al. Optimism-pessimism assessed in the 1960s and self-reported health status 30 years later. Mayo Clin Proc. 2002;77:748–753. doi: 10.4065/77.8.748. [DOI] [PubMed] [Google Scholar]
- 3.Peterson C, Seligman ME. Causal explanations as a risk factor for depression: theory and evidence. Psychol Rev. 1984;91:347–374. [PubMed] [Google Scholar]
- 4.Kung S, Rummans TA, Colligan RC, et al. Association of optimism-pessimism with quality of life in patients with head and neck and thyroid cancers. Mayo Clin Proc. 2006;81:1545–1552. doi: 10.4065/81.12.1545. [DOI] [PubMed] [Google Scholar]
- 5.Petersen LR, Clark MM, Novotny P, et al. Relationship of optimism-pessimism and health-related quality of life in breast cancer survivors. J Psychosoc Oncol. 2008;26:15–32. doi: 10.1080/07347330802359578. [DOI] [PubMed] [Google Scholar]
- 6.Raikkonen K, Matthews KA, Flory JD, et al. Effects of optimism, pessimism, and trait anxiety on ambulatory blood pressure and mood during everyday life. J Pers Soc Psychol. 1999;76:104–113. doi: 10.1037//0022-3514.76.1.104. [DOI] [PubMed] [Google Scholar]
- 7.Scheier MF, Matthews KA, Owens JF, et al. Optimism and rehospitalization after coronary artery bypass graft surgery. Arch Intern Med. 1999;159:829–835. doi: 10.1001/archinte.159.8.829. [DOI] [PubMed] [Google Scholar]
- 8.Allison PJ, Guichard C, Fung K, et al. Dispositional optimism predicts survival status 1 year after diagnosis in head and neck cancer patients. J Clin Oncol. 2003;21:543–548. doi: 10.1200/JCO.2003.10.092. [DOI] [PubMed] [Google Scholar]
- 9.Yang P, Allen MS, Aubry MC, et al. Clinical Features of 5,628 Primary Lung Cancer Patients: Experience at Mayo Clinic from 1997–2003. Chest. 2005;128:452–462. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z, Aubry MC, Deschamps C, et al. Histologic Grade is an Independent Prognostic Factor for Survival in Non-Small Cell Lung Cancer: An Analysis of 5018 Hospital- and 712 Population-Based Cases. Journal of Thoracic & Cardiovascular Surgery. 2006;131:1014–1020. doi: 10.1016/j.jtcvs.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 11.Sugimura H, Yang P. Long-term Survivorship in Lung Cancer. A review Chest. 2006;29:1088–1097. doi: 10.1378/chest.129.4.1088. [DOI] [PubMed] [Google Scholar]
- 12.Jatoi A, Williams B, Nichols FC, et al. Is Voluntary Vitamin and Mineral Supplementation Associated with Better Outcome in Non-Small Cell Lung Cancer? Results from the Mayo Clinic Lung Cancer Cohort. Lung Cancer. 2005;49:77–84. doi: 10.1016/j.lungcan.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Visbal AL, Williams BA, Nichols FC, et al. Gender Differences in Non-Small Cell Lung Cancer Survival: An Analysis of 4,618 Patients Diagnosed Between 1997–2002. Ann Thorac Surg. 2004;78(1):209–215. doi: 10.1016/j.athoracsur.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111(6):1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 15.Clark MM, Croghan IT, Reading S, et al. The relationship of body image dissatisfaction to cigarette smoking in college students. Body Image. 2005;2:263–270. doi: 10.1016/j.bodyim.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Hathaway S, McKinley J. The Minesota Multiphasic Personality Inventory. Minneapolis, Minnesota: University of Minnesota; 1943. [Google Scholar]
- 17.Butcher JN, Dahlstrom WG, Graham JR, et al. Manual for Administration and Scoring: MMPI-2, Minnesota Multiphasic Personality Inventory-2 (Revised Edition) Minneapolis: University of Minnesota; 2001. [Google Scholar]
- 18.Butcher JN. A Beginner’s Guide to the MMPI-2. 2. Washington: American Psychological Association; 2005. [Google Scholar]
- 19.Gillham JE, Shatte AJ, Reivich KJ, et al. Optimism, Pessimism, and Explanatory Style. In: Chang EC, editor. Optimism & Pessimism: Impllicaitons for Theory, Research, and Practice. Washington: American Pyschological Association; 2001. [Google Scholar]
- 20.Colligan RC, Offord KP, Malinchoc M, et al. CAVEing the MMPI for an Optimism-Pessimism Scale: Seligman’s attributional model and the assessment of explanatory style. J Clin Psychol. 1994;50:71–95. doi: 10.1002/1097-4679(199401)50:1<71::aid-jclp2270500107>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Seligman MEP. Explanatory styple: Predicting depression, achievemnt, and health. In: Yapko MD, editor. Brief Therapy Approaches to Treating Anxiety and Depression. New York: Brunner; 1989. [Google Scholar]
- 22.Therneau TM, Atkinson EJ. Technical Report #61. Rochester: Mayo Clinic; 1997. An Introduction to Recursive Partitioning Using the RPART Routines. [Google Scholar]
- 23.Efron B, Tibshirani RJ. An introduction to the bootstrap. London: Chapman & Hall; 2003. [Google Scholar]
- 24.McLeod TG, Costello BA, Colligan RC, et al. Personality Characteristics of Healthcare Satisfaction Survey Non-respondents. International Journal of Health Care Quality Assurance. 2009;22:145–156. doi: 10.1108/09526860910944638. [DOI] [PubMed] [Google Scholar]
- 25.Rausch SM, Auerbach SA, McCain NL, et al. The relationship between psychosocial and immune variables in American women with breast cancer. Tokoyo, Japan. 10th International Congress of Behavioral Medicine Meeting; 2008. [Google Scholar]
- 26.Cohen F, Kearney KA, Zegans LS, et al. Differential immune system changes with acute and persistent stress for optimists vs pessimists. Brain Behav Immun. 1999;13:155–174. doi: 10.1006/brbi.1998.0531. [DOI] [PubMed] [Google Scholar]
- 27.Segerstrom SC, Taylor SE, Kemeny ME, et al. Optimism is associated with mood, coping, and immune change in response to stress. J Pers Soc Psychol. 1998;74:1646–1655. doi: 10.1037//0022-3514.74.6.1646. [DOI] [PubMed] [Google Scholar]
- 28.Byrnes DM, Antoni MH, Goodkin K, et al. Stressful events, pessimism, natural killer cell cytotoxicity, and cytotoxic/suppressor T cells in HIV+ black women at risk for cervical cancer. Psychosom Med. 1998;60:714–722. doi: 10.1097/00006842-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Coyne JC, Pajak TF, Harris J, et al. Emotional well-being does not predict survival in head and neck cancer patients: a Radiation Therapy Oncology Group study. Cancer. 2007;110:2568–2575. doi: 10.1002/cncr.23080. [DOI] [PubMed] [Google Scholar]
- 30.Hermann BP, Trenerry MR, Colligan RC. Learned Helplessness, Attributional Style, and Deprssion in Epilepsy. Epilepsia. 1996;37:680–686. doi: 10.1111/j.1528-1157.1996.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 31.Malinchoc M, Rocca WA, Colligan RC, et al. Premorbid Personality Characteristics of Alzheimer’s Disease: An Exploatory Case-Control Study. European Journal of Neurology. 1997;4:227–230. doi: 10.3233/BEN-1997-10403. [DOI] [PubMed] [Google Scholar]
- 32.Ames SC, Vickers KS, Decker PA, et al. Select Minnesota Multiphasic Personality Inventory (MMPI) sclaes as predicotrs of tobacco abstinence following treatment for niotine dependence. Psychology and Health. 2005;20:331–351. [Google Scholar]
- 33.Brummett BH, Helms MJ, Dahlstrom WG, et al. Prediction of all-cause mortality by the Minnesota Multiphasic Personality Inventory Optimism-Pessimism Scale scores: study of a college sample during a 40-year follow-up period. Mayo Clin Proc. 2006;81:1541–1544. doi: 10.4065/81.12.1541. [DOI] [PubMed] [Google Scholar]
- 34.Grossardt BR, Bower JH, Geda YE, et al. Pessimistic, anxious, and depressive personality traits predict all-cause mortality: the Mayo Clinic cohort study of personality and aging. Psychosom Med. 2009;71:491–500. doi: 10.1097/PSY.0b013e31819e67db. [DOI] [PMC free article] [PubMed] [Google Scholar]