Abstract
Pax3 and Pax7 are closely related paired-boxed family transcription factors that are known to play important roles in embryonic and adult myogenesis. Previous reports describing the expression of Pax3 and Pax7 transcripts reveal expression in many overlapping domains. In this manuscript, we extend these studies by examining the protein expression profiles for Pax3 and Pax7 in developing chick somites and limbs with cellular resolution. Our studies show the existence of distinct subpopulations of cells in the somite and developing limb that are defined by the relative expression levels of Pax3 and Pax7. We also show that Pax3 and Pax7 negatively regulate each other's expression in the dermomyotome, thus providing a possible mechanism for the maintenance of observed expression patterns in the dermomyotome. Further characterization of Pax3 and/or Pax7 positive cells in the dermomyotome and myotome with respect to proliferation and differentiation reveals subpopulations of cells with distinct properties.
Keywords: Pax3, Pax7, MyoD, Myogenin, phosphohistone H3, BrdU, chick, somite, dermomyotome, myotome, limb, myogenesis
INTRODUCTION
Pax3 and Pax7 belong to the paired-box family of transcription factors (subgroup III), which is defined by the presence of a “paired” domain that functions in DNA binding. In addition to the paired domain, Pax3 and Pax7 also have a homeodomain, which is separated from the paired domain by an octapeptide region, and a transactivation domain. The transactivation domain is thought to associate with transcriptional co-activators (Murakami et al., 2006), transcriptional co-repressors (Hollenbach et al., 1999; Lehembre et al., 2001; Hsieh et al., 2006) and/or chromatin remodeling proteins (Magnaghi et al., 1998; Hsieh et al., 2006; McKinnell et al., 2007).
The participation of Pax3 and Pax7 in the generation and regeneration of skeletal muscle has stimulated wide interest in the biological roles of these two members of the paired box family of transcription factors. The role of Pax3 in the generation of skeletal muscle, particularly that of the limb, has long been appreciated as deficiencies in Pax3 lead to profound defects in limb musculature (Franz et al., 1993; Bober et al., 1994; Goulding et al., 1994). By contrast, deficiencies in Pax7 lead to no overt defects in early myogenesis and patterning (Seale 2000). Nonetheless, the importance of Pax7 in the generation of embryonic muscle is demonstrated by the observation that secondary muscle development is completely ablated in mice lacking functional Pax3 and Pax7 (Relaix 2005).
Regeneration of skeletal muscle cells is thought to occur via activation of satellite cells and/or CD45+:ScaI+ stem cells. Until recently, the embryonic origin of satellite cells was unknown. It is now thought that satellite cells of the body wall originate from a population of proliferative Pax3+/Pax7+ stem cells in the central dermomyotome (CD) of developing chicks and mice (Ben-Yair and Kalcheim, 2005; Gros et al., 2005; Kassar-Duchossoy et al., 2005; Relaix et al., 2005) while satellite cells of the limb are primarily derived from Pax3+/Pax7+ cells of the hypaxial dermomyotome (Schienda et al., 2006). Consistent with the expression of Pax7 in these satellite cell progenitor cells, knockout studies in mice show that Pax7 participates in the maintenance and/or specification of adult satellite cells (Seale et al., 2000; Oustanina et al., 2004; Relaix et al., 2006). Recently, roles for Pax3 in skeletal muscle regeneration have also been reported. Two reports suggest that Pax3 positive cells in interstitial (Kuang et al., 2006) and/or satellite (Relaix et al., 2006) cell niches can partially, but not fully, compensate for the loss of Pax7. In contrast to satellite cells, CD45+/ScaI+ stem cells are still present in Pax7−/− mice. Nonetheless, Pax7 is sufficient and required for myogenic specification of these cells (Seale et al., 2004).
While the importance of Pax3 and Pax7 in skeletal muscle development and regeneration is clear, the molecular and cellular mechanisms that are mediated by Pax3 and Pax7 are less well understood. This gap in our knowledge is exacerbated by that fact that although Pax3 and Pax7 are functionally and spatially redundant in a number of tissues (Relaix et al., 2004; Horst et al., 2006; Otto et al., 2006) and are able to act as upstream regulators of myogenic regulatory factors (Maroto et al., 1997; Tajbakhsh et al., 1997; Seale et al., 2004; Zammit et al., 2006; McKinnell et al., 2007), divergent roles exist (Relaix et al., 2004). While early studies with Pax3 suggested roles in cell proliferation, survival, and/or the inhibition of differentiation (Epstein et al., 1995; Mennerich et al., 1998; Borycki et al., 1999), studies pertaining to Pax7 have reached somewhat disparate conclusions. While one group found that ectopic expression of Pax7 inhibited the expression of MyoD and promoted withdrawal from the cell cycle (Olguin and Olwin, 2004), another group found that ectopic expression of Pax7 had no effect on MyoD expression and possibly acted to maintain proliferation and/or prevent the precocious differentiation of myoblasts (Zammit et al., 2006). In mice lacking both Pax3 and Pax7, cell survival, but not cell proliferation, is altered (Relaix et al., 2005).
In order to continue the dissection of the molecular and cellular function of Pax3 and Pax7 in embryos, it is imperative to first delineate the Pax3 and Pax7 expression profiles in cells of interest. Although the expression of Pax3 and Pax7 transcripts and proteins in developing chick and mouse embryos has been nicely described by a number of groups (Jostes et al., 1990; Johnson et al., 1994; Williams and Ordahl, 1994; Tajbakhsh et al., 1997; Venters et al., 2004; Ben-Yair and Kalcheim, 2005; Gros et al., 2005; Kassar-Duchossoy et al., 2005; Horst et al., 2006; Otto et al., 2006; Schienda et al., 2006), none of these studies showed the direct co-localization of Pax-3 and Pax-7 proteins by immunohistochemistry. The objective of the work shown in this paper was to extend these studies by simultaneously mapping the expression profiles for Pax3 and Pax7 proteins in developing chick paraxial mesoderm and limb. Our immunohistochemical analysis of the segmental plate, dermomyotome, myotome and limb identifies previously underappreciated subpopulations of Pax3 and/or Pax7 positive cells. In regions that have overlapping patterns of Pax3 and Pax7 expression, high levels of Pax3 expression correlated with low levels of Pax7 expression and vice versa. To investigate the mechanism by which unequal expression of Pax3 and Pax7 is maintained, we ectopically expressed Pax3 or Pax7 in the dermomyotome and evaluated the effects on Pax7 and Pax3 expression, respectively. Our results, showing cross inhibition of expression, suggest a possible mechanism for the maintenance of the unequal expression patterns observed in the localization studies.
To characterize the possible cellular functions of Pax3 and Pax7, we also analyzed the proliferation and differentiation status of subpopulations Pax3 and/or Pax7 expressing cells in the dermomyotome and myotome. We found that in the dermomyotome, there is not a correlation between high levels of Pax3 expression in the dorsomedial lip (DML) and ventrolateral lip (VLL) and proliferation. Instead, we observed a medial to lateral proliferation gradient. In the myotome of HH st 18 embryos, however, Pax7 expression positively correlates with proliferation and negatively correlates with myogenic differentiation. And in the myotome of HH st 18 embryos, Pax3 and Pax7 expression are inversely correlated with that of MyoD. In summary, we have identified and characterized unique subpopulations of Pax3 and/or Pax7 expressing cells in the developing paraxial mesoderm and limbs with respect to proliferation and differentiation. Our studies provide new insights about the basic properties of these subpopulations of cells.
MATERIALS AND METHODS
Materials
In situ cell proliferation kit (BrdU; Roche); Fertile eggs (Rhode Island Red; Petaluma Farms).
Plasmids and antibodies
Plasmids were kindly provided by the following labs: cPax3 cDNA was from Dr. Michael Stark (BYU), cPax7 cDNA was from Dr. Atsushi Kawakami (Nagoya University; Kawakami et al., 1997), and pCIG was from Dr. Andy McMahon (Harvard University; Megason and McMahon, 2002). Antibodies were generously provided by the following sources: MyoD and Myogenin antibodies were kindly provided by Dr. Zipora Yablonka-Reuveni (University of Washington, Yablonka-Reuveni and Paterson, 2001; Halevy et al., 2004), Pax3 MAbs were from Dr. Charlie Ordahl (UCSF) and Dr. Marianne Bronner-Fraser (CalTech), Pax7 MAbs were from the Developmental Studies Hybridoma Bank. Anti-BrdU (clone BU-1) and anti-phosphohistone H3 were purchased from Upstate Biochemicals. Goat-anti-mouse IgG1-Cy5 and goat-anti-mouse IgG2a-FITC were obtained from Southern Biotech while Goat-anti-mouse IgG (H+L) Cy3 and Goat-anti-Rabbit IgG (H+L) Cy3 were purchased from Jackson Labs.
Electroporations
Fertilized Rhode Island Red eggs (Gallus Gallus domesticus) were set in a 39°C humidified incubator for 50–56 hours preceding electroporation. After windowing eggs, a small gauge needle was used to inject 0.2 ml 10% India ink in Ringer's solution (123 mM NaCl, 1.5 mM CaCl2, 5 mM KCl, 0.4 mM Na2HPO4, pH 7.4) beneath the embryo to allow for visualization of the embryonic structures. The embryo was staged according to Hamburger and Hamilton (Hamburger and Hamilton, 1951). For somite electroporations, embryos were injected between HH stage 14 and 16. A tungsten knife was used to tear a hole in the vitelline membrane over the posterior somites, and a fine tungsten knife was used to incise the dorsal ectoderm between somites II and III. Ringer's solution was applied to the embryo. The injection mixture was prepared by combining 2.5 μl plasmid DNA at 2–10mg/ml and 1 μl of a solution containing 1.3% methyl cellulose (w/v) and 0.7 mg/ml fast green. The DNA mixture was injected underneath the ectoderm, dorsal to somites III, IV and V. The embryo was electroporated using the BTX electroporation system (BTX Electro Square Porator T820). Four 50-msec pulses of 27 V were applied across a gold-tungsten wire pair of electrodes. The negative tungsten electrode was positioned underneath (ventrally) the embryo while the positive gold electrode was positioned on top of the somites (dorsally). Additional Ringer's solution was applied to the embryo. After resealing the egg, the embryo was returned to the incubator for 24 hours.
BrdU labeling
Embryos in windowed eggs were labeled with BrdU by pipetting 50 ml of a 100X solution of BrdU (Roche) around the heart. Embryos were incubated for 10 minutes prior to harvesting and fixing in cold PBS containing 4% paraformaldehyde. Embryos were embedded and cryosectioned as previously described (Galli et al., 2004).
Immunohistochemistry and Microscopy
Immunohistochemical analysis was performed essentially as described (Galli 2004). For Pax3 and Pax7 double labeling, we used subclass specific antibodies (anti-IgG2a for Pax3 and anti IgG1 for Pax7). Sections labeled with BrdU were denatured in 0.25N HCl (diluted in PBS) for 10 minutes at 37°C and then washed with 0.1M borax for 10 minutes at room temperature prior to immunostaining. Confocal microscopy was carried out as previously described (Galli et al., 2006). To prevent bleed through, each channel was independently scanned. The intensity of labeling for individual cells in different channels was assessed with software from Adobe Photoshop version 9.0.2.
RESULTS
Analysis of Pax3 and Pax7 expression in HH stage 10 chick embryos
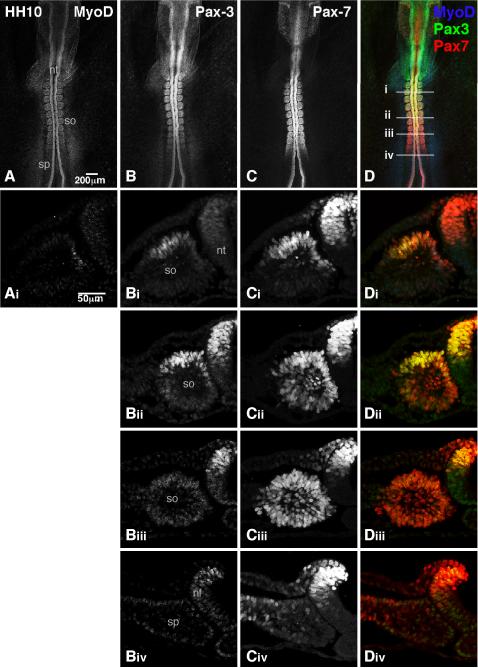
To define the expression patterns of Pax3 and Pax7 with cellular resolution, sections from HH stage 10 chick embryos were simultaneously immunostained for both Pax3 and Pax7 and then analyzed by confocal microscopy (Fig 1). It is important to note that the relative affinities of the Pax3 and Pax7 antibodies for Pax3 and Pax7 have not been determined. Thus, it is impossible to directly compare the protein levels of Pax3 to Pax7 in single cells. However, we can compare the relative levels of Pax3 in different cells and the relative levels of Pax7 in different cells. We first analyzed Pax3 and Pax7 expression in HH stage 10 embryos in whole mount (Fig 1B–D). MyoD was also analyzed as a reference (Fig 1A, Ai; Yablonka-Reuveni and Paterson, 2001). Our analysis shows that the relative levels of Pax7 are fairly constant along the anterior/posterior axis while the relative levels of Pax3 increase from posterior to anterior (Fig 1B–D). The expression of MyoD is not readily visible in whole mount analysis (Fig 1A).
Figure 1. Differential expression of Pax3 and Pax7 in HH stage 10 chick embryo.
Whole (A–D) or sectioned (Ai–Div) HH stage 10 embryos were immunostained with MyoD (blue), Pax3 (green), and Pax7 (red). Images were collected by confocal microscopy. A dorsal view of the trunk is shown. The axial levels of the sections are shown by the lines in D. nf=neural fold; nt=neural tube; sp=segmental plate; so=somite. A total of three embryos were analyzed in whole mount and two embryos were analyzed in sections.
We then performed similar analysis on sections derived from HH stage 10 embryos (Fig 1Ai–Di, Bii–Dii, Biii–Diii, Biv–Div). While little or no Pax3 protein is detectable in the segmental plate and early somites (Fig 1Biv), Pax7 is readily detectable in a number of cells in the segmental plate, particularly in the lateral segmental plate (Fig 1Civ). It should be noted that while our data is consistent with the localization of Pax3 and Pax7 transcripts in HH stage 10 embryos (Otto et al., 2006), Pax3 expression is much more apparent in the segmental plate of HH stage 12 embryos (Venters et al., 2004; Otto et al., 2006).
Progressing anteriorly, Pax7 retains robust expression in epithelial somites, including the somitocoel (Fig 1Cii and Ciii). As the somites mature, Pax7 restricts to the dorsal and medial quadrants of the somite (Fig 1Ci); this restriction is accompanied by a significant increase in the levels of Pax3 expression in the dorsal somite (Fig 1Bi and Bii). In somite XIII, the expression of Pax3 and Pax7 overlaps with MyoD in the medial quadrant and further extends into the dorsal quadrant, where no MyoD is detected (Fig 1Ai–Di). While the Pax3 and Pax7 expression profiles are almost entirely overlapping at this point, some differences in the levels of expression are observed. For example, while Pax7 levels are relatively uniform in the medial and dorsal quadrants, Pax3 levels are lower in the medial quadrant than in the dorsal quadrant. Cumulatively, these data suggest that while Pax3 and Pax7 expression are largely overlapping in early embryos, differences in relative levels of expression arise early in the development of the paraxial mesoderm.
Pax3 and Pax7 expression defines subpopulations of cells in the somites and limbs of HH stage 18 chick embryos
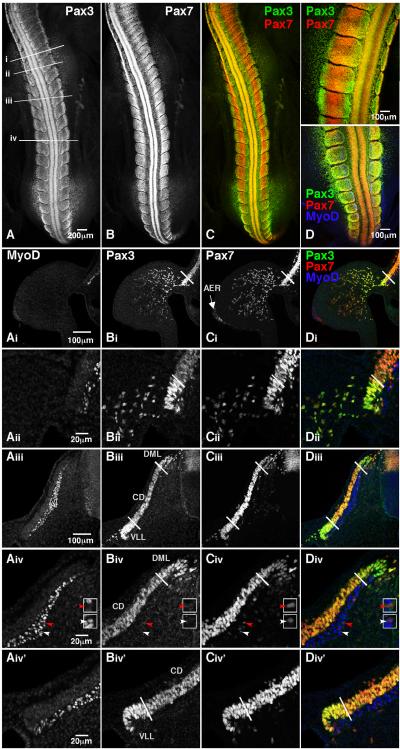
We then analyzed Pax3 and Pax7 expression in the somites of HH stage 18 embryos, which were actively undergoing the primary and secondary waves of myogenesis (Fig 2). As in the HH stage 10 embryos, we observed distinct populations of cells that can be characterized by their relative levels of Pax3 and Pax7 expression. In HH stage 18 embryos, both Pax3 and Pax7 are expressed throughout the dermomyotome (Fig 2Aiii–Diii). However, Pax3 expression in the DML and VLL is high as compared to the CD (Fig 2A, Bi, Bii, Biii, Biv, Biv'). By contrast, Pax7 expression in the DML and VLL is subtly lower as compared to the CD (Fig 2B, Ci, Cii, Ciii,Civ,Civ'). Analysis of the intensity of Pax3 and Pax7 labeling for individual cells in the DML, CD, and VLL confirms these results (data shown in Fig 4). MyoD is robustly expressed in the myotome (Fig 2Ai, Aii, Aiii, Aiv, Aiv'). We also observed a few Pax7 positive cells in the myotome (Fig 2Ciii, Civ). Some are these Pax7 positive cells are MyoD positive (white arrowheads) while others are not (red arrowheads; Fig 2Aiv, Civ, Div). Little or no Pax3 is detected in the myotome at this stage of development (Fig 2Bi, Bii, Biii, Biv, Biv'). Analysis of Pax3 and Pax7 expression in the forelimb also shows cell populations with differing relative amounts of Pax3 and Pax7 (Fig 2Ai–Di). Comparison of the intensity of labeling in 45 randomly chosen cells in both the dorsal and ventral compartments reveals that Pax7 levels are approximately 10% lower in the ventral compartment (data not shown). A Student's T-test suggests that these data are moderately significant (p<0.07). By contrast, the difference in Pax3 expression in the dorsal and ventral compartments is much more striking as Pax3 levels are 47% lower in the ventral compartment. These data are highly significant (p<0.001) and suggest that loss of Pax3 expression precedes that of Pax7 as cells move away from the VLL. One area with a very distinct expression profile is the apical ectodermal ridge (AER), where Pax7 expression is apparent, but no Pax3 is detected (Fig 2Bi–Ci).
Figure 2. Pax3 and Pax7 are expressed in spatially distinct subpopulations of cells in HH stage 18 embryos.
Whole (A–D) or sectioned (Ai–Div') HH stage 18 embryos were immunostained with MyoD (blue), Pax3 (green) and Pax7 (red). Images were collected by confocal microscopy. A dorsal view of the trunk is shown in A–D. The axial levels of the sections are shown by the lines in A. The forelimb is shown in Ai–Di while a close-up of the VLL at the level of the forelimb is shown in Aii–Dii. The entire dermomyotome and myotome of a flank level somite are shown in panels Aiii–Diii. A close-up view of the DML is shown in Aiv–Div while a close up view of the VLL is shown in Aiv'–Div'. White lines demarcate the DML, CD and VLL as defined by Pax3 and Pax7 expression. The white arrowhead points to a cell that is positive for MyoD and Pax7 while the red arrowhead marks a cell expressing only Pax7. Enlarged versions of these two cells are shown in the white boxes located to the right of each panel (Aiv–Div). MyoD (blue) staining in the limb mesenchyme and apical ectodermal ridge appears to be nonspecific. M=myotome; DML=dorsomedial lip; CD=central dermomyotome; VLL=ventrolateral lip; AER=apical ectodermal ridge. A total of 2 embryos were analyzed in whole mount and 7 embryos were analyzed in sections.
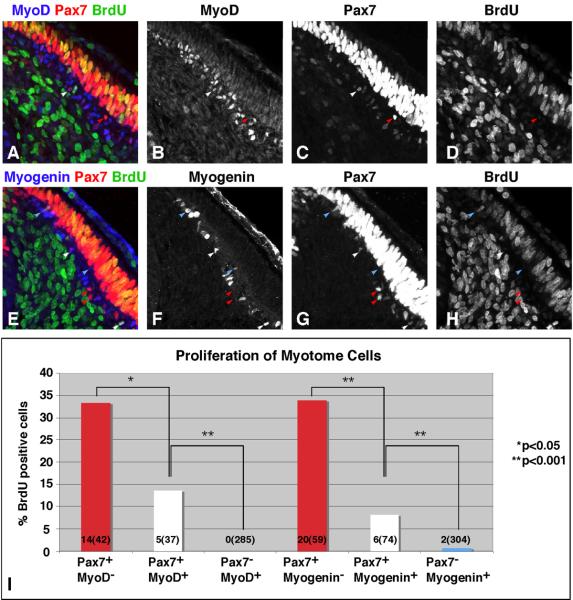
Figure 4. Pax7 expression in the myotome is positively correlated with proliferation and negatively correlated with differentiation.
HH stage 18 embryos were labeled in ovo with a 10 minute pulse of BrdU. Sections were immunostained with antibodies against MyoD, Pax7 and BrdU (top panels) or Myogenin, Pax7, and BrdU (middle panels). Images were collected by confocal microscopy. Red arrowheads mark cells that express Pax7, but not MyoD or Myogenin. White arrowheads mark cells that express Pax7 and MyoD or Myogenin. Blue arrowheads mark cells that express MyoD or Myogenin, but not Pax7. The percentage of BrdU positive cells in each of these populations was quantified and plotted in panel I. The numbers in each bar of the graph represent number of BrdU positive cells (total number of cells with that particular expression profile). For the MyoD stained sections, a total of 3 embryos (14 sections) were analyzed. For Myogenin stained sections, a single embryo (16 sections) was analyzed. Chi-square analysis was used to determine statistical significance.
Cells in the DML are more proliferative than those in the CD or VLL
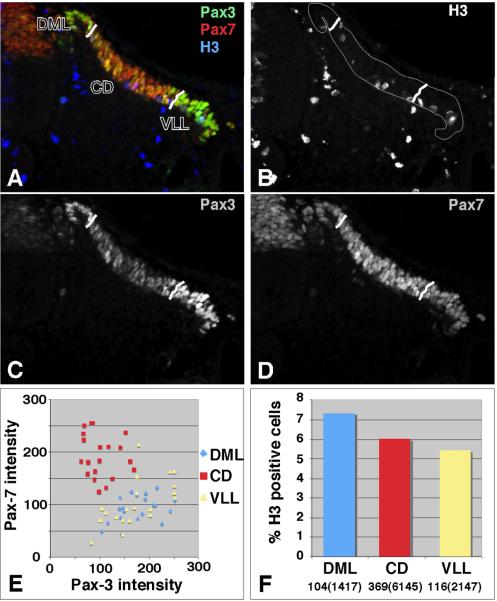
While the DML and VLL have generally been defined by morphological criteria, our studies provide an alternate means of defining the DML and VLL based on reciprocal Pax3 and Pax7 expression profiles. Two lines of evidence led us to test the hypothesis that cells of the DML and VLL, which express high levels of Pax3, would be more proliferative than cells of the CD, which expresses low levels of Pax3. First, ectopic expression of Pax3 in somite explants caused a dramatic increase in proliferation (Mennerich et al., 1998). Second, lineage tracing studies have suggested that cells of the DML and VLL represent the leading edges of the morphogenetic expansion of the dermomyotome and myotome (Ordahl and Le Douarin, 1992; Denetclaw et al., 1997; Denetclaw and Ordahl, 2000; Denetclaw et al., 2001; Ordahl et al., 2001; Gros et al., 2004). To test this hypothesis, we mapped the proliferative capacity of the DML, CD, and VLL, as defined by Pax3 and Pax7 expression. As a precursor to this study, we first needed to insure that we could reliably mark a definitive boundary between the DML, CD, and VLL. To do this, we immunostained sections and then marked the boundaries as shown in Fig 3 (A–D). We then measured the intensity of Pax3 and Pax7 expression in these three compartments. A plot of the Pax3 intensity vs the Pax7 intensity in single cells in each domain shows that that DML and VLL have virtually indistinguishable Pax3/Pax7 expression profiles while that of the CD is radically different. These results gave us confidence in our ability to use Pax3 and Pax7 expression to define three compartments in the somite. We then tested the proliferative capacity of cells in somites between the forelimbs and hindlimbs in HH stage 18 embryos (Fig 3A–D, F). To do this, the total number of mitotic cells, as defined by the expression of anti-phosphohistone H3, was divided by the total number of cells in each compartment. The average percentage of cells in M phase in somites of HH stage 9–13 embryos has been reported to be 4.7 +/− 2.5% (Stern and Bellairs, 1984). As the antibody to phosphohistone H3 used in this study labels cells in both late G2 and M phases, we expected to observe a higher percentage of positively labeled cells (Hendzel et al., 1997; Wei et al., 1999). Our analysis shows that while the cells in the dermomyotome as a whole are highly proliferative (5–7% are labeled with anti-phosphohistone H3), a greater percentage of cells in the DML are proliferative as compared to cells in the CD and the VLL. This difference in proliferation between the DML and CD is moderately significant (p<0.06) as assessed by chi-square analysis while the difference between the DML and VLL is more so (p<0.02). There was no significant difference between proliferation in the CD and VLL. As only the DML shows enhanced proliferation, our data do not support the theory that the relatively greater expression of Pax3 in the DML and VLL promotes the “hyperproliferation” of these tissues, but are consistent with DML housing the proliferative progenitors that are key for the expansion of the dermomyotome (Venters and Ordahl, 2002).
Figure 3. Analysis of proliferation in dermomyotomal compartments as defined by Pax3 and Pax7 expression.
HH stage 18 embryos were sectioned and immunostained with antibodies that recognize Pax3 (green), Pax7 (red) and Phosphohistone H3 (blue). Images were collected by confocal microscopy. The DML and VLL were defined by their high expression of Pax3 and low expression of Pax7. Lines in A–D demarcate the DML, CD, and VLL. Panels B, C, and D show phosphohistone H3, Pax3 and Pax7 immunostaining respectively. The merged image is shown in panel A. The relative intensity of staining for individual cells in each compartment was determined in Adobe Photoshop and plotted in panel E. To insure uniformity of staining and imaging in panel E, data were collected from two sections from the same embryo that were stained and imaged under identical conditions on the same day. The percent of Phosphohistone H3 positive cells in each compartment was determined in plotted in panel F. The number of H3 positive cells and the total number of cells (in parentheses) in each compartment are shown at the bottom. A total of 4 embryos and 63 sections were analyzed for the proliferation study. Chi-square analysis shows that the difference between proliferation in the DML and CD is moderately significant (p<0.06). DML=dorsomedial lip; CD=central dermomyotome; VLL=ventrolateral lip.
The expression of Pax7 is inversely correlated with myogenic differentiation in the myotome of HH stage 18 embryos
As Pax3 and Pax7 have also been characterized as inhibitors of differentiation, we further assessed the correlation between Pax7 expression and proliferation/differentiation in the myotome of HH stage 18 embryos (Fig 4). Because proliferating cells are quite rare in the myotome, we pulsed embryos with BrdU for 10 minutes to label cells in S phase (Fig 4A–H), which is substantially longer than M phase. We then measured the proliferative capacity of cells at different points in the differentiation pathway, as assessed by Pax7, MyoD, and Myogenin staining. Cells that were positive for Pax7, but not MyoD or Myogenin were actively proliferating as evidenced by the observation that 33.3% of the MyoD− and 33.8% of the Myogenin− cells were in S phase (Fig 4I). By contrast, 13.5% of the Pax7+/MyoD+ cells were in S phase and slightly fewer (8.1%) of the Pax7+/Myogenin+ cells were BrdU positive. The difference in proliferation between Pax7+/MyoD+ cells and Pax7+/Myogenin+ cells is not statistically significant. More differentiated cells expressing either MyoD or Myogenin, but not Pax7, incorporated even less BrdU into their DNA. While we were unable to identify any Pax7−/MyoD+ cells that were also BrdU positive, we did identify 2 (out of 304) Pax7−/Myogenin+ cells that were BrdU positive (Fig 4I). Our results indicate that there is a strong correlation between Pax7 expression and the ability of cells to proliferate. Coupled with the observation that only 11.5% of the total MyoD positive cells co-express Pax7 (37/322) while the remaining 88.5% of the total MyoD positive cells do not co-express Pax7, our results further suggest that there is an inverse correlation between Pax7 expression and differentiation. A similar trend was observed for Pax7 and Myogenin.
MyoD protein levels are known to fluctuate during the cell cycle with the lowest levels at the G1/S transition and the highest levels at the G2/M transition (Kitzmann et al., 1998). Intermediate levels are observed during S and G2 phases. Thus, it is possible that our determination of the number of cells co-expressing MyoD and BrdU may reflect a slight underestimation of the true number of MyoD positive cells that are in S phase. This caveat does not affect our basic conclusions.
Pax3 and Pax7 expression in HH stage 22 chick embryos
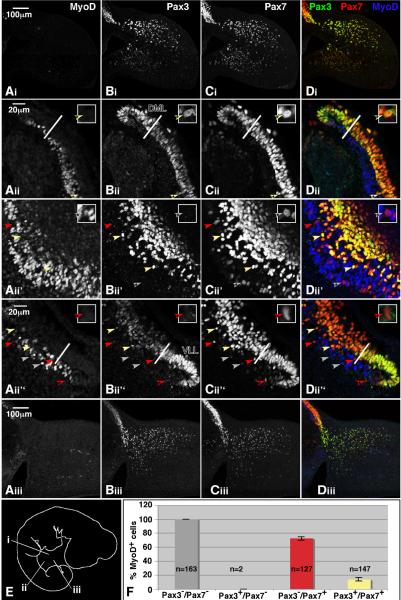
In HH stage 22 chick embryos, the Pax3 and Pax7 expression patterns are similar to those observed in HH stage 18 embryos except a new population of Pax3+/Pax7+ cells is now detected in the myotome (Fig 5). Pax3 continues to be enriched in the DML and VLL as compared to the CD, while Pax7 is similarly enriched in the CD (Fig 5 Aii–Dii, Aii”–Dii”). Previous reports have shown that Pax7 positive cells undergo an asymmetric division that results in the delamination of cells that are then deposited in the myotome (Ben-Yair and Kalcheim, 2005; Gros et al., 2005; Kassar-Duchossoy et al., 2005; Relaix et al., 2005). Though these studies suggested that the cells were also Pax3 positive, the detection methods used were indirect or lacking in cellular resolution. We now show that some of the Pax7 positive cells that are apparently migrating into the myotome are indeed Pax3 positive while others are not (Fig 5Bii'–Dii', 5Bii”–Dii”).
Figure 5. Pax3 and Pax7 expression in HH stage 22 embryos.
Sectioned HH stage 22 embryos were immunostained with antibodies against MyoD (blue), Pax3 (green) and Pax7 (red). Images were collected by confocal microscopy. The axial levels of the sections are shown in panel E. Red arrowheads mark Pax3 negative/Pax7 positive cells that do not express MyoD. White arrowheads mark Pax3 negative/Pax7 positive cells that do express MyoD. Yellow arrowheads mark cells positive for Pax3 and Pax7 that do not express MyoD. Enlarged versions of characteristic cells are shown in the upper right hand corner of Aii–Dii, Aii'–Dii', and Aii”–Dii”. The arrow pointing to the cell shown in the blown up version is marked by an asterisk. MyoD staining in the limb mesenchyme and apical ectodermal ridge is nonspecific. Images obtained from 3 different embryos were used for the qualitative analysis. Quantitative analysis of the percentage of MyoD expressing cells in the different Pax3/Pax7 subpopulations is shown in panel F. Error bars represent the standard error from 6 sections (2 embryos). n=the number of cells counted for each subpopulation; DML=dorsomedial lip; VLL=ventrolateral lip.
We then analyzed MyoD expression as a function of Pax3 and Pax7 expression. Experiments in which DAPI was used to label nuclei (data not shown) show that the vast majority of cells in the myotome express at least one of the three markers assayed (MyoD, Pax3 and Pax7). For this quantitative study, we analyzed the expression of MyoD, Pax3, and Pax7 in 6 sections from 2 different embryos. A total of 439 cells in the myotome were analyzed. The axial level of all sections was limited to the flank region between the forelimb and hindlimb. Whereas 100% of Pax3−/Pax7− cells expressed MyoD, only 72.1 +/− 2.4% of Pax3−/Pax7+ cells expressed MyoD (Fig 5Aii'–Dii'). Only 2 Pax3+/Pax7− cells were observed; neither expressed MyoD (data not shown). Only 13.8 +/− 2.4% of Pax3+/Pax7+ cells expressed MyoD. These data are consistent with our analysis of the proliferation and differentiation status of Pax7+ cells in the myotome of HH stage 18 embryos. And, consistent with the observation that satellite cell progenitors in the myotome are marked by the expression Pax3 and Pax7, the expression of Pax3 and/or Pax7 is negatively correlated with that of MyoD.
In the limbs, individual cells once again show different relative levels of Pax3 and Pax7 (Fig 3Ai–Di, Aiii–Diii). In the forelimb, Pax7 and Pax3 expression is more robust dorsally (Fig 3Ai–Di). Analysis of the intensity of expression in 45 randomly chosen cells in the dorsal and ventral halves shows that the average intensity of labeling for both Pax3 and Pax7 in the dorsal compartment is roughly double that of the intensity of labeling in the ventral compartment. This difference is statistically significant (p<0.001; data not shown). A similar trend is evident in the hindlimb (Fig 5Aiii–Diii). A population of Pax7 expressing cells remain in the AER (Fig 5Ci–Di). There is little or no detectable MyoD in the limbs at this stage of development.
Pax3 and Pax7 are negative regulators of one another
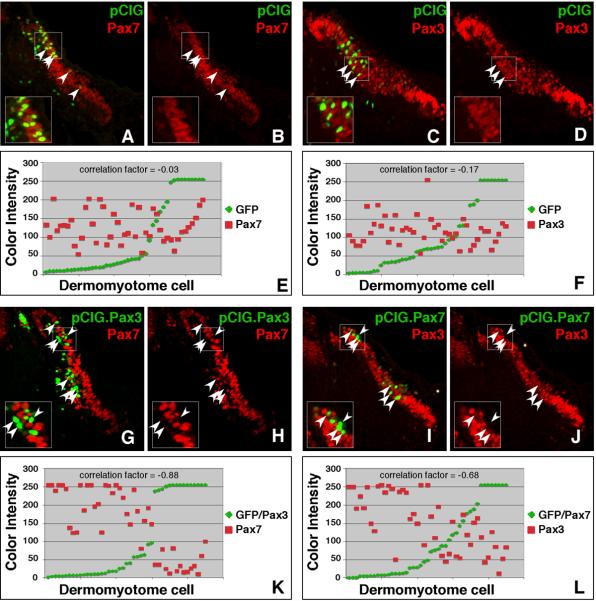
Coupled with the observation that Pax7 is upregulated in Splotch embryos (Borycki et al., 1999), the differences in relative expression levels observed for Pax3 and Pax7 in the dermomyotome suggested the possibility that Pax3 and Pax7 may be negative regulators of one another. To test this prediction, we analyzed the expression of Pax3 and Pax7 in the dermomyotome of somites that were electroporated with constructs encoding Pax7 or Pax3, respectively (Fig 6). Our constructs were designed to co-express GFP; thus, cells labeled with GFP also express ectopic Pax3 or Pax7. Controls in which GFP alone was expressed in somites show no alteration of endogenous Pax3 and Pax7 expression patterns (Fig 6A–D). However, when electroporated with Pax3, GFP positive cells in the dermomyotome expressed less Pax7 than neighboring cells (Fig 6G–H). Analysis of the correlation between GFP expression (which reflects ectopic Pax3) and Pax7 expression in a single section (45 cells counted) reveals an inverse relationship with a correlation factor of −0.88 (Fig 6K) while parallel studies in control embryos reveal a correlation factor of −0.03 (Fig 6E). Likewise, when electroporated with Pax7, GFP positive cells in the dermomyotome expressed less Pax3 than neighboring cells (Fig 6I–J). Analysis of the correlation between GFP expression (which reflects ectopic Pax7) and Pax3 expression in a single section (45 cells counted) reveals an inverse relationship with a correlation factor of −0.68 (Fig 6L) while parallel studies in control embryos show a correlation factor of −0.17 (Fig 6F). These correlation factors suggest that ectopic Pax3 and Pax7 negatively regulate the expression of endogenous Pax7 and Pax3, respectively, and are consistent with previous studies showing the upregulation of Pax7 in Splotch embryos (Borycki et al., 1999).
Figure 6. Pax3 and Pax7 cross-regulate each other's expression.
Embryos were electroporated with constructs designed to overexpress either Pax3 or Pax7. The expression construct (pCIG) generates a bicistronic transcript encoding either Pax3 or Pax7 along with GFP. Somites were electroporated at HH stage 14–16. Embryos were incubated for 24 hours prior to harvesting and immunostaining for Pax3 and Pax7. Images were collected by confocal microscopy. Images of the dermomyotome on the right side of the embryo are shown (A–D; G–J). Insets show a magnified version of the boxed region. Panels A, C, G, and I show a merge of the green and red channels while panels B, D, H and J show only the red channel. Arrowheads point to GFP positive cells (electroporated cells). When Pax3 is overexpressed, GFP positive cells (with ectopic Pax3) show a decline in Pax7 expression (E–F) and when Pax7 is overexpressed, GFP positive cells (with ectopic Pax7) show a similar decline in Pax3 expression (G–K). In panels E, F, K, and L, the intensity of fluorescence in the green and red channels was measured for embryos electroporated with pCIG alone (E and F) or Pax3 (K) and Pax7 (L). The values for each of 45 cells were plotted in ascending order according to GFP expression. All cells analyzed for each panel were on the same section and thus, were stained and imaged under identical conditions.
DISCUSSION
Many parallels exist between the molecular mechanisms underlying the generation and regeneration of skeletal muscle. Although the roles of Pax3 and Pax7 in satellite cells have recently become a topic of intense investigation, much less is known about the roles of Pax3 and Pax7 in developing somites. As an initial step toward closing this gap in our knowledge, we have used immunohistochemistry to directly localize Pax3 and Pax7 in developing segmental plate, somites, and limb buds and have correlated these patterns of expression with proliferation and differentiation in the somite. In this paper, we show: 1) the existence of different subpopulations of cells in the developing segmental plate and somite that can be defined based on their relative levels of Pax3/Pax7 expression 2) that high Pax3 expression in the DML and VLL does not correlate with proliferation 3) that there is a medial to lateral proliferation gradient in the dermomyotome 4) that Pax7 expression in the myotome correlates with proliferation and the inhibition of differentiation and 5) that Pax3 and Pax7 are negative regulators of one another.
After mapping the expression patterns of Pax3 and Pax7 in the segmental plate, somites and limbs, we then sought to evaluate the functional consequences of different Pax3/Pax7 expression profiles. First, we tested whether the expression of Pax3 in the dermomyotome was correlated with proliferation. Contrary to our prediction that the DML and VLL would exhibit elevated levels of proliferation, we discovered a medial to lateral proliferation gradient in the dermomyotome. It is important to note that this result is for somites in the interlimb region only; we do not know if it is also true for limb level somites. Though this gradient is consistent with data reported by Ben-Yair et al (Ben-Yair et al., 2003), the existence of this gradient does not contradict the importance of the DML and the VLL in the morphogenetic expansion. It is hard to ignore the similarity between this proliferation gradient and the proliferation gradient identified in the neural tube (Megason and McMahon, 2002). In the neural tube, the dorsal expression of Wnt-1 and Wnt-3a drives the expansion of the neural tube along the dorsoventral axis (Megason and McMahon, 2002). Could it be that medial signals, such as Wnt-1/Wnt-3a (in the dorsal neural tube) or Wnt-5a/Wnt-5b/Wnt-11 (in the DML) also drive the expansion of the dermomyotome along the mediolateral axis? Although our data showing that Wnt-3a stimulates proliferation in the dermomyotome is consistent with this notion (Galli et al., 2004), further studies are needed to rigorously test this hypothesis.
We then went on to evaluate the role of Pax3 and Pax7 in the myotome. In HH stage 18 embryos, we rarely observed Pax3 positive cells and thus, focused our analysis on Pax7. We show that the expression of Pax7 in the myotome of a HH stage 18 chick embryos correlates with the ability of a cell to undergo proliferation. Furthermore, we also demonstrate that although Pax7 and Myogenin are co-expressed in a small number of cells, the percentage of myotomal cells expressing Pax7 declined dramatically as cells progressed down the differentiation pathway. This observation is consistent with previous studies showing downregulation of Pax genes upon differentiation (Halevy et al., 2004; Zammit et al., 2004; Relaix et al., 2005).
Because of the many similarities between the mechanisms of embryonic myogenesis and regeneration, a comparison of our data to that obtained in chick and mouse satellite cells is relevant. The data we describe in this manuscript closely parallels that observed for differentiating chick satellite cells (Halevy et al., 2004). Specifically, as Pax7 positive progenitor cells differentiate, they sequentially give rise to cells expressing Pax7 and MyoD and/or Myogenin and then cells expressing MyoD and/or Myogenin, but not Pax7.
In HH stage 22 embryos, a new Pax3+/Pax7+ subpopulation of cells is observed in the myotome. Previous studies indicate that these cells delaminate from the CD and represent progenitors to satellite cells (Ben-Yair and Kalcheim, 2005; Gros et al., 2005; Kassar-Duchossoy et al., 2005; Relaix et al., 2005). Consistent with the idea that Pax3+/Pax7+ cells are progenitor cells, we show that Pax3+/Pax7+ cells rarely express MyoD while Pax3−/Pax7+ cells are often co-express MyoD.
Our studies define subpopulations of cells that can be defined based on the spatiotemporal expression patterns of Pax3 and Pax7. How are these unique patterns set up and maintained? Although we and others have shown that several Wnt family members can induce or maintain the expression of Pax3 and Pax7 (Fan et al., 1997; Maroto et al., 1997; Capdevila et al., 1998; Galli et al., 2004), the presence of this signaling pathway alone fails to explain the finely tuned expression patterns observed. However, once the expression patterns have been established, our data suggests that the cross regulation of Pax3 expression by Pax7 and vice versa will play a role in the maintenance of these expression patterns. An additional note is that Pax3 and Pax7 are capable of forming both homodimers and heterodimers (Schafer et al., 1994). These data further suggest a variety of possible combinations based on the relative expression levels. Elucidation of the significance of these different combinations will shed additional light on the molecular and cellular underpinnings of Pax3 and Pax7 functions.
ACKNOWLEDGEMENTS
Many thanks to Dr. Sara Venters (UCSF) and Dr. Graciela Unguez (NMSU) for providing comments on this manuscript. Thanks also to Cathy Krull's lab (University of Michigan) for technical assistance with somite electroporations. We would like to thank Dr Annette Chan of SFSU CMIC for her assistance with imaging. This work was funded by MDA (3989) and NIH-MBRS (SO6 GM52588) grants to L.W.B. and a NIH-RIMI (P20 MD000262) grant to SFSU.
REFERENCES
- Ben-Yair R, Kahane N, Kalcheim C. Coherent development of dermomyotome and dermis from the entire mediolateral extent of the dorsal somite. Development. 2003;130:4325–4336. doi: 10.1242/dev.00667. [DOI] [PubMed] [Google Scholar]
- Ben-Yair R, Kalcheim C. Lineage analysis of the avian dermomyotome sheet reveals the existence of single cells with both dermal and muscle progenitor fates. Development. 2005;132:689–701. doi: 10.1242/dev.01617. [DOI] [PubMed] [Google Scholar]
- Borycki AG, Li J, Jin F, Emerson CP, Epstein JA. Pax3 functions in cell survival and in pax7 regulation. Development. 1999;126:1665–1674. doi: 10.1242/dev.126.8.1665. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Tabin C, Johnson RL. Control of dorsoventral somite patterning by Wnt-1 and beta-catenin. Dev Biol. 1998;193:182–194. doi: 10.1006/dbio.1997.8806. [DOI] [PubMed] [Google Scholar]
- Cinnamon Y, Kahane N, Kalcheim C. Characterization of the early development of specific hypaxial muscles from the ventrolateral myotome. Development. 1999;126:4305–4315. doi: 10.1242/dev.126.19.4305. [DOI] [PubMed] [Google Scholar]
- Denetclaw WF, Jr., Berdougo E, Venters SJ, Ordahl CP. Morphogenetic cell movements in the middle region of the dermomyotome dorsomedial lip associated with patterning and growth of the primary epaxial myotome. Development. 2001;128:1745–1755. doi: 10.1242/dev.128.10.1745. [DOI] [PubMed] [Google Scholar]
- Denetclaw WF, Jr., Christ B, Ordahl CP. Location and growth of epaxial myotome precursor cells. Development. 1997;124:1601–1610. doi: 10.1242/dev.124.8.1601. [DOI] [PubMed] [Google Scholar]
- Denetclaw WF, Ordahl CP. The growth of the dermomyotome and formation of early myotome lineages in thoracolumbar somites of chicken embryos. Development. 2000;127:893–905. doi: 10.1242/dev.127.4.893. [DOI] [PubMed] [Google Scholar]
- Epstein JA, Lam P, Jepeal L, Maas RL, Shapiro DN. Pax3 inhibits myogenic differentiation of cultured myoblast cells. J Biol Chem. 1995;270:11719–11722. doi: 10.1074/jbc.270.20.11719. [DOI] [PubMed] [Google Scholar]
- Fan CM, Lee CS, Tessier-Lavigne M. A role for WNT proteins in induction of dermomyotome. Dev Biol. 1997;191:160–165. doi: 10.1006/dbio.1997.8713. [DOI] [PubMed] [Google Scholar]
- Galli LM, Barnes T, Cheng T, Acosta L, Anglade A, Willert K, Nusse R, Burrus LW. Differential inhibition of Wnt-3a by Sfrp-1, Sfrp-2, and Sfrp-3. Dev Dyn. 2006;235:681–690. doi: 10.1002/dvdy.20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli LM, Willert K, Nusse R, Yablonka-Reuveni Z, Nohno T, Denetclaw W, Burrus LW. A proliferative role for Wnt-3a in chick somites. Dev Biol. 2004;269:489–504. doi: 10.1016/j.ydbio.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Gros J, Manceau M, Thome V, Marcelle C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 2005;435:954–958. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- Gros J, Scaal M, Marcelle C. A two-step mechanism for myotome formation in chick. Dev Cell. 2004;6:875–882. doi: 10.1016/j.devcel.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Halevy O, Piestun Y, Allouh MZ, Rosser BW, Rinkevich Y, Reshef R, Rozenboim I, Wleklinski-Lee M, Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn. 2004;231:489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Hollenbach AD, Sublett JE, McPherson CJ, Grosveld G. The Pax3-FKHR oncoprotein is unresponsive to the Pax3-associated repressor hDaxx. Embo J. 1999;18:3702–3711. doi: 10.1093/emboj/18.13.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst D, Ustanina S, Sergi C, Mikuz G, Juergens H, Braun T, Vorobyov E. Comparative expression analysis of Pax3 and Pax7 during mouse myogenesis. Int J Dev Biol. 2006;50:47–54. doi: 10.1387/ijdb.052111dh. [DOI] [PubMed] [Google Scholar]
- Hsieh MJ, Yao YL, Lai IL, Yang WM. Transcriptional repression activity of PAX3 is modulated by competition between corepressor KAP1 and heterochromatin protein 1. Biochem Biophys Res Commun. 2006;349:573–581. doi: 10.1016/j.bbrc.2006.08.064. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Laufer E, Riddle RD, Tabin C. Ectopic expression of Sonic hedgehog alters dorsal-ventral patterning of somites. Cell. 1994;79:1165–1173. doi: 10.1016/0092-8674(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Jostes B, Walther C, Gruss P. The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech Dev. 1990;33:27–37. doi: 10.1016/0925-4773(90)90132-6. [DOI] [PubMed] [Google Scholar]
- Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomes D, Tajbakhsh S. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 2005;19:1426–1431. doi: 10.1101/gad.345505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami A, Kimura-Kawakami M, Nomura T, Fujisawa H. Distributions of PAX6 and PAX7 proteins suggest their involvement in both early and late phases of chick brain development. Mech Dev. 1997;66:119–130. doi: 10.1016/s0925-4773(97)00097-x. [DOI] [PubMed] [Google Scholar]
- Kitzmann M, Carnac G, Vandromme M, Primig M, Lamb NJ, Fernandez A. The muscle regulatory factors MyoD and myf-5 undergo distinct cell cycle-specific expression in muscle cells. J Cell Biol. 1998;142:1447–1459. doi: 10.1083/jcb.142.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehembre F, Muller S, Pandolfi PP, Dejean A. Regulation of Pax3 transcriptional activity by SUMO-1-modified PML. Oncogene. 2001;20:1–9. doi: 10.1038/sj.onc.1204063. [DOI] [PubMed] [Google Scholar]
- Magnaghi P, Roberts C, Lorain S, Lipinski M, Scambler PJ. HIRA, a mammalian homologue of Saccharomyces cerevisiae transcriptional co-repressors, interacts with Pax3. Nat Genet. 1998;20:74–77. doi: 10.1038/1739. [DOI] [PubMed] [Google Scholar]
- Maroto M, Reshef R, Munsterberg AE, Koester S, Goulding M, Lassar AB. Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell. 1997;89:139–148. doi: 10.1016/s0092-8674(00)80190-7. [DOI] [PubMed] [Google Scholar]
- McKinnell IW, Ishibashi J, Le Grand F, Punch VG, Addicks GC, Greenblatt JF, Dilworth FJ, Rudnicki MA. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol. 2007 doi: 10.1038/ncb1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Mennerich D, Schafer K, Braun T. Pax-3 is necessary but not sufficient for lbx1 expression in myogenic precursor cells of the limb. Mech Dev. 1998;73:147–158. doi: 10.1016/s0925-4773(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Murakami M, Tominaga J, Makita R, Uchijima Y, Kurihara Y, Nakagawa O, Asano T, Kurihara H. Transcriptional activity of Pax3 is co-activated by TAZ. Biochem Biophys Res Commun. 2006;339:533–539. doi: 10.1016/j.bbrc.2005.10.214. [DOI] [PubMed] [Google Scholar]
- Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordahl CP, Berdougo E, Venters SJ, Denetclaw WF., Jr. The dermomyotome dorsomedial lip drives growth and morphogenesis of both the primary myotome and dermomyotome epithelium. Development. 2001;128:1731–1744. doi: 10.1242/dev.128.10.1731. [DOI] [PubMed] [Google Scholar]
- Ordahl CP, Le Douarin NM. Two myogenic lineages within the developing somite. Development. 1992;114:339–353. doi: 10.1242/dev.114.2.339. [DOI] [PubMed] [Google Scholar]
- Otto A, Schmidt C, Patel K. Pax3 and Pax7 expression and regulation in the avian embryo. Anat Embryol (Berl) 2006;211:293–310. doi: 10.1007/s00429-006-0083-3. [DOI] [PubMed] [Google Scholar]
- Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. Embo J. 2004;23:3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev. 2004;18:1088–1105. doi: 10.1101/gad.301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Schafer BW, Czerny T, Bernasconi M, Genini M, Busslinger M. Molecular cloning and characterization of a human PAX-7 cDNA expressed in normal and neoplastic myocytes. Nucleic Acids Res. 1994;22:4574–4582. doi: 10.1093/nar/22.22.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienda J, Engleka KA, Jun S, Hansen MS, Epstein JA, Tabin CJ, Kunkel LM, Kardon G. Somitic origin of limb muscle satellite and side population cells. Proc Natl Acad Sci U S A. 2006;103:945–950. doi: 10.1073/pnas.0510164103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Ishibashi J, Scime A, Rudnicki MA. Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+ stem cells from injured muscle. PLoS Biol. 2004;2:E130. doi: 10.1371/journal.pbio.0020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Stern CD, Bellairs R. Mitotic activity during somite segmentation in the early chick embryo. Anat Embryol (Berl) 1984;169:97–102. doi: 10.1007/BF00300591. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- Venters SJ, Argent RE, Deegan FM, Perez-Baron G, Wong TS, Tidyman WE, Denetclaw WF, Jr., Marcelle C, Bronner-Fraser M, Ordahl CP. Precocious terminal differentiation of premigratory limb muscle precursor cells requires positive signalling. Dev Dyn. 2004;229:591–599. doi: 10.1002/dvdy.20016. [DOI] [PubMed] [Google Scholar]
- Venters SJ, Ordahl CP. Persistent myogenic capacity of the dermomyotome dorsomedial lip and restriction of myogenic competence. Development. 2002;129:3873–3885. doi: 10.1242/dev.129.16.3873. [DOI] [PubMed] [Google Scholar]
- Wei Y, Yu L, Bowen J, Gorovsky MA, Allis CD. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell. 1999;97:99–109. doi: 10.1016/s0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]
- Williams BA, Ordahl CP. Pax-3 expression in segmental mesoderm marks early stages in myogenic cell specification. Development. 1994;120:785–796. doi: 10.1242/dev.120.4.785. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Paterson BM. MyoD and myogenin expression patterns in cultures of fetal and adult chicken myoblasts. J Histochem Cytochem. 2001;49:455–462. doi: 10.1177/002215540104900405. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, Partridge TA, Beauchamp JR. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]