Abstract
Ceruloplasmin (Cp) is a multicopper oxidase and the most abundant copper binding protein in vertebrate plasma. Loss of function mutations in humans or experimental deletion in mice result in iron overload consistent with a putative ferroxidase function. Prior work suggested plasma may contain multiple ferroxidases. Studies were conducted in Holtzman rats (Rattus novegicus), albino mice (Mus musculus), Cp -/- mice, and adult humans (Homo sapiens) to investigate the copper-iron interaction. Dietary copper-deficient (CuD) rats and mice were produced using a modified AIN-76A diet. Results confirmed that o-dianisidine is a better substrate than paraphenylene diamine (PPD) for assessing diamine oxidase activity of Cp. Plasma from CuD rat dams and pups, and CuD and Cp -/- mice contained no detectable Cp diamine oxidase activity. Importantly, no ferroxidase activity was detectable for CuD rats, mice, or Cp -/- mice compared to robust activity for copper-adequate (CuA) rodent controls using western membrane assay. Immunoblot protocols detected major reductions (60-90%) in Cp protein in plasma of CuD rodents but no alteration in liver mRNA levels by qRT-PCR. Data are consistent with apo-Cp being less stable than holo-Cp. Further research is needed to explain normal plasma iron in CuD mice. Reduction in Cp is a sensitive biomarker for copper deficiency.
Keywords: copper-deficient, rat, mouse, ceruloplasmin, ferroxidase, assay
1. Introduction
Copper is an essential metal for mammals because approximately a dozen proteins require stoichiometric amounts of copper to catalyze key reactions (cuproenzymes) (Prohaska, 2006). One such cuproenzyme is ceruloplasmin (Cp) 1 a plasma α-2-globulin multicopper oxidase originally discovered and characterized by Holmberg in the 1940s (Frieden and Hsieh, 1976). Cp is the major plasma copper binding protein but it is generally agreed that the main function of Cp is to participate in iron homeostasis due to its ferroxidase activity by converting ferrous to ferric iron (Hellman and Gitlin, 2002).
A role for copper in iron biology, has been appreciated since the 19th century as described in detail elsewhere (Fox, 2003). However, there are still many unanswered questions. Copper deficient rats and mice both develop symptoms of severe anemia (low hematocrit and low mean corpuscular hemoglobin) similar to iron deficiency. Both copper deficient rats and mice have non-detectable Cp diamine oxidase activity and elevated liver iron suggesting loss of function; yet only in copper-deficient rats is there a consistent and robust hypoferremia, consistent with impaired iron efflux to plasma (Pyatskowit and Prohaska, 2008a).
One suggestion to explain these observations was that mouse plasma may have ferroxidase activity that is not impacted by copper deficiency (Prohaska, 1981). In support of this finding was a recent study reporting ferroxidase activity in ceruloplasmin null (Cp -/-) mice (Gray et al., 2009). However, an earlier study on Cp -/- mice did not detect ferroxidase using a similar gel technique (Cherukuri et al., 2004). Since both rats and mice are often used as models of nutritional metal deficiencies a comparison of the Cp response to limiting copper or iron seems apropos. Thus, one goal of the current work was to investigate plasma ferroxidase activity following dietary copper deficiency and following deletion of mouse Cp.
Cp function is often assessed by its diamine oxidase activity (Frieden and Hsieh, 1976). In fact, human Cp was discovered by isolating the paraphenylene diamine (PPD) oxidase of plasma (Fox, 2003). The accuracy of Cp activity quantification depends on the fact that azide inhibits nearly all Cp activity of human and rat enzymes (Frieden and Hsieh, 1976). However, the PPD oxidase of mouse plasma is not inhibited totally under these same conditions (Prohaska, 1981; Gray et al., 2009). An accurate assessment of mammalian Cp using diamines as substrates is a worthwhile goal. Thus, a second goal of this research was to compare the diamine oxidase activity, and azide sensitivity in human, rat, and mouse plasma.
Furthermore, some use Cp as a biomarker of human copper status because it is easy to assay and plasma is readily available. Some, in fact measure both Cp enzyme activity and Cp protein and their ratio to evaluate copper status (Milne, 1994). Previous work in adult rats using immunoelectrophoresis and ELISA protocols suggest that dietary copper deficiency results in lower Cp protein (Holtzman and Gaumnitz, 1970a; Gitlin et al., 1992). This issue has not been evaluated in other mammals, in younger rats, or female rats. Thus, a third goal of the current studies was to assess plasma Cp protein in several models of copper deficiency to confirm,, and extend previous research on older rats.
The underlying hypothesis was that Cp function is necessary to prevent anemia via the role of Cp in iron metabolism.
2. Materials and methods
2.1 Animal care and induction of copper deficiency
Sperm-positive Holtzman rats (Rattus norvegicus) and Hsd:ICR (CD-1) outbred albino mice (Mus musculus) were purchased commercially (Harlan Sprague Dawley, Indianapolis, IN, USA) and received either copper-adequate (CuA) or copper-deficient (CuD) dietary treatment consisting of a copper-deficient modified AIN-76A diet (Teklad Laboratories, Madison, WI, USA) that contained 0.32 mg Cu/kg by analysis. Normal AIN-76A diet contains approximately 6 mg Cu/kg. All dams and offspring were fed the CuD diet. CuA groups drank water supplemented with cupric sulfate, 20 mg Cu/L, and CuD groups drank deionized water. All animals were maintained at 24°C with 55% relative humidity on a 12-h light cycle (0700-1900 h). All protocols were formally approved by the University of Minnesota Animal Care Committee.
Several nutritional paradigms were designed similar to prior work with rodents (Pyatskowit and Prohaska, 2007). Mouse dams (n=5 per group) were placed on treatment the day pups were born and at postnatal day 20 male pups were continued on treatment of their respective dams for 8 additional days, P28. Rat dams, five CuD and four CuA, were placed on treatment on embryonic day 7 and were killed on P20. Rat pups were weaned to treatment of their dams and selected males were killed on P25 and females on P26. Also at P25 10 CuA males were placed in stainless steel cages and half were continued on CuA treatment and half switched to CuD treatment for four weeks. These 10 males were killed on P53 and constitute a postweanling CuD model. Additionally, at P26 five CuD female pups and 5 CuA female pups were switched to a non purified rodent chow containing 12 mg Cu/kg by analysis. This repletion protocol was conducted until rats were P100. Both rat and mouse dams were maintained on the rodent chow and tap water prior to the onset of semipurified dietary treatments.
2.2 Tissue collection
Rodents were anesthetized by ketamine/xylazine injection and killed by cardiac puncture. Blood was removed with a heparinized needle and an aliquot was used for hemoglobin and the remainder saved for plasma collected after centrifugation. A piece of liver was dissected, rinsed, blotted dry, weighed and placed in a pre-weighed acid-washed flask to process for metal content. We are grateful to Dr. Z. L. Harris who sent tissue from C57BL mice missing Cp (Cp -/-) and wild-type controls (Cp +/+). Several plasma samples from normal human volunteers were used to compare Cp enzyme activity with rodent data and to verify Cp antibody specificity. These subjects were part of a clinical study and only placebo control subject samples were used.
2.3 Biochemical analyses
Reagents were purchased from Sigma-Aldrich unless specified otherwise. A 5μL aliquot of blood was added to Drabkin's reagent and used to measure hemoglobin spectrophotometrically as metcyanhemoglobin (Prohaska, 1983). Portions of liver were wet-digested with HNO3 (Trace Metal grade; Fisher Scientific, Pittsburgh, PA, USA) and samples were analyzed for total copper and iron content by flame atomic absorption spectroscopy (Model 1100B, Perkin-Elmer, Norwalk, CT) (Prohaska, 1991). Protein concentration of plasma samples was determined using a modified version of the Lowry method (Markwell et al., 1978).
2.4 Cp diamine oxidase and ferroxidase activity assays
Plasma diamine oxidase activity is a common way to assess Cp function. Two separate assays were compared to revisit the issue of azide sensitivity, first reported for mouse Cp in 1981 (Prohaska, 1981) and recently again (Gray et al., 2009). Plasma from humans and rats was assayed at pH 5.0 and mice at pH 5.5 either at 30 °C, human, or 37 °C for rodents in 0.1 mol/l sodium acetate. One substrate was paraphenylene diamine (PPD, 1,4-diaminobenzene) used at 4 mM final concentration. Commericial PPD was purified by sublimation and dissolved in acetate buffer to initiate the reaction. The reaction also contained 30 μM EDTA. Absorbance was read at 533nm to measure the oxidation product of PPD, N,N’-Bis(2,5diaminophenyl)-p-quinonediimine, Bandrowski's base) (Rice, 1962). Molar absorptivity of 11,600 was used to calculate Cp activity units(μmol/min)/L plasma. The second substrate used was o-dianisidine (ODA). It was purchased commercially as the hydrochloride salt and used without further purification. Conditions for human and rodent Cp assay with ODA are described in detail elsewhere (Schosinsky et al., 1974; Prohaska, 1991). When azide sensitivity was evaluated reactions included 1 mM sodium azide. Appropriate non-enzymatic blanks were run simultaneously. All measurements were made on Beckman DU 640 spectrophotmeter.
Cuvette assay of Cp ferroxidase activity following ferritransferrin formation using mouse plasma yielded unreliable results (Prohaska, 1981). Therefore, plasma ferroxidase activity was evaluated by a western blot membrane method. Plasma, usually 5μL, was mixed with a non-denaturing non-reducing loading buffer (Huster et al., 2006). Proteins were separating by non-denaturing 8% polyacrylamide gel electrophoresis using the Laemmli buffer system without SDS. Proteins were electroblotted to PVDF (Biorad) membranes using Tris glycine pH 8.3 buffer with 20% methanol (Biorad). Membranes were then processed for ferroxidase activity according to an established protocol that incubates first with ferrous iron and then with potassium ferrocyanide (Mazumder et al., 1997). The Prussian blue image, where ferric ion was produced, was captured by camera.
2.5 Analysis of ceruloplasmin mRNA expression in rodent liver
Total RNA was isolated from fast frozen liver chemically using TRI reagent (Ambion, Austin, TX, USA) following manufacturer recommendations including suggested optional steps. Concentration of purified RNA was measured with a Nanodrop spectrophotometer and integrity was evaluating by denaturing agarose gel electrophoresis. DNAse treatment used DNA-free kit (Ambion) and cDNA was synthesized with Omniscript Reverse Transcriptase (Qiagen, Valencia, CA, USA). Liver cDNA was amplified and quantified by qRT-PCR using either SYBR Green I kit (Roche, Indianapolis, IN, USA) or Rotor-Gene SYBR Green PCR Kit (Qiagen), amplification used either a Roche LightCycler or Corbett RotorGene RG-3000. Copy number of mRNAs for Cp and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), used as a control, was determined. Agarose gel analysis showed each primer pair to yield a single band of the expected size. Resultant products were purified, quantified using absorbance at 260 nm, and used in standard curves for qRT-PCR. Each reaction contained cDNA synthesized from 10 ng total RNA in a 10 μl total reaction volume. Cp primers, derived from NM_012532, were reverse: 5’-TAT CCC ATG CAC GTC TGC CTC ATT-3’ and forward: 5’-TGC ACC TGA GAA TGT GGA CAA GGA-3’. Amplicon size was 169 bp. Forward and reverse primers used for GAPDH analysis, NM_017008, were 5’-TTC CTA CCC CCA ATG TAT CCG-3’ and 5’-ACC ACC CTG TTG CTG TAG CCA -3’, respectively. This produced a 271 bp product. For each RNA sample, the Cp/GAPDH value was determined. Within an experimental group the relative expression of Cp was calculated by determining abundance of individual Cp/GAPDH relative to the mean CuA ratio value.
2.6 Western immunoblot analysis
Plasma samples were stored at -20 °C until analyzed for Cp and trasferrin (Tf). Fractionation was carried out by loading 1 μL or less of plasma on 8% SDS-PAGE gels. Samples were boiled and denatured in Laemmli loading buffer prior to electrophoresis. Proteins were transferred to 0.2 μm nitrocellulose membranes and processed for immunoblotting as described elsewhere (Prohaska and Brokate, 2001). Membranes were stained with Ponceau S to confirm equal loading and transfer of plasma proteins. Membranes were reprobed for Tf after incubation of membranes with buffer containing 2-mercaptoethanol and SDS at 55 °C.
Plasma Cp levels were evaluated using goat anti-humanCp antisera at a 1:1000 dilution (Sigma C0911). Plasma Tf protein levels were analyzed using goat anti-humanTf at 1:5000 dilution (Sigma T6265). All membranes were incubated in blocking buffer containing 5% powdered milk, and overnight with primary antibodies. All secondary species-specific antibodies were diluted 1:10,000. SuperSignal West Pico chemiluminescent substrate (Thermo Scientific, Rockford, IL, USA) was used for detection. Chemiluminescence detection and densitometry was carried out using the FluorChem™ system (Alpha Innotech, San Leandro, CA, USA).
2.7 Statistical analysis
Means ± SEM were calculated. Student's unpaired two-tailed t-test was used to compare data between the two dietary treatments, α=0.05. Variance equality was evaluated by F-test. All data were processed using Microsoft Excel™.
3. Results
3.1 Confirmation of copper deficiency
Several rodent models of dietary copper deficiency were designed to confirm and extend previous work on Cp and copper status. When copper deficiency was created during perinatal development, either gestation and lactation or lactation alone, CuD offspring were smaller in size whereas when copper deficiency was induced in older animals, rat dams or post-weanling male rats, there was no impact on body weight (Table 1). However, dietary copper deficiency in all cases resulted in lower hemoglobin concentration. In some cases, this deficiency was rather mild as in adult male rats and rat dams and was most severe in CuD male mice. Dietary copper deficiency also reduced concentration of copper in liver of all CuD groups that were evaluated. Since liver synthesizes Cp, low copper is an important observation suggesting the copper pool for Cp synthesis may be limiting. One of the hallmarks of dietary copper deficiency is an accumulation of iron in the liver, particularly after weanling. Animals in the current dietary model all show an enhancement of liver iron following copper deficiency with one notable exception (Table 1). Concentration of iron in liver of CuD female rats was not different than controls. Plasma iron was not measured in rats in this study. Plasma iron was not impacted by copper deficiency in mice following lactational and postweaning copper deficency: CuA 4.28 ± 0.65 μg/mL compared to CuD 4.38 ± 0.51 mL. In general, the rodents that were deprived of copper showed signs characteristic of CuD rats and CuD mice reported previously.
Table 1.
Characteristics of rats and mice following copper deficiency and repletion
| Characteristic | |||||
|---|---|---|---|---|---|
| Group | Diet | BW (g) | Hb (g/L) | L-Cu (μg/g) | L-Fe (μg/g) |
| Rat Dams | CuA | 330 ± 2.9 | 166 ± 2.1 | 5.33 ± 1.23 | 68 ± 9.5 |
| CuD | 348 ± 8.0 | 139 ± 2.8* | 1.12 ± 0.02* | 180 ± 33.4* | |
| P25 Male rats | CuA | 80.1 ± 7.0 | 108 ± 4.5 | 6.44 ± 0.36 | 33.1 ± 2.3 |
| CuD | 62 ± 3.0* | 67.7 ± 4.3* | 0.38 ± 0.08* | 56.3 ± 5.8* | |
| P26 Female rats | CuA | 71.5 ± 4.1 | 124 ± 8.3 | 6.86 ± 0.55 | 76.2 ± 7.7 |
| CuD | 53.2 ± 2.9* | 70.5 ± 5.5* | 0.42 ± 0.09* | 68.3 ± 4.75 | |
| P53 Male rats | CuA | 288 ± 16.5 | 141 ± 12 | 5.26 ± 0.88 | 73.5 ±4.3 |
| CuD | 283 ± 30.1 | 111 ± 15.6* | 1.01 ± 0.22* | 149 ± 19.8* | |
| P100 Female rats | CuA | 308 ± 8.1 | 156 ± 0.3 | 4.45 ± 0.07 | 111 ± 54.1 |
| CuR | 313 ± 15.4 | 158 ± 4.1 | 4.02 ± 0.27 | 132 ± 57.0 | |
| P28 Male mice | CuA | 23.8 ± 0.3 | 151 ± 4.4 | 4.31 ± 0.07 | 101 ± 6.9 |
| CuD | 18.5 ± 0.7* | 46.6 ± 6.5* | 1.40 ± 0.05* | 324 ± 19.3* | |
Values are means ± SEM (n=4 or 5). Body weight (BW), hemoglobin (Hb), liver copper (L-Cu), and liver iron (L-Fe) were determined in copper-adequate (CuA), copper-deficient (CuD), or copper-repleted (CuR) rodents as described in Methods.
Different from Cu-adequate (CuA) within group, P < 0.05 (Student's t-test).
When CuD female rats were repleted with a diet containing 12 mg Cu/kg for over two months their biochemical characteristics were not different than CuA rats (Table 1). This included body weight, hemoglobin, and liver metal concentrations suggesting that these rats recovered biochemically from perinatal copper deficiency.
3.2 Cp diamine oxidase activity depends on substrate choice
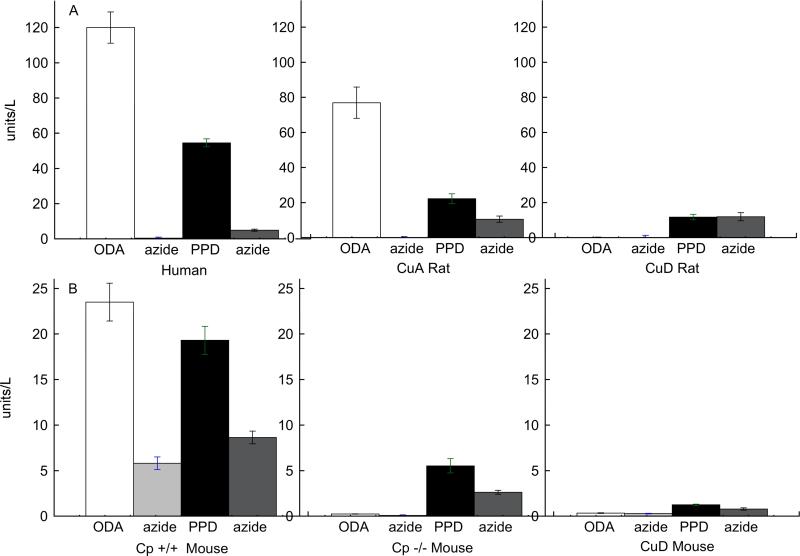
There has been some uncertainty in the literature regarding the use of an appropriate substrate for the detection of Cp diamine oxidase activity. Thus, a comparison was made in humans, rats, and mice of diamine oxidase activity of plasma using either o-dianisidine (ODA) or paraphenylene diamine (PPD) (Figure 1). Plasma samples were also analyzed with azide, since previous work suggested that azide inhibits Cp diamine oxidase activity, in fact the PPD assay depends on this fact. When human plasma was assayed with either ODA or PPD, most of the oxidase activity was inhibited by the presence of azide (Figure 1). It is customary, in the case of PPD, to subtract residual azide activity from total activity and report that as net Cp activity. For human plasma, both substrates appeared to work well. For CuA rats, all of the diamine oxidase with ODA was inhibited by azide, but a much smaller portion of the activity of PPD was inhibited by azide. Interestingly this azide-resistant activity also persisted in CuD rat plasma whereas all of the ODA activity, with or without azide, was below the limits of detection (Figure 1A). This suggests that the azide resistant diamine oxidase activity of rat plasma is not copper-dependent.
Fig. 1.
Impact of diet and azide on plasma diamine oxidase activity. A. Normal human and CuA and CuD rat plasma was assayed for Cp activity with either o-dianisidine (ODA) or paraphenylene diamine (PPD) as substrate with or without azide. B. Mouse plasma from wild-type (Cp +/+) and null (Cp -/-) mice and CuD mice was assayed for Cp activity with either ODA or PPD with or without azide. Activity (unit = μmol/min) was determined on a minimum of 4 samples of each group and all data was repeated. Bars represent mean ± SEM. Note the scale difference in A compared to B.
When analyzing diamine oxidase activity of mouse plasma, a different result was obtained (Figure 1B). In mice, Cp was assayed both in C57BL wild type (Cp+/+) and in outbred white albino CuA males with similar results. Only Cp+/+ data is shown for simplification. As can be seen, there was significant diamine oxidase that was resistant to azide (Figure 1B). This was true for both substrates, and is different than either human or rat. However, the azide resistant activity is likely copper-dependent in mice since CuD mice had almost non-detectable Cp activity with either ODA or PPD although certainly the activity was greater with PPD than ODA. Plasma diamine oxidase activity of Cp -/- mice was nondetectable with ODA, but was clearly detectable with PPD, suggesting that if PPD is used as a substrate for mouse Cp, it falsely detects activity when Cp is absent. Collectively, these results suggest that ODA is a more specific substrate for Cp plasma enzyme activity than PPD.
3.3 Cp ferroxidase activity
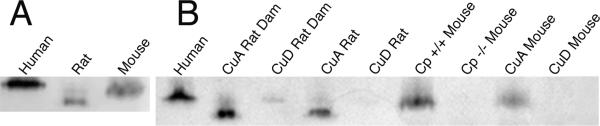
Ferroxidase activity of plasma from humans, rats, and mice was also evaluated in these studies. Previous work with a cuvette based ferroxidase assay yielded inconsistent results with mice and so the ferroxidase activity in these experiments was probed using a Western blot activity assay (Figure 2). Plasma was fractionated on polyacrylamide gels and transferred to membranes that were exposed to ferrous iron and then an agent to detect ferric iron production, i.e., ferroxidase activity. A robust band was detected for human, rat, and mouse (Figure 2A). The mobility of ferroxidase in the rat was faster than the human or mouse, and the mouse perhaps slightly faster than human.
Fig. 2.
Ferroxidase activity of plasma. Plasma, 5 μL, from normal human or CuA rat or mouse (panel A) or from other treatment groups (B) was subjected to non-denaturing electrophoresis and transfer to PVDF membranes as assayed for membrane ferroxidase activity. Mobility of the Cp in rat is different from human or mouse. Ferroxidase activity in CuD rat, CuD mouse, and Cp -/- mouse plasma was not detected.
When plasma from rats and mice with varying degrees of copper status were evaluated, it was quite clear that ferroxidase activity was only detected in CuA and wildtype animals (Figure 2B). Ferroxidase was not detected in CuD rat pups, Cp-/-mice, or CuD mice. A faint ferroxidase band with a different mobility was observed in CuD rat dam plasma.
3.4 Cp protein levels are markedly lower following copper deficiency
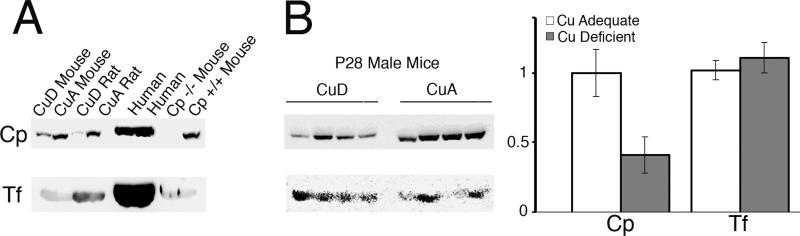
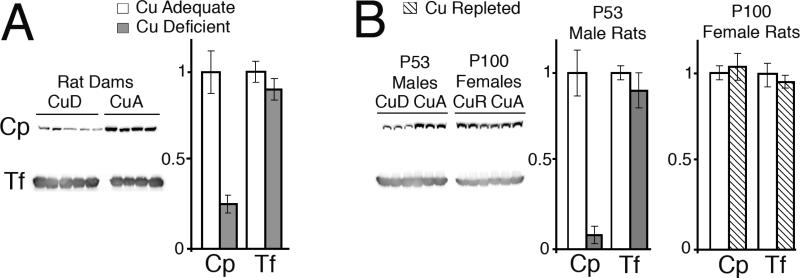
Previous work with postweanling rats suggested that dietary copper deficiency resulted in secretion of apoceruloplasmin, and that the level of Cp immunoreactive protein was decreased (Holtzman and Gaumnitz, 1970a; Gitlin et al., 1992). Current experiments were designed to extend this observation using Western immunoblot technology. The first step was to characterize the antisera used to detect Cp protein in rodents (Figure 3A). Goat anti-humanCp antibody, commercially available, detected a robust signal in human plasma and was able to detect a similar migrating band, though weaker, in mouse and rat plasma (Figure 3A). Importantly, this antibody failed to detect an immunoreactive band in the Cp-/- mouse plasma confirming specificity of this antisera. However, when reprobed this membrane showed that Tf, another plasma protein, was present equally in CuD and CuA animals and was present in the Cp-/- mouse plasma. To compare with prior work in rats, plasma from P28 CuA and CuD male mice was subjected to Cp and Tf detection by western blot (Figure 3B). A highly significant reduction in the abundance of Cp in CuD mouse plasma was detected, whereas Tf abundance was equivalent between CuA and CuD samples.
Fig. 3.
A. Characterization of the goat anti-human Cp antibody against human, mouse, and rat plasma. Plasma, 1 μL, was subjected to denaturing SDS PAGE, 8% gel, and probed for Cp. The blot was stripped and reprobed for transferrin (Tf). A single band, MW 135 kDa, was detected in human, CuA rat and mouse and Cp +/+ mouse. No Cp band was detected in the plasma from Cp -/- mice but a robust band for Tf was present. B. Abundance of Cp and Tf in mouse plasma following copper deficiency. Each lane contained 1 μL plasma from CuA or CuD mice. A 60% reduction in Cp abundance was detected in CuD mouse samples, P < 0.05, whereas Tf levels were comparable.
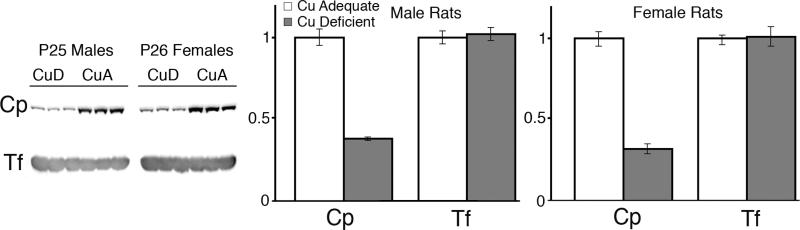
To determine whether CuD rat pups may also experience a similar diminution in plasma Cp, three CuD and three CuA male and female pups were evaluated (Figure 4). In confirmation of the mouse work, there was an approximate 70% reduction in the abundance of total Cp immunoreactivity in both CuD male and female pups whereas Tf expression was equivalent to levels in CuA pups. Experiments were extended to older rats (Figure 5). The diminution of Cp abundance in CuD rat dams, generally considered as less copper deficient than their pups, also showed a remarkable and significant reduction in Cp abundance (Figure 5A); whereas, Tf abundance was not affected. In confirmation of previous work, the Cp abundance in post weanling CuD male rats was also significantly reduced in current studies (Figure 5B) whereas Tf abundance was not affected. Also assessed was whether copper repletion could reverse the Cp reduction phenomena. Female rats were repleted with copper for over two months (Figure 5B). Abundance of Cp was equivalent between repleted CuR and CuA females as was Tf expresssion (Figure 5B).
Fig. 4.
Abundance of Cp and Tf in rat pup plasma following copper deficiency. Each lane contained 1 μL plasma from CuA or CuD male or female rat pups. A 70% reduction in Cp abundance was detected in CuD rat samples, P < 0.05, whereas Tf levels were comparable.
Fig. 5.
A. Abundance of Cp and Tf in rat dam plasma following copper deficiency. Each lane contained 1 μL plasma from CuA or CuD rat dams. A 75% reduction in Cp abundance was detected in CuD rat samples, P < 0.05, whereas Tf levels were comparable. B. Abundance of Cp and Tf in adult male rat plasma following copper deficiency and adult female rat plasma following copper repletion (CuR). Each lane contained 1 μL plasma. A 90% reduction in Cp abundance was detected in CuD male rat samples, P < 0.05, whereas Tf levels were comparable. Cp abundance was not different between CuA and CuR female rats, P > 0.05.
3.5 Cp mRNA levels are not altered following Cu deficiency
To evaluate whether the reduction in abundance of Cp protein might be due to an impaired synthesis, despite previous work showing that this was not the case in postweanling male rats, messenger RNA content of CuD and CuA rodents was evaluated. When compared to expression in CuA animals, there appeared to be a consistent yet small diminution in abundance of Cp messenger RNA. However this was only significant in one situation—that of the female CuD rat pups (Table 2). This trend was not due to differences in GAPDH abubundance as equivalent levels of GAPDH mRNA were detected in all comparisons. Analyses were repeated on a different set of female rat pups from another perinatal copper depletion experiment. In that study we failed to detect a significant difference between CuA and CuD females for Cp mRNA. Thus, it appeared that the diminution in messenger RNA content for Cp cannot explain the profound decreases in Cp protein observed in all of the experimental paradigms.
Table 2.
Relative expression of ceruloplasmin mRNA in liver of rats and mice following copper deficiency
| Relative mRNA Abundance | |||
|---|---|---|---|
| Group | Diet | Cp/GAPDH | GAPDH |
| Rat Dams | CuA | 1.00 ± 0.32 | 1.00 ± 0.25 |
| CuD | 0.72 ± 0.09 | 1.09 ± 0.25 | |
| P25 Male rats | CuA | 1.00 ± 0.28 | 1.00 ± 0.26 |
| CuD | 0.92 ± 0.29 | 1.00 ± 0.29 | |
| P26 Female rats | CuA | 1.00 ± 0.18 | 1.00 ± 0.28 |
| CuD | 0.39 ± 0.06* | 0.92 ± 0.29 | |
| P53 Male rats | CuA | 1.00 ± 0.28 | 1.00 ± 0.03 |
| CuD | 0.92 ± 0.25 | 1.00 ± 0.03 | |
| P28 Male mice | CuA | 1.00 ± 0.12 | 1.00 ± 0.09 |
| CuD | 0.68 ± 0.13 | 1.04 ± 0.15 | |
Values are means ± SEM (n=4 or 5). Total RNA was isolated from fast frozen liver and cDNA prepared and evaluated by qRT-PCR. Ceruloplasmin (Cp) abundance relative to glyceraldehyde phosphate dehydrogenase (GAPDH) was calculated and expressed relative to CuA means. Liver GAPDH mRNA abundance was determined and normalized to CuA means.
Different from Cu-adequate (CuA) within group, P < 0.05 (Student's t-test).
4. Discussion
One of the greatest opportunities and challenges of the copper-iron interaction field is to determine the mechanism for anemia associated with copper deficiency. One objective of the current work was to aid in this quest by evaluating Cp following copper deficiency. Results clearly show that anemic rodents have little, if any, Cp activity either diamine oxidase (ODA or PPD) or ferroxidase when evaluated by western blot iron assay. This was true both for CuD rats and CuD mice. Both CuD male rodents had elevated hepatic iron. In this study P26 CuD female rat pups did not have elevated liver Fe, similar to other studies with CuD males of similar age (Pyatskowit and Prohaska, 2008b). Perhaps, the variability in perinatal hepatic Fe overload in CuD rodents occurs because of time consuming solid diets containing high Fe versus nursing during lactation on milk with lower Fe content. However, older anemic P53 male CuD rats in the current study had liver Fe levels twice that of CuA controls consistent with a role for low Cp activity associated with anemia and tissue Fe retention. Humans with copper deficiency also have hepatic iron overload, anemia, low ferroxidase activity and low serum iron, supporting the current experimental models (Videt-Gibou et al., 2009). However, the Cp driven mechanism is not totally clear.
Cp -/- mice evaluated in the current study had no detectable ODA activity or ferroxidase activity by membrane iron assay. However, previous work on these null mice did not detect anemia (Harris et al., 1999). Cp -/- mice did display an accumulation of hepatic iron consistent with an impairment in iron flux due to loss of ferroxidase, however, serum iron was not lower. How can serum iron be normal if Cp ferroxidase is absent and iron efflux impaired as suggested by hepatic iron overload?
In support of this earlier work on Cp -/- mice, our previous work on CuD mice, lacking Cp activity, also reported normal plasma iron, yet hepatic iron elevation (Pyatskowit and Prohaska, 2008a). Importantly, that study documented low plasma iron in CuD rats. Data in the current study also appear to rule out differences in plasma transferrin, the iron transport protein, as a mechanism to explain low plasma iron in CuD rats and normal plasma iron in CuD mice as transferrin was not impacted by copper deficiency in any of the models in these studies.
One possibility to explain the plasma iron data in mice is that mice may have additional ferroxidase activity. Our current gel work and that by others with Cp -/- mice and copper-deficient mice do not support this view, as only one ferroxidase was detected, with mobility equal to Cp (Cherukuri et al., 2004; Chen et al., 2006). However, a recent study did detect ferroxidase activity in Cp -/- mice by substrate assay, in gel assay, and following gel permeation chromatography (Gray et al., 2009). The estimated size of this other “ferroxidase” was larger than Cp. Note current data on rat plasma appeared to detect a weak ferroxidase band above the Cp band on blots. Our lab studied mouse plasma ferroxidase nearly three decades ago and concluded that spectrophotometric ferroxidase assay (holotransferrin formation) for mouse, but not rat, Cp was unreliable and that most mouse plasma ferroxidase eluted in void volume not with Cp following gel filtration (Prohaska, 1981). Thus, the issue of mouse Cp ferroxidase remains somewhat unclear in regards to explaining the lack of impact on serum iron following copper deficiency.
Data in the current experiments clearly show that if Cp activity is to be assessed with diamines, ODA is a better substrate choice than PPD. Results with humans and rats indicated near total loss of Cp activity in the presence of azide with either substrate, as expected (Frieden and Hsieh, 1976). However, as confirmed in the current studies and as reported previously, mouse Cp activity is not eliminated totally by azide (Prohaska, 1981). This is likely due to subtle structural differences in the azide binding site of mouse Cp, as reported recently (Gray et al., 2009). Importantly, when mouse plasma Cp was assayed with ODA there was no detectable activity in Cp -/- mice or CuD mice suggesting that the residual azide resistant ODA activity in Cp +/+ mice and CuA mice (not shown) is also Cp. Note that Cp -/- mice had detectable PPD oxidase activity with or without azide. The nature of this azide resistant PPD oxidase is not known, but likely to be copper-dependent since lower activity was detected in plasma of CuD mice. Interestingly, this was not true for rat plasma as azide resistant PPD oxidase was equivalent in CuA and CuD rats.
Reductions in cuproenzyme activity following dietary copper restriction are not just the result of limiting cofactor. Current data demonstrate a profound reduction in plasma Cp protein following copper deficiency. This result in CuD mice, CuD rat pups, and CuD rat dams confirms prior work in adult copper deficient rats (Holtzman and Gaumnitz, 1970a; Gitlin et al., 1992). In those seminal studies it was shown that copper deficiency did not impair Cp biosynthesis (Gitlin et al., 1992). Rather, apo-Cp was released by liver and its half-life was shorter than holo-Cp resulting in significant reduction in Cp protein (Holtzman and Gaumnitz, 1970b). Our western immunoblot technique did not distinguish apo- from holo-Cp but is consistent with this concept. Interestingly, when Cp synthesis is limited by deletion of ATP7B, the gene mutated in Wilson's disease that is essential for metallation of Cp prior to secretion, the resulting null mice have no detectable Cp activity but normal levels of immunoreactive Cp, largely in the apo-form (Huster et al., 2006). This is clearly different from our data with CuD mice lacking Cp activity that demonstrate a 60% reduction in immunoreactive Cp using the same commercial antibody. Perhaps liver copper content impacts Cp turnover as it would be low in CuD liver and high in ATP7b -/- mice.
Lower Cp protein following copper deficiency is similar to observations on a number of other cuproenzymes. For example, lower levels of Cu, Zn-superoxide dismutase (Levieux et al., 1991), hephaestin (Nittis and Gitlin, 2004; Reeves et al., 2005), peptidylglycine α-amidating monoxygenase (Prohaska et al., 2005), and cytochrome c oxidase (Medeiros et al., 1997) have all been reported previously. Perhaps a general concept of increased turnover of cuproenzymes in the absence of cofactor copper applies. It is also possible that clearance of plasma apo-Cp is accelerated by binding to the extracellular chaperone clusterin (Wyatt and Wilson, 2010).
The possibility that Cp levels might be regulated transcriptionally by cellular copper content seems slight. Current work using qRT-PCR with rodent liver, past work using northern blots and rat and mouse liver, and work with copper chelators and hepatocyte cultures all failed to detect a diminuition in Cp mRNA (McArdle et al., 1990; Gitlin et al., 1992; Chen et al., 2006).
Dogma on the copper-iron interaction clearly support a role for Cp (ferroxidase) in iron efflux from liver (Osaki et al., 1966). However, the lack of frank anemia in Cp -/- mice and humans with aceruloplasminemia compared to severe anemia following dietary copper deficiency suggests that other copper-dependent factors besides Cp must be responsible for assuring that iron transport and erythropoiesis work properly. Understanding these mechanisms will be important to treat micronutrient anemia properly.
Acknowledgements
This research was supported by NIH grant HD 039708 and National Research Initiative grant 2006-35200-17378 from the USDA National Institute for Food and Agriculuture. Plasma from Cp -/- mice and their wild-type littermates were kindly donated by Dr. Z. Leah Harris, Vanderbilt University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations used: Cu, copper; CuA, copper-adequate; CuD, copper-deficient; Cp, ceruloplasmin
References
- Chen H, Huang G, Su T, Gao H, Attieh ZK, McKie AT, Anderson GJ, Vulpe CD. Decreased hephaestin activity in the intestine of copper-deficient mice causes systemic iron deficiency. J. Nutr. 2006;136:1236–1241. doi: 10.1093/jn/136.5.1236. [DOI] [PubMed] [Google Scholar]
- Cherukuri S, Tripoulas NA, Nurko S, Fox PL. Anemia and impaired stress-induced erythropoiesis in aceruloplasminemic mice. Blood Cells Mol. Dis. 2004;33:346–355. doi: 10.1016/j.bcmd.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Fox PL. The copper-iron chronicles: the story of an intimate relationship. Biometals. 2003;16:9–40. doi: 10.1023/a:1020799512190. [DOI] [PubMed] [Google Scholar]
- Frieden E, Hsieh HS. Ceruloplasmin: the copper transport protein with essential oxidase activity. Adv Enzymol Rel Areas Mol Biol. 1976;44:187–236. doi: 10.1002/9780470122891.ch6. [DOI] [PubMed] [Google Scholar]
- Gitlin JD, Schroeder JJ, Lee-Ambrose LM, Cousins RJ. Mechanisms of caeruloplasmin biosynthesis in normal and copper- deficient rats. Biochem. J. 1992;282:835–839. doi: 10.1042/bj2820835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LW, Kidane TZ, Nguyen A, Akagi S, Petrasek K, Chu YL, Cabrera A, Kantardjieff K, Mason AZ, Linder MC. Copper proteins and ferroxidases in human plasma and that of wild-type and ceruloplasmin knockout mice. Biochem. J. 2009;419:237–245. doi: 10.1042/BJ20081983. [DOI] [PubMed] [Google Scholar]
- Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc. Natl. Acad. Sci. USA. 1999;96:10812–10817. doi: 10.1073/pnas.96.19.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu. Rev. Nutr. 2002;22:439–458. doi: 10.1146/annurev.nutr.22.012502.114457. [DOI] [PubMed] [Google Scholar]
- Holtzman NA, Gaumnitz BM. Identification of an apoceruloplasmin-like substance in the plasma of copper-deficient rats. J. Biol. Chem. 1970a;245:2350–2353. [PubMed] [Google Scholar]
- Holtzman NA, Gaumnitz BM. Studies on the rate of release and turnover of ceruloplasmin and apoceruloplasmin in rat plasma. J. Biol. Chem. 1970b;245:2354–2358. [PubMed] [Google Scholar]
- Huster D, Finegold MJ, Morgan CT, Burkhead JL, Nixon R, Vanderwerf SM, Gilliam CT, Lutsenko S. Consequences of copper accumulation in the livers of the Atp7b-/- (Wilson disease gene) knockout mice. Am. J. Pathol. 2006;168:423–434. doi: 10.2353/ajpath.2006.050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levieux A, Levieux D, Lab C. Immunoquantitation of rat erythrocyte superoxide dismutase: its use in copper deficiency. Free Radic. Biol. Med. 1991;11:589–595. doi: 10.1016/0891-5849(91)90140-x. [DOI] [PubMed] [Google Scholar]
- Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mazumder B, Mukhopadhyay CK, Prok A, Cathcart MK, Fox PL. Induction of ceruloplasmin synthesis by IFN-gamma in human monocytic cells. J. Immunol. 1997;159:1938–1944. [PubMed] [Google Scholar]
- McArdle HJ, Mercer JF, Sargeson AM, Danks DM. Effects of cellular copper content on copper uptake and metallothionein and ceruloplasmin mRNA levels in mouse hepatocytes. J. Nutr. 1990;120:1370–1375. doi: 10.1093/jn/120.11.1370. [DOI] [PubMed] [Google Scholar]
- Medeiros DM, Shiry L, Samelman T. Cardiac nuclear encoded cytochrome c oxidase subunits are decreased with copper restriction but not iron restriction: gene expression, protein synthesis and heat shock protein aspects. Comp. Biochem. Physiol. A. Physiol. 1997;117:77–87. doi: 10.1016/s0300-9629(96)00365-9. [DOI] [PubMed] [Google Scholar]
- Milne DB. Assessment of copper nutritional status. Clin. Chem. 1994;40:1479–1484. [PubMed] [Google Scholar]
- Nittis T, Gitlin JD. Role of copper in the proteosome-mediated degradation of the multicopper oxidase hephaestin. J. Biol. Chem. 2004;279:25696–25702. doi: 10.1074/jbc.M401151200. [DOI] [PubMed] [Google Scholar]
- Osaki S, Johnson DA, Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J. Biol. Chem. 1966;241:2746–2751. [PubMed] [Google Scholar]
- Prohaska JR. Comparison between dietary and genetic copper deficiency in mice: copper-dependent anemia. Nutr. Res. 1981;1:159–167. [Google Scholar]
- Prohaska JR. Changes in tissue growth, concentrations of copper, iron, cytochrome oxidase and superoxide dismutase subsequent to dietary or genetic copper deficiency in mice. J. Nutr. 1983;113:2048–2058. doi: 10.1093/jn/113.10.2048. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Changes in Cu,Zn-superoxide dismutase, cytochrome c oxidase, glutathione peroxidase and glutathione transferase activities in copper- deficient mice and rats. J. Nutr. 1991;121:355–363. doi: 10.1093/jn/121.3.355. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Copper. In: Bowman BA, Russell RM, editors. Present Knowledge In Nutrition. Vol. 1. International Life Sciences Institute; Washington, DC: 2006. pp. 458–470. [Google Scholar]
- Prohaska JR, Brokate B. Dietary copper deficiency alters protein levels of rat dopamine-bet a - monooxygenase and tyrosine monooxygenase. Experimental biology and medicine (Maywood, N.J. 2001;226:199–207. doi: 10.1177/153537020122600307. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Gybina AA, Broderius M, Brokate B. Peptidylglycine-alpha-amidating monooxygenase activity and protein are lower in copper-deficient rats and suckling copper-deficient mice. Arch. Biochem. Biophys. 2005;434:212–220. doi: 10.1016/j.abb.2004.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyatskowit JW, Prohaska JR. Rodent brain and heart catecholamine levels are altered by different models of copper deficiency. Comp. Biochem. Physiol. C. 2007;145:275–281. doi: 10.1016/j.cbpc.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyatskowit JW, Prohaska JR. Copper deficient rats and mice both develop anemia but only rats have lower plasma and brain iron levels. Comp. Biochem. Physiol. C. 2008a;147:316–323. doi: 10.1016/j.cbpc.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyatskowit JW, Prohaska JR. Multiple mechanisms account for lower plasma iron in young copper deficient rats. Biometals. 2008b;21:343–352. doi: 10.1007/s10534-007-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PG, Demars LC, Johnson WT, Lukaski HC. Dietary copper deficiency reduces iron absorption and duodenal enterocyte hephaestin protein in male and female rats. J. Nutr. 2005;135:92–98. doi: 10.1093/jn/135.1.92. [DOI] [PubMed] [Google Scholar]
- Rice EW. Standardization of ceruloplasmin activity in terms of International Enzyme Units. Oxidative formation of “Bandrowski's base” from p-phenylenediamine by ceruloplasmin. Anal. Biochem. 1962;3:452–456. doi: 10.1016/0003-2697(62)90076-3. [DOI] [PubMed] [Google Scholar]
- Schosinsky KH, Lehmann HP, Beeler MF. Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin. Chem. 1974;20:1556–1563. [PubMed] [Google Scholar]
- Videt-Gibou D, Belliard S, Bardou-Jacquet E, Troadec MB, Le Lan C, Jouanolle AM, Loreal O, Rivalan J, Brissot P. Iron excess treatable by copper supplementation in acquired aceruloplasminemia: a new form of secondary human iron overload? Blood. 2009;114:2360–2361. doi: 10.1182/blood-2009-06-226175. [DOI] [PubMed] [Google Scholar]
- Wyatt AR, Wilson MR. Identification of human plasma proteins as major clients for the extracellular chaperone clusterin. J. Biol. Chem. 2010;285:3532–3539. doi: 10.1074/jbc.M109.079566. [DOI] [PMC free article] [PubMed] [Google Scholar]