Abstract
OBJECTIVE
The survival rates after pediatric intestinal transplant according to underlying disease are unknown. The objective of our study was to describe the population of pediatric patients receiving an intestinal transplant and to evaluate survival according to specific disease condition.
PATIENTS
Pediatric patients (≤21 years of age) with intestinal failure meeting criteria for intestinal transplant were included in the study.
METHODS
A retrospective review of the United Network for Organ Sharing intestinal transplant database (January 1, 1991, to May 16, 2008), including all pediatric transplant centers participating in the United Network for Organ Sharing, was conducted. The main outcome measures were survival and mortality.
RESULTS
Eight hundred fifty-two children received an intestinal transplant (54% male). Median age and weight at the time of transplant were 1 year (interquartile rage: 1–5) and 10.7 kg (interquartile rage: 7.8–21.7). Sixty-nine percent of patients also received a simultaneous liver transplant. The most common diagnoses among patients who received a transplant were gastroschisis (24%), necrotizing enterocolitis (15%), volvulus (14%), other causes of short-gut syndrome (19%), functional bowel syndrome (16%), and Hirschsprung disease (7%). The Kaplan-Meier curves demonstrated variation in patient survival according to diagnosis. Cox regression analysis confirmed a survival difference according to diagnosis (P < .001) and demonstrated a survival advantage for those patients listed with a diagnosis of volvulus (P < .01) compared with the reference gastroschisis. After adjusting for gender, recipient weight, and concomitant liver transplant, children with volvulus had a lower hazard ratio for survival and a lower risk of mortality.
CONCLUSIONS
Survival after intestinal transplant was associated with the underlying disease state. The explanation for these findings requiresadditionalinvestigationintothedifferencesincharacteristics of the population of children with intestinal failure.
Keywords: intestinal failure, intestinal transplant, outcomes, survival, mortality, pediatrics, children
Intestinal failure in the pediatric population may be a consequence of a variety of diseases, including gastroschisis, volvulus, enteropathies, and motility disorders. Regardless of etiology, the pathophysiology of the condition is similar: inadequate bowel length or absorptive capacity to provide for appropriate nutrition and hydration. Advancements in clinical management (total parenteral nutrition, fluids, and antibiotics) have led to an increase in the number of children surviving with intestinal failure. When a child’s condition fails to respond to total parenteral nutrition, intestinal transplant is an option at certain centers. Outcomes after intestinal transplant are central to understanding the risks and benefits of this procedure. There have been analyses of the international experience with intestinal transplant for both patient and graft survival, including subanalyses of survival comparing intestinal and multivisceral transplant.1,2 Additional analyses have been performed at single centers, and the relationship between specific diagnoses and survival was evaluated. Bueno et al3 noted that “surgical” conditions had poorer survival compared with those with “nonsurgical” conditions, while the Miami, Florida, group4 demonstrated that children with necrotizing enterocolitis (NEC) could enjoy survival rates similar to those of other conditions. Information on survival across multiple centers after intestinal transplant for specified disease diagnoses is lacking. The primary aim of this study was to describe the population of pediatric patients receiving an intestinal transplant and analyze their survival rates with respect to disease diagnosis.
METHODS
Database and Patients
The United Network of Organ Sharing (UNOS)/Organ Procurement and Transplantation Network (OPTN) is charged by the Human Resources and Services Administration to maintain the US transplantation network.5 This prospectively collected database documents pretransplant, transplant, and follow-up information for patients who need or have had a transplant and contains information on all intestinal transplants performed in the United States and reported to the OPTN. Data are available to any citizen. Because data in the file were deidentified, the University of Washington Human Subjects Division granted a certificate of exemption (HSD 34595) for our work.
The database contains unique entries that represent a listing event. An individual patient may have more than 1 entry within the database. Individuals may be listed simultaneously at 2 centers to maximize their opportunity for transplant, and patients who receive an initial graft may suffer graft failure and require another listing. In both situations, patients may have more than 1 entry in the database, but all entries contain the same unique identification number.
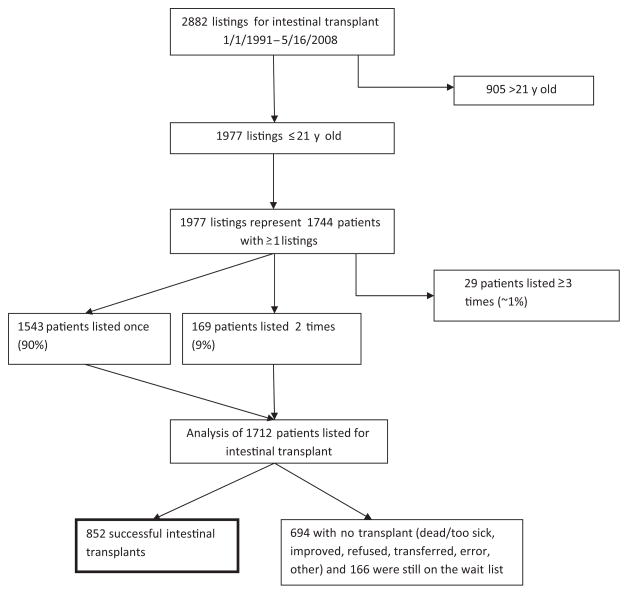
On the basis of OPTN data of May 16, 2008, we identified 2882 listings for intestinal transplantation. Of these, 1977 listings were subjects 21 years of age or younger at the time of the listing, representing 1744 individual patients. Patients listed 3 or more times (29 patients, 93 listings, ~1% of the population) were excluded from the study. Reconciling the listings on 3 occasions coupled with statistical analysis of patients listed 3 or more times, including the possibilities of transplant, relisting, retransplant, and death, was outside the parameters of our specific aims. The remaining 1712 patients who were listed once or twice represent the population for our analysis (Fig 1). We chose subjects 21 years of age or younger at listing as the age cutoff with the belief that pediatric conditions leading to intestinal transplant may continue to affect individuals beyond 18 years of age.
FIGURE 1.
Study subject selection outline from UNOS database (≤21 years of age at listing).
Entries in which date data were chronologically improbable (eg, cases in which the transplant date preceded the listing date), had the transplant date designated as the listing date (6 cases). Instances of multiple listing (as described previously) were reconciled (198 subjects). The earliest listing date was chosen as the representative listing date for subjects with multiple or overlapping listings at 2 or more centers. When subjects required relisting, follow-up data were updated to reflect retransplants, deaths, or those patients still waiting.
Patients younger than 1 year of age were categorized as <1 year old. Weights used are in kilograms and are specific to the listing or transplant. Patients with multiorgan transplants were included in the study.
Diagnostic Definitions
The underlying disease leading to intestinal failure was determined by using the most specific entry among all diagnosis categories provided in the database. For cases in which an “other/unknown” diagnosis was indicated or if a diagnosis was missing, either a secondary or text diagnosis was used, if reasonable. For situations in which both the secondary and text diagnosis were not reconcilable, the diagnosis was left as “other.” Data for diagnoses were ascertained “at discharge,” if available; if not, then “at transplant.” If no data were available at transplant, then data “at registration” were ascertained.
In the OPTN database, “short-gut syndrome” is a large category encompassing gastroschisis, atresia, NEC, and volvulus, as well as shortened gut due to resection and other unspecified reasons (Table 1). We separated patients with NEC, gastroschisis, and volvulus from the larger category of short-gut syndrome for our analysis. Patients within the short-gut syndrome category in this study included the remaining OPTN-defined patients and are referred to in this article as patients with short-gut syndrome (Table 1). Similarly, OPTN-defined “functional bowel problem” comprises a group of several conditions, including Hirschsprung disease, which was analyzed separately. The remaining conditions contributing to the study category of functional bowel problems are outlined in Table 1. Graft failure and retransplant represent their own category.
TABLE 1.
UNOS and Study Diagnostic Categories
| OPTN Diagnosis Categories | Our Diagnosis Categories |
|---|---|
| SGS | |
| Gastroschisis | Gastroschisis |
| Intestinal volvulus due to adhesion, malrotation, persistent obstruction | Volvulus |
| NEC | NEC |
| Intestinal atresia; mass resection due to inflammatory bowel/Crohn disease, mesenteric arterial or venous thrombus, or tumor; other; unspecified | SGS |
| FBP | |
| Hirschsprung disease | Hirschsprung disease |
| Microvillus, inclusion disease, neuronal intestinal dysplasia, protein-losing enteropathy, myopathic pseudo-obstruction, neuropathic pseudo-obstruction, other, unspecified | FBP |
| Graft failure | Other/unknown/missing/graft failure (intractable diarrhea, trauma, multiple polyposis) |
| Intestinal disease, other, specify | |
| Retransplant/graft failure | |
| Other | |
| Not reported | |
SGS indicates short-gut syndrome; FBP, functional bowel problem.
Statistical Analysis
Descriptive statistics were used to evaluate subjects who received an intestinal transplant. Demographic data from the time of listing and time of transplant were analyzed for this cohort. Categorical data were summarized with absolute numbers and percentages. Numeric data were summarized with means and SDs or medians and interquartile range (IQR) statistics, where appropriate. Weight, rather than age, was used in the analysis, given the collinearity of these 2 variables.
For those children with available follow-up data, Kaplan-Meier survival curves were used to analyze survival after intestinal transplant according to receipt of concomitant liver transplant, specific disease conditions, and before and after the introduction of the model for end-stage liver disease (MELD) and pediatric end-stage liver disease (PELD) criteria for liver transplant allocation in 2002. Greenwood variance was used to calculate confidence intervals (CIs) for survival proportions at 1, 3, and 5 years, respectively. We analyzed the proportion of children who received a concomitant liver transplant according to diagnosis using the χ2 test. Multiple logistic regression analysis analyzed factors (weight at transplant, gender, diagnostic category, and concomitant liver transplant) associated with mortality among children who received a transplant. Cox regression analysis was used to examine differences in the time of survival after transplant according to diagnostic category, including adjustment for recipient weight and concomitant liver transplant. Both multiple logistic and Cox regressions used gastroschisis as the reference category, because it was the most common condition and the largest diagnostic group. Analyses were performed by using Stata 10.0 (Stata Corp, College Station, TX).
RESULTS
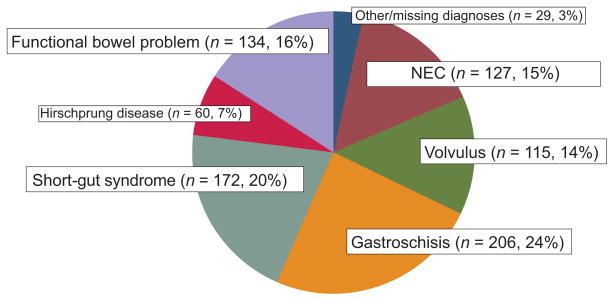
We identified 852 children who received an intestinal transplant (54% male). The median age, weight, and height of these children at the time of the listing were 1 year, 9.2 kg, and 70 cm, respectively (Table 2). Demographic data for those same children at the time of the transplant demonstrated a median age of 1 year with a median weight and height of 10.7 kg and 74 cm, respectively. The most common diagnosis at the time of listing among those who received a transplant was gastroschisis (24%) followed by short-gut syndrome (20%), functional bowel syndrome (16%), and NEC (15%) (Fig 2). Nearly 70% of intestinal transplant recipients received a concomitant liver transplant. Almost 90% of the children with NEC required a concomitant liver transplant, whereas only 60% of the children with volvulus or a functional bowel problem received a liver transplant. A χ2 analysis of the proportion of children who received a concomitant liver transplant demonstrated significant differences according to disease condition (P < .001) (Table 3).
TABLE 2.
Demographic Characteristics of Children Who Received a Transplant
| Characteristic | No. (%)/Median (IQR) |
With Liver, n (%) | Multiple Logistic Regression |
|||
|---|---|---|---|---|---|---|
| At Listing | At Transplant | OR | 95% CI | P | ||
| Age, y | 1 (0–5) | 1 (1–5) | a | a | a | |
| Height, cm | 70 (61–101.3) | 74 (65–109) | a | a | a | |
| Weight, kg | 9.2 (6.7–18.3) | 10.7 (7.8–21.7) | 0.99 | 0.98–1.00 | .489 | |
| Female, % | 43 | 46 | 0.99 | 0.72–1.36 | .967 | |
| Simultaneous liver transplant | 572 (70) | 1.46 | 0.99–2.13 | .050 | ||
| NEC | 127 | 112 (88) | 1.08 | 0.66–1.77 | .734 | |
| Volvulus | 115 | 66 (57) | 0.38 | 0.21–0.69 | .001 | |
| Short-gut syndrome | 172 | 164 (80) | 1.01 | 0.63–1.60 | .966 | |
| Hirschsprung disease | 60 | 113 (66) | 0.80 | 0.41–1.56 | .526 | |
| Functional bowel problem | 134 | 40 (67) | 0.85 | 0.51–1.41 | .543 | |
| Gastroschisis (reference) | 206 | 164 (80) | ||||
IQR indicates interquartile range. Includes concomitant liver transplant by diagnosis and multiple logistic regression analysis for mortality following intestinal transplant by diagnosis (reference: gastroschisis).
Not used in logistic regression because of collinearity with weight.
FIGURE 2.
Diagnoses of children who received a transplant (at listing) (N = 810).
TABLE 3.
Kaplan-Meier 1-, 3-, and 5-Year Survival Rates After Transplant According to Diagnosis
| Diagnosis | Kaplan-Meier Survival Rate, % (95% CI) |
||
|---|---|---|---|
| 1 y | 3 y | 5 y | |
| All (N = 814) | 73 (69–76) | 61 (57–64) | 55 (51–59) |
| NEC (n = 127) | 61 (51–69) | 47 (36–57) | 43 (32–54) |
| Volvulus (n = 115) | 82 (73–88) | 79 (69–86) | 73 (62–82) |
| Gastroschisis (n = 206) | 71 (64–77) | 57 (48–64) | 49 (41–57) |
| Short-gut syndrome (n = 172) | 71 (63–78) | 55 (45–63) | 50 (40–59) |
| Hirschsprung disease (n = 60) | 76 (62–85) | 69 (55–80) | 56 (37–71) |
| Functional bowel problem (n = 134) | 75 (66–81) | 62 (52–70) | 57 (46–66) |
Follow-up time was not available for all children.
Kaplan-Meier Curves
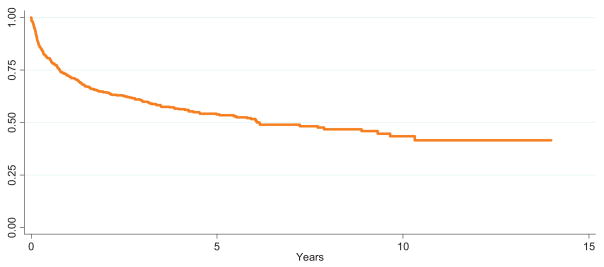
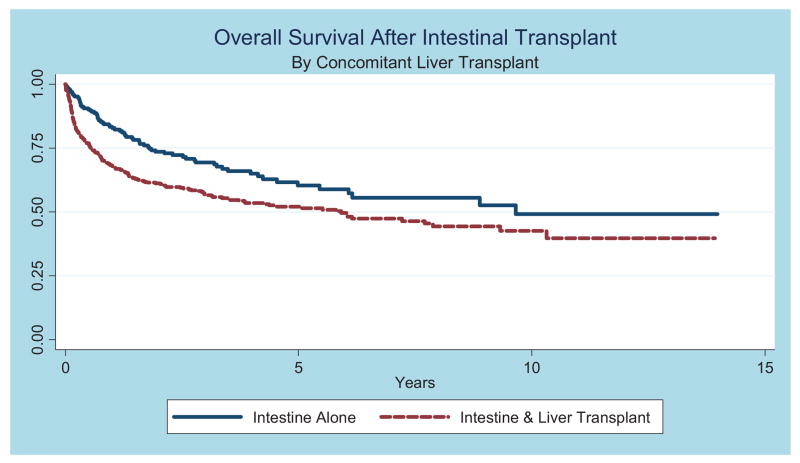
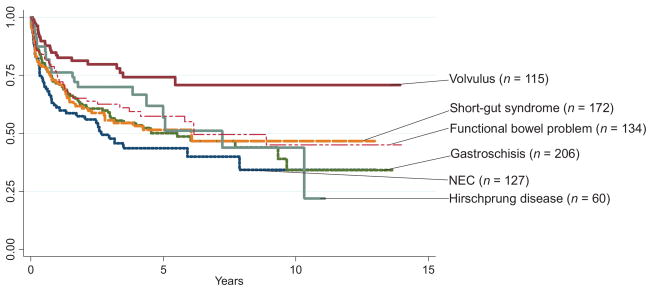
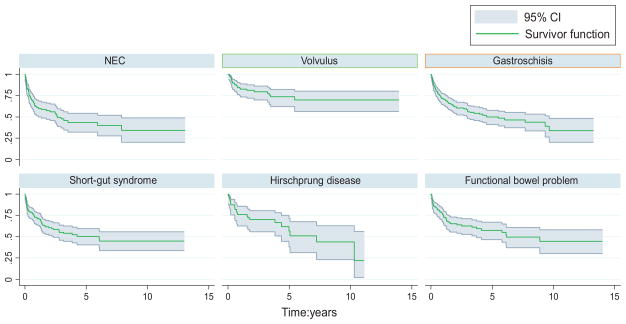
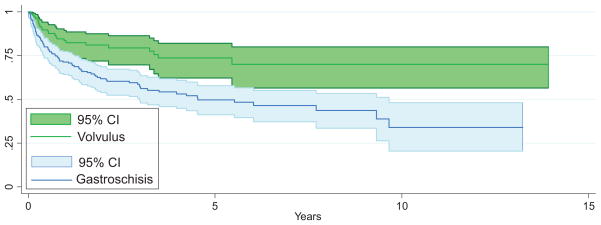
Among children with available follow-up data(n = 814), the overall survival rate was 73% (95% CI: 69%–76%) at 1 year, 61%(95% CI: 57%–64%) at 3 years, and 55% (95% CI: 51%–59%) at 5 years (Fig 3 and Table 4). Analysis of survival before and after MELD/PELD criteria (2002) for liver transplant demonstrated an increase in survival rates after the implementation of MELD/PELD, with the 3-year survival rate improving from 54% to 65% (Table 5). Cox regression analysis confirmed a significant improvement in survival rates among transplants performed during and after 2002 compared with those performed before 2002 (Table 5). There is evidence for improved survival in those children who did not need a concomitant liver transplant (Fig 4). After performing a graphical analysis of overall survival according to diagnosis, there was the suggestion of a difference among diagnostic categories (Fig 5). Individual Kaplan-Meier curves with 95% CIs were calculated and graphed for the 6 most common diagnoses to further illustrate differences in survival rates (Fig 6). There seemed to be a difference in survival among these diagnostic categories. Compared with patients in our reference group of gastroschisis, patients with a diagnosis of volvulus demonstrated significantly better survival rates (Fig 7).
FIGURE 3.
Overall survival rates after transplant (not all children had available follow-up time).
TABLE 4.
Kaplan-Meier 1-, 3-, and 5-Year Survival Rates, Including Additional Analysis of Survival Before and After 2002 After the Introduction of the MELD/PELD Allocation Criteria for Liver Transplants
| Survival, y | Kaplan-Meier Survival Rate, % (95% CI) |
||
|---|---|---|---|
| Overall | Before 2002a | 2002 and Laterb | |
| 1 | 73 (69–76) | 65 (60–71) | 77% (72–80) |
| 3 | 61 (57–64) | 54 (48–60) | 65% (59–69) |
| 5 | 55 (51–59) | 50 (43–55) | 54% (46–62) |
Cox proportional hazards of transplants before and after 2002: 0.69 (95% CI: 0.56–0.88); P < .002.
n = 303.
n = 532.
TABLE 5.
Cox Proportional Hazard Ratios (Adjusted and Unadjusted) for Survival According to Diagnosis
| Diagnosis | Unadjusted Hazard Ratio (Overall P < .01) | 95% CI | P | Adjusted Hazard Ratioa (Overall P < .001) | 95% CI | P |
|---|---|---|---|---|---|---|
| NEC | 1.29 | 0.927–1.82 | .128 | 1.25 | 0.87–1.79 | .223 |
| Volvulusb | 0.451 | 0.286–0.710 | .001 | 0.46 | 0.28–0.71 | .003 |
| Short-gut syndrome | 1.03 | 0.747–1.42 | .844 | 1.14 | 0.81–1.63 | .44 |
| Hirschsprung disease | 0.810 | 0.507–1.29 | .380 | 0.810 | 0.507–1.29 | .380 |
| Functional bowel problem | 0.865 | 0.607–1.23 | .422 | 0.91 | 0.61–1.35 | .637 |
Gastroschisis was used as the reference category.
Adjusted for both weight at transplant and concomitant liver transplant.
Weight at transplant: adjusted Cox hazard ratio for volvulus versus gastroschisis: 0.444 (0.268–0.736); P < .01.
FIGURE 4.
Overall survival rates after transplant stratified according to concomitant liver transplant among the 6 most common diagnostic conditions.
FIGURE 5.
Intestinal transplant recipient survival rates according to diagnosis (not all children have available follow-up time).
FIGURE 6.
Intestinal transplant recipient survival rates according to 6 most common diagnoses, with 95% CIs.
FIGURE 7.
Intestinal transplant recipient survival rates, with 95% CIs: volvulus and gastroschisis.
Multiple Logistic Regression
Multiple logistic regression analysis of survival after intestinal transplant measured the proportionate increase in the odds of mortality after intestinal transplant given a variety of factors (Table 2). Gender and weight at transplant were not significantly associated with mortality. There was a trend in the association between concomitant liver transplant and higher odds of mortality that was just shy of statistical significance (odds ratio [OR]: 1.46; P = .050). Almost all diagnostic conditions were not significantly different in posttransplant mortality compared with the reference category. An exception was the category of children who received a transplant for volvulus. These children were associated with a lower risk of mortality compared with those in the reference category, after adjusting for gender, recipient weight, and concomitant liver transplant (OR: 0.38; P < .01).
Cox Proportional Hazards
Analysis of survival using Cox regression demonstrated a statistically significant difference in survival when comparing the reference category with all other disease states (P < .01). The specific hazard ratio for survival for the volvulus group was 0.451 (95% CI: 0.286–0.710; Table 5). Another regression analysis that was adjusted for recipient weight at transplant and for concomitant liver transplant did not change the findings of a lower hazard ratio for survival among the volvulus group. Cox proportional hazards regression analysis seemed to provide hazard ratios that explained the findings in the Kaplan-Meier curves outlined previously (Fig 7). The hazard ratios for other diagnoses were not statistically significant.
DISCUSSION
The number of children who develop intestinal failure and are referred for intestinal transplant for definitive treatment has doubled over the past decade.6 Improving survival rates in this group of patients is important to maximize the scarce resource of donor intestines.
This study represents the largest analysis of survival after pediatric intestinal transplant. The closest comparison is the Intestinal Transplant Registry (ITR), an international registry that includes data from US centers spanning nearly 20 years, with slightly more than 600 transplants.7 The ITR demonstrates a slight preference for transplant in males (57%), which resonates with our findings (54%). In addition, our data were in agreement on the indication of gastroschisis for transplant (21%). The majority of their children who received a transplant were between the ages of 1 and 13 year, whereas in our study, the median age at the time of transplant was 1 year. This discrepancy may be a result of recipient selection, availability of donor organs, or our inclusion criteria. The ITR analysis revealed the following factors to be significantly associated with survival: pretransplant status, re-transplant, center size, maintenance therapy, and type of transplant.
The UNOS experience has established the use of transplant to cure children with intestinal failure from diverse underlying conditions. Survival after intestinal transplant may be associated with the disease condition underlying the development of intestinal failure; however, this concept is not unique to intestinal transplant. The pediatric cardiac surgery literature has demonstrated a difference in perioperative survival rates among different disease conditions.8 Although Kaplan-Meier survival curves after intestinal transplant seemed to be similar across groups, the volvulus group seemed to have an improved survival rate. Cox proportional hazards analysis of survival demonstrated that the volvulus group had a 30% to 70% reduction in risk compared with the referent group. Improved outcomes among the volvulus group persisted after adjusting for weight at transplant and after adjusting for concomitant liver transplant. Similarly, multivariate regression analysis showed that volvulus was associated with a lower chance of mortality after intestinal transplant after adjusting for gender, weight, and concomitant liver transplant.
Reasons underlying the disparities in survival rates after intestinal transplant among diagnostic categories, specifically volvulus, are unknown. An evaluation of survival in patients with intestinal failure, including transplant as a treatment option, showed an association between the diagnosis of “pseudo-obstruction” and improved outcomes.9,10 Similar work determined that in children (<18 months of age), the diagnosis of intestinal atresia and the presence of an ileocecal valve may confer an increased risk of mortality.11 When examining the outcomes of patients who received an intestinal transplant alone, previous studies focused on pretransplant status, number of transplants, center size, or “surgical” and “nonsurgical” causes of intestinal failure as determinants of outcomes.3,7 In addition, individual centers have evaluated their experience with intestinal transplant in the setting of only NEC or intestinal pseudo-obstruction.4,12,13 No study has compared the outcomes after intestinal transplant across the spectrum of diseases that lead to intestinal failure.
The 2007 OPTN/Scientific Registry of Transplant Recipients annual report noted that “there does not appear to be a significant difference between patient or graft survival when comparing intestine alone to liver-intestine.”6 Our Kaplan-Meier analysis including concomitant liver transplant seemed to demonstrate a difference in survival rates between these groups (Fig 4). Multiple logistic regression analysis further supported this idea by demonstrating a trend toward mortality among patients who received a concomitant liver transplant, after adjusting for gender, weight at transplant, and diagnosis. Our data seemed to agree with the ITR analysis from 2003, which demonstrated a difference in pediatric patient survival rates among the type of transplant (intestine only, intestine and liver, and multivisceral).7 The discrepancy in our findings compared with the OPTN/Scientific Registry of Transplant Recipients may be because of their use of only the most recent decade of transplant data. In addition, their data include both children and adults, whereas we excluded those older than 21 years of age. Our choice to focus on younger patients gives our population a larger percentage of children <1 year old. Outcomes in this group have traditionally been poor, perhaps as a result of an increased need for liver transplant.6
In 2002, the UNOS created new listing criteria for patients waiting for liver transplants to establish more equality in allocation. Because children with intestinal failure often develop liver disease secondary to parenteral nutrition, we analyzed the survival rate of children who received a transplant before and after 2002. Comparing intestinal transplant survival according to era, there is evidence of improved survival rates among those transplanted after 2002, also shown to be significant by Cox regression analysis. A similar improvement in patient survival is demonstrated in the ITR analysis when evaluating survival in eras from 1991 to 2003. Although this may be because of improvements in clinical care of the posttransplant patients in the international setting, it may also be caused by improved allocation of livers among children awaiting intestinal transplant in the United States.
There are several limitations to our study. The primary limitation is our dependence on a clinical database. There were dates and entries that were either logically or chronologically improbable that required identification and reconciliation. Although the UNOS and its members make every effort to ensure the quality of the data, we were dependent on others to reliably diagnose, code, and record diseases, dates, and events. Despite that, the UNOS database is the largest database for intestinal transplant in North America and provides information on patients from 1987. Although our data spanned nearly 2 decades and provided for a relatively large number of patients, there were drawbacks to using data drawn from 20 years. Improvements in care and technology in the management of intestinal failure have taken place. Wait times, donor availability, immunosuppression, surgical transplantation techniques, and medical, diagnostic, and management methods have all changed during this time. Changes in allocation policies have affected eligibility and priorities. Specifically, changes in OPTN policy, such as providing additional points for liver-intestine candidates and the allocation of pediatric donor organs have changed. Follow-up time after transplant is limited by the number of years that this procedure has been used. Long-term analysis of survival is subject to the possibility of a type I error, given the relatively small numbers of children surviving to reach the long-term follow-up period. Lastly, the opportunity for in- or out-migration from the database was possible; however, nearly all patients in the United States are captured by this national database. Despite these limitations, this comprehensive database allowed us to analyze the largest cohort of patients with intestinal failure and who have received a transplant to date.
CONCLUSIONS
A recent national conference to determine research priorities in pediatric solid organ transplantation determined 2 areas of study specific to intestinal transplantation: (1) identifying the best immunosuppression “protocol” and (2) determining the best methods to discriminate between infection and rejection in the allograft.14 Given the findings of our study, we would suggest the addition of a third priority. Just as the disparity in wait-list mortality between patients with hepatocellular carcinoma and patients with end-stage liver disease has allowed for differential listing criteria for those conditions, identifying differences in outcomes among patients receiving intestinal transplantation may compel listing criteria specific to underlying disease states. Ultimately, intestine and organ allocation must balance the risks of the wait list with the benefits after transplant. The determination of differential outcomes among intestinal transplant recipients is not meant to create a group in which transplant should be contraindicated. On the contrary, our data have demonstrated successful transplant in a variety of conditions. Future research should identify what factors (preexisting bowel length, length of time on parenteral nutrition, degree of liver dysfunction, or other comorbid conditions) allow certain groups to demonstrate improved outcomes. In doing so, we may be able to improve the outcomes of all children receiving an intestinal transplant.
WHAT’S KNOWN ON THIS SUBJECT: Intestinal transplant is increasingly being used to treat children with intestinal failure. The survival rates after pediatric intestinal transplant according to underlying disease are unknown. Information on survival across multiple centers after intestinal transplant for specified disease diagnoses is lacking.
WHAT THIS STUDY ADDS: There are disparities in survival rates after intestinal transplant on the basis of the underlying etiology leading to intestinal failure.
Acknowledgments
This work was supported by National Institutes of Health grant T32 CA09168. This work was also supported in part by the Health Resources and Services Administration contract 234-2005-370011C.
ABBREVIATIONS
- NEC
necrotizing enterocolitis
- UNOS
United Network for Organ Sharing
- OPTN
Organ Procurement and Transplantation Network
- IQR
interquartile range
- MELD
model for end-stage liver disease
- PELD
pediatric end-stage liver disease
- CI
confidence interval
- OR
odds ratio
- ITR
Intestinal Transplant Registry
Footnotes
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or National Institutes of Health or the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Fryer JP. Intestinal transplantation: an update. Curr Opin Gastroenterol. 2005;21(2):162–168. doi: 10.1097/01.mog.0000153313.43574.1b. [DOI] [PubMed] [Google Scholar]
- 2.Grant D. Intestinal transplantation: 1997 report of the international registry. Intestinal Transplant Registry. Transplantation. 1999;67(7):1061–1064. doi: 10.1097/00007890-199904150-00021. [DOI] [PubMed] [Google Scholar]
- 3.Bueno J, Ohwada S, Kocoshis S, et al. Factors impacting the survival of children with intestinal failure referred for intestinal transplantation. J Pediatr Surg. 1999;34(1):27–32. 32–23. doi: 10.1016/s0022-3468(99)90223-3. [DOI] [PubMed] [Google Scholar]
- 4.Vennarecci G, Kato T, Misiakos EP, et al. Intestinal transplantation for short gut syndrome attributable to necrotizing enterocolitis. Pediatrics. 2000;105(2) doi: 10.1542/peds.105.2.e25. Available at: www.pediatrics.org/cgi/content/full/105/2/e25. [DOI] [PubMed]
- 5.US Department of Health and Human Services, Health Resources and Services Administration/Organ Procurement and Transplantation Network. [Accessed May 16, 2008];Uniting people and information to help save lives. Available at: http://optn.transplant.hrsa.gov.
- 6.Magee JC, Krishnan SM, Benfield MR, Hsu DT, Shneider BL. Pediatric transplantation in the United States, 1997–2006. Am J Transplant. 2008;8(4 pt 2):935–945. doi: 10.1111/j.1600-6143.2008.02172.x. [DOI] [PubMed] [Google Scholar]
- 7.Intestinal Transplant Registry. [Accessed May 16, 2008];An international collaboration of centers performing intestinal transplant. Available at: www.intestinaltransplant.org.
- 8.Dionigi B, Razzouk AJ, Hasaniya NW, Chinnock RE, Bailey LL. Late outcomes of pediatric heart transplantation are independent of pre-transplant diagnosis and prior cardiac surgical intervention. J Heart Lung Transplant. 2008;27(10):1090–1095. doi: 10.1016/j.healun.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Vantini I, Benini L, Bonfante F, et al. Survival rate and prognostic factors in patients with intestinal failure. Dig Liver Dis. 2004;36(1):46–55. doi: 10.1016/j.dld.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Reyes J, Mazariegos GV, Bond GM, et al. Pediatric intestinal transplantation: historical notes, principles and controversies. Pediatr Transplant. 2002;6(3):193–207. doi: 10.1034/j.1399-3046.2002.02003.x. [DOI] [PubMed] [Google Scholar]
- 11.Mian SI, Dutta S, Le B, Esquivel CO, Davis K, Castillo RO. Factors affecting survival to intestinal transplantation in the very young pediatric patient. Transplantation. 2008;85(9):1287–1289. doi: 10.1097/TP.0b013e31816dd236. [DOI] [PubMed] [Google Scholar]
- 12.Iyer K, Kaufman S, Sudan D, et al. Long-term results of intestinal transplantation for pseudo-obstruction in children. J Pediatr Surg. 2001;36(1):174–177. doi: 10.1053/jpsu.2001.20046. [DOI] [PubMed] [Google Scholar]
- 13.Sigurdsson L, Reyes J, Kocoshis SA, et al. Intestinal transplantation in children with chronic intestinal pseudo-obstruction. Gut. 1999;45(4):570–574. doi: 10.1136/gut.45.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartosh SM, Ryckman FC, Shaddy R, Michaels MG, Platt JL, Sweet SC. A national conference to determine research priorities in pediatric solid organ transplantation. Pediatr Transplant. 2008;12(2):153–166. doi: 10.1111/j.1399-3046.2007.00811.x. [DOI] [PubMed] [Google Scholar]