Recent breakthroughs in fluorescence microscopy are producing notable improvements in imaging resolution.[ 1 ] These ongoing technical advances should have a major positive impact on the emerging field of cell imaging using single molecule methods, and help merge the sub-disciplines of cell and molecular biology.[2] However, the full potential of many of these new high-resolution imaging methods will only be achieved if they employ extremely bright and highly stable luminescent probes that do not undergo photobleaching or photoblinking.
Luminescent probes can be grouped into two major classes; synthetic systems such as organic dyes, inorganic nanoparticles, and lanthanide coordination complexes,[3] and biological systems such as fluorescent and bioluminescent proteins.[4] It is unlikely that any one luminescent system will emerge as the universal solution for all optical applications and each class warrants further development. This report focuses on fluorescent organic dyes that emit in the near-infrared (NIR) region of 650-900 nm. NIR dyes are particularly attractive for fluorescence imaging of biomedical samples because there is very little undesired absorption and autofluorescence by common biomolecules, and diminished Rayleigh-Tyndall scattering of light.[5] Indeed, wavelengths above 650 nm can penetrate through skin and tissue,[ 6 ] and fluorescent NIR imaging probes are increasingly used for in vivo optical imaging of live animals.[7]At present, the most commonly used fluorescent NIR dyes are sulfonated carbocyanine dyes (Cy-5, Cy-5.5, Cy-7) and their derivatives.[8] Although quite popular, the performance of Cy dyes is limited by molecular properties such as moderate to poor photostability, undesired reactivity with nucleophiles, and a propensity to self-quench upon dye aggregation.[9] Furthermore, cyanine dyes are known to undergo photoblinking[10] and light-driven fluorescence switching,[11] which makes them inherently
 unsuitable as FRET acceptors in single molecule studies.[12] New classes of high-performance NIR fluorophores are under development by various commercial and academic research groups around the world.[13,14] In particular, significant progress has been made with Bodipy,[15] and Rylene[16] dyes that exhibit excellent photostability, however, most of these fluorophores have not been incorporated into molecular probes for cell imaging.
unsuitable as FRET acceptors in single molecule studies.[12] New classes of high-performance NIR fluorophores are under development by various commercial and academic research groups around the world.[13,14] In particular, significant progress has been made with Bodipy,[15] and Rylene[16] dyes that exhibit excellent photostability, however, most of these fluorophores have not been incorporated into molecular probes for cell imaging.
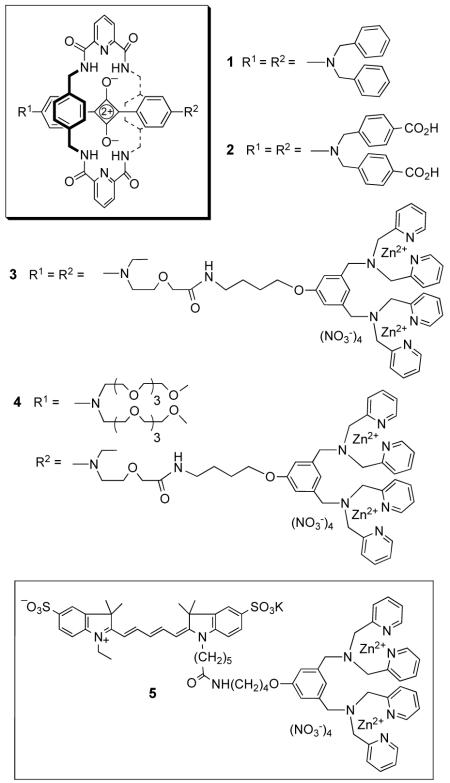
Our interest is in squaraine dyes, which are well-known to have very attractive photophysical properties for biological imaging.[17] However, they suffer from two important limitations; chemical instability due to attack by strong biological nucleophiles and a tendency to form non-fluorescent self-aggregates. We have discovered that both problems can be greatly attenuated by encapsulating the dye as the thread component inside an interlocked rotaxane structure.[18] The synthetic strategy to produce squaraine-rotaxanes has already been described;[19] the key reaction is a templated Leigh-type macrocyclization that connects five building blocks in a single step. X-ray crystal structures show that the surrounding macrocycle sits perfectly over both faces of the electrophilic cyclobutene core of the squaraine thread and blocks nucleophilic attack.[ 20 ] The steric protection provided by the surrounding macrocycle also explains why there is no aggregation-induced broadening of absorption or self-quenching of fluorescence. Even when aggregated, the inner squaraine chromophores are simply unable to get close enough to interact. Furthermore, non-halogenated squaraine-rotaxanes are poor photosensitizers of singlet oxygen and they do not react readily with it;[21] thus, squaraine-rotaxanes are likely to be non-phototoxic and highly resistant to photobleaching. Here, we demonstrate that squaraine-rotaxanes are versatile, high-performance molecular probes for in vitro and in vivo fluorescence imaging of cells. Three different types of probe structures are shown to target different cellular locations, i.e., organelle membrane, internal aqueous phase, and exterior cell surface.
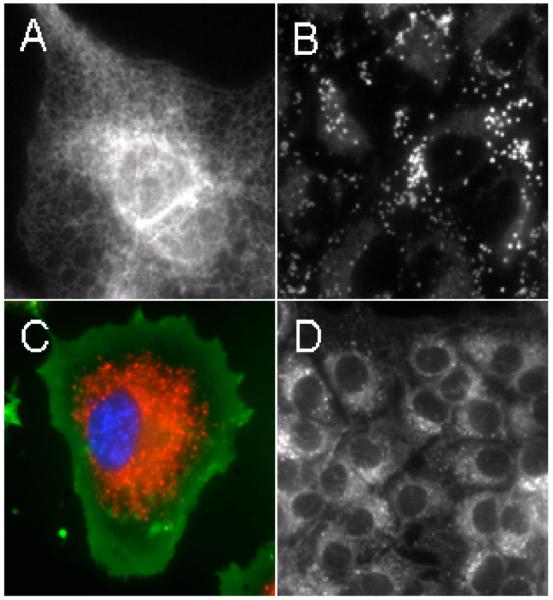
Squaraine-rotaxanes 1-4 have absorption and emission profiles that closely match the Cy-5 chromophore in control compound 5 (Table 1). Thus, all probes can be conveniently imaged using an epifluorescence microscope and a standard Cy-5 filter set (Exciter: HQ620/60X, Dichroic: 660LP, Emitter: HQ700/75m). We find that the relatively non-polar squaraine-rotaxane 1 interacts with cells in a very similar way as the well-known lipophilic stain Nile Red.[22] As shown in Figure 1, probe 1 rapidly accumulates at lipophilic sites inside a living cell such as the endoplasmic reticulum (ER) and intracellular lipid droplets. For example, probe localization with Chinese hamster ovary (CHO) cells is primarily in the ER (panel A), whereas, with human lung carcinoma (A549) cells, uptake is mainly in lipid droplets (panel B). Confirmation of these localization sites was obtained by co-staining the lipid droplets with Nile Red, and the ER with blue-emitting ER Tracker™ Blue-White DPX (Molecular Probes)[23] (see supporting information). The red emission band for probe 1 is quite narrow and permits the acquisition of multicolor images. For example, shown in panel C is a monkey kidney (COS-7) cell that has been treated with the DNA stain H33342 (blue), a lipophilic stain administered to label the plasma membrane, FM1-43 (green), and probe 1 (red). Panel D illustrates the high chemical stability and low toxicity of 1. Even after eight days, the staining pattern in live human breast cancer (MCF7) cells remains the same as obtained on day one, while fluorescence intensity remains strong in all cells; thus, it appears that the dye is transferred efficiently to subsequent daughter cells. Another notable feature with probe 1 is that its staining is unaffected by cell fixation with paraformaldehyde (see supporting information).
Table 1.
Photophysical properties in water
| Compound | λabs nm | λem nm | Φ f[b] |
|---|---|---|---|
| 1 [a] | 639 | 659 | 0.70 |
| 2 [b] | 653 | 675 | 0.25 |
| 3 [c] | 653 | 675 | 0.20 |
| 4 | 650 | 669 | 0.08 |
| 5 | 648, 603 | 671 | 0.25 |
|
| |||
Measurements were carried out in THF.
Excited state lifetime in water was found to be 1.5 ns.
Molar absorbtivity in water was found to be 121,052 M−1L (log ε = 5.1)
Solutions were excited at optically matching wavelengths and emission monitored in the region 600-900 nm. Fluorescence quantum yields were determined using 4,4-[bis-(N,N-dimethylamino)phenyl]squaraine dye as the standard (Φf = 0.7 in CHCl3), error limit ± 5%.
Figure 1.
Fluorescence microscopy images of live mammalian cells treated with 1. Panel A: Endoplasmic reticulum staining of a single CHO cell. Panel B: Lipid droplet staining in A549 cells. Panel C: Multicolor image of a single COS-7 cell treated with H33342 (blue), FM1-43 (green), and 1 (red). Panel D: MCF7 cells 144 hours after a single treatment with 1. Single color images were left in grayscale to preserve image detail and contrast. Each scale bar corresponds to 20 μM.
The tetracarboxylic acid squaraine-rotaxane 2 is soluble in physiological solution and acts as an excellent fluorescent marker of encapsulated aqueous phases inside living cells. For example, treatment of COS-7 cells with 2 leads to accumulation of the dye in small punctate compartments within the cell that are trafficked quite rapidly. The high photostability of 2 allows this fascinating trafficking process to be monitored as a real-time movie, with constant sample irradiation, over many minutes (see supporting information). This type of movie cannot be acquired with currently available NIR fluorescent probes, such as the amphiphilic styryl dye FM 4-64 and water-soluble dextran Alexa-647 conjugate, because they are rapidly photobleached.[16]
Fluorescent probes 3-5 have appended zinc(II)-dipicolylamine (Zn-DPA) coordination complexes as cell targeting ligands. We have recently demonstrated that fluorescent conjugates with attached Zn-DPA groups will selectively associate with the anionic surfaces of bacterial cells, in preference to the zwitterionic membrane surfaces of healthy animal cells.[24] In order to directly compare the targeting ability and photostability of a squaraine-rotaxane with a related Cy-5 probe, we prepared three fluorophore Zn-DPA conjugates; the squaraine-rotaxane 3 with two Zn-DPA targeting ligands, the squaraine-rotaxane 4 with one Zn-DPA targeting ligand, and the Cy-5 probe 5 with one Zn-DPA targeting ligand. As expected, all three probes strongly associate with the periphery of bacterial cells, which allowed photobleaching experiments to be conducted. Three separate samples of bacterial cells (E. coli), each stained with equal amounts of probes 3, 4, or 5 were irradiated continuously with light (filtered to permit λ = 620±30 nm) from an X-cite 120 fluorescence illumination system through a Nikon TE2000-U epifluorescence microscope. The observed photobleaching half-lives were: 1080 seconds for 3, 197 seconds for 4, and 11 seconds for the Cy5 conjugate, 5. Thus, the direct comparison between squaraine-rotaxane 4 and Cy-5 probe 5 suggests that the squaraine-rotaxane is almost twenty times more photostable. The additional five-fold increase in half-life with doubly valent squaraine-rotaxane 3 is attributed to its stronger cell surface affinity, leading to a slower off rate for the probe. In other words, there is diminished signal loss with 3 due to slower probe diffusion away from the cell surface.
The remarkable stability of probe 3 permits fluorescence imaging experiments that are impossible with probes based on NIR cyanine dyes. For example, we have acquired real-time fluorescence microscopy movies of bacteria undergoing cell division. Shown in Figure 2 is a montage of images at various time points from a 30 minute real-time movie that monitors binary fission of E. coli cells stained with 3. The morphological changes associated with binary fission, such as formation of the septum between the daughter cells, are easily resolved. These microscopy images of cell division highlight the extreme photostability of squaraine-rotaxane probe 3 and also its low phototoxicity. Since the NIR fluorescence from probe 3 can penetrate through the skin and tissue of a living animal, it can be used to image bacteria in vivo. To demonstrate this imaging property, separate populations of E. coli and S. aureus bacteria were incubated with 3, washed, and then injected subcutaneously near the posterior thigh muscles of a living nude mouse. A fluorescence image of the entire animal was subsequently acquired using a Kodak 4000MM Fluorescence Imaging Station. As illustrated in Figure 3, both sites of bacterial inoculation are very apparent, due to fluorescence emission intensities that are about 100 times greater than the background signal from other anatomical parts of the mouse.
Figure 2.
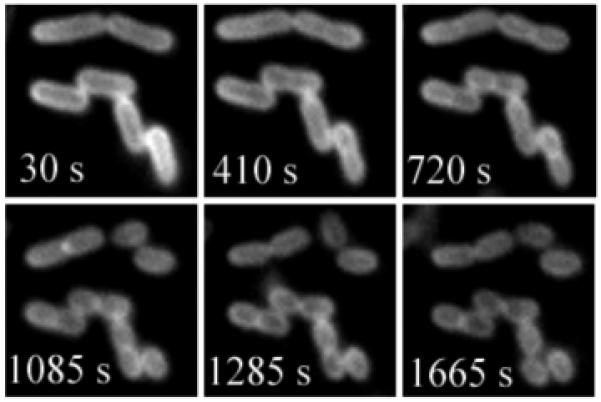
Montage from a larger movie showing binary fission of E. coli cells stained with 3. The cells were imaged, using fluorescence microscopy, every 5 seconds for 30 minutes. The times stated in each panel correspond to the movie time point and the scale bar represents 2 μM.
Figure 3.
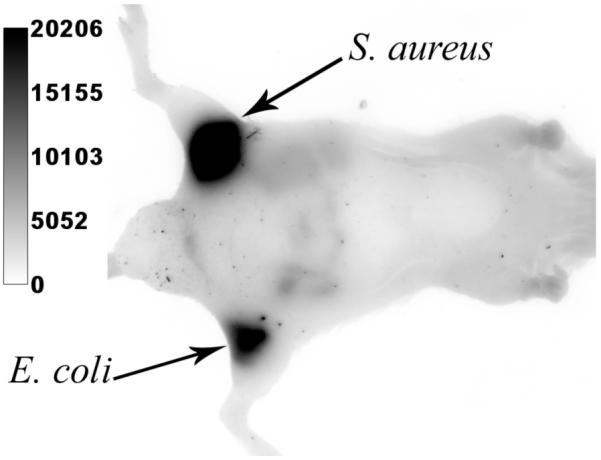
Optical image of a live mouse with subcutaneous injections of S. aureus and E. coli that were pre-labeled with 3. The entire animal was irradiated with filtered light of wavelength 625±40 nm and an image of emission intensity at 670±20 nm was collected by a CCD camera during a 5 s acquisition period.
In summary, squaraine-rotaxane dyes can be readily converted into extremely bright and highly stable NIR fluorescent probes for in vitro and in vivo optical imaging of live and fixed cells. The probes can be structurally modified for targeting to quite different cellular locations. Squaraine-rotaxanes are likely to be superior substitutes for Cy-5 in many biotechnology and imaging applications that require NIR fluorescent dyes.
Supplementary Material
Footnotes
We warmly thank the NIH (GM059078 and P50 CA94056) for their support.
References
- [1].a) Amato I. Chem. Eng. News. 2006 Sept 4;:49. [Google Scholar]; b) Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]; c) Rust MJ, Bates M, Zhuang X. Nat. Methods. 2006;3:793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Willig KI, Rizzoli SO, Westphal V, Jahn R, Hell SW. Nature. 2006;440:935–939. doi: 10.1038/nature04592. [DOI] [PubMed] [Google Scholar]
- [2].a) Jaiswal J, Simon SM. Nat. Chem. Biol. 2007;3:92–98. doi: 10.1038/nchembio855. [DOI] [PubMed] [Google Scholar]; b) Xie XS, Yu J, Yang WY. Science. 2006;312:228–230. doi: 10.1126/science.1127566. [DOI] [PubMed] [Google Scholar]
- [3].a) Sapsford KE, Berti L, Medintz IL. Angew. Chem. Intl. Ed. 2006;45:4562–4588. doi: 10.1002/anie.200503873. [DOI] [PubMed] [Google Scholar]; b) Wang F, Tan WB, Zhang Y, Fan X, Wang M. Nanotechnology. 2006;17:R1–R13. [Google Scholar]
- [4].Giepmans BN, Adams SR, Ellisman MH, Tsien R. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- [5].Licha K. Topics in Curr. Chem. 2002;222:1–29. [Google Scholar]
- [6].Bashkatov AN, Genina EA, Kochubey VI, Tuchin VV. J. Phys. D: Appl. Phys. 2005;38:2543–2555. [Google Scholar]
- [7].a) Gross S, Piwnica-Worms D. Curr. Opin. Chem. Biol. 2006;10:334–342. doi: 10.1016/j.cbpa.2006.06.028. [DOI] [PubMed] [Google Scholar]; b) Keller PJ, Pampaloni F, Stelzer EH. Curr. Opin. Cell Biol. 2006;18:117–143. doi: 10.1016/j.ceb.2005.12.012. [DOI] [PubMed] [Google Scholar]
- [8].a) Mishra A, Behera RK, Behera PK, Mishra BK, Behera GB. Chem. Rev. 2000;100:1973–2011. doi: 10.1021/cr990402t. [DOI] [PubMed] [Google Scholar]; b) Ballou B, Erst LA, Waggoner AS. Curr. Med. Chem. 2005;12:795–805. doi: 10.2174/0929867053507324. [DOI] [PubMed] [Google Scholar]
- [9].a) Soto CM, Blum AS, Vora GJ, Lebedev N, Meador CE, Won AP, Chatterji A, Johnson JE, Ratna BR. J. Am. Chem. Soc. 2006;128:5184–5189. doi: 10.1021/ja058574x. [DOI] [PubMed] [Google Scholar]; b) Gruber HJ, Hahn CD, Kada G, Riener CK, Harms GS, Ahrer W, Dax TG, Knaus H. Bioconj. Chem. 2000;11:696–704. doi: 10.1021/bc000015m. [DOI] [PubMed] [Google Scholar]; c) Song F, Peng X, Lu E, Zhang R, Chen X, Song B. J. Photochem. Photobiol. A. 2004;168:53–57. [Google Scholar]
- [10].Rasnik I, McKinney SA, Taekjip H. Nature Methods. 2006;11:891–893. doi: 10.1038/nmeth934. [DOI] [PubMed] [Google Scholar]
- [11].Sauer M. Proc. Nat. Acad. Sci. 2005;102:9433–9434. doi: 10.1073/pnas.0504264102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].a) Heilemann M, Margeat E, Kasper R, Sauer M, Tinnefeld P. J. Am. Chem. Soc. 2005;127:3801–3806. doi: 10.1021/ja044686x. [DOI] [PubMed] [Google Scholar]; b) Eggeling C, Widengren J, Brand L, Schaffer J, Felekyan S, Seidel CAM. J. Phys. Chem. A. 2006;110:2979–2995. doi: 10.1021/jp054581w. [DOI] [PubMed] [Google Scholar]
- [13].a) Salama G, Choi B–R, Azour G, Lavasani M, Tumbev V, Salzberg BM, Patrick MJ, Ernst LA, Waggoner AS. J. Membr. Biol. 2005;208:125–140. doi: 10.1007/s00232-005-0826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Daltrozzo E, Fischer GM, Ehlers AP, Zumbusch A. Angew. Chem. Int. Ed. 2007;46:3750–3753. doi: 10.1002/anie.200604763. [DOI] [PubMed] [Google Scholar]
- [14].NIR fluorophores that are commercial competitors to Cy-5™ (Amersham Biosciences) include Alexa-647™ (Invitrogen), Dy-647™ (Dyomics), DiLight-649™ (Pierce Biotechnology), HiLyte-647™ (Anaspec), Seta-650 (Setabiomedicals), and Atto-647™ (Sigma-Aldrich). The structures of these dyes are proprietary which makes it difficult to conduct meaningful comparison studies.
- [15].a) Goze C, Urich G, Ziessel R. J. Org. Chem. 2007;72:313–322. doi: 10.1021/jo060984w. [DOI] [PubMed] [Google Scholar]; b) Kim TG, Castro JC, Loudet A, Jiao JG, Hochstrasser RM, Burgess K, Topp MR. J. Phys. Chem. A. 2006;110:20–27. doi: 10.1021/jp053388z. [DOI] [PubMed] [Google Scholar]; c) Zhoa W, Carreira EM. Angew. Chem. Int. Ed. 2005;44:1677–1679. doi: 10.1002/anie.200461868. [DOI] [PubMed] [Google Scholar]; d) Hall MJ, Allen LT, O’Shea DF. Org. Biomol. Chem. 2006;4:776–780. doi: 10.1039/b514788c. [DOI] [PubMed] [Google Scholar]; e) Yu YH, Descalzo AB, Shen Z, Rohr H, Liu Q, Wang YW, Spieles M, Li YZ, Rurack K, You XZ. Chem. Asian J. 2006;1:176–187. doi: 10.1002/asia.200600042. [DOI] [PubMed] [Google Scholar]
- [16].a) Jung C, Muller BK, Lamb DC, Nolde F, Müllen K, Bräuchle C. J. Am. Chem. Soc. 2006;128:5283–5291. doi: 10.1021/ja0588104. [DOI] [PubMed] [Google Scholar]; b) Langhals H, El-Shishtawy R, von Unold P, Rauscher M. Chem. Eur. J. 2006;12:4642–4645. doi: 10.1002/chem.200501439. [DOI] [PubMed] [Google Scholar]
- [17].a) Oswald B, Patsenker L, Duschl J, Szmacinski H, Wolfbeis OS, Terpetschnig E. Bioconj. Chem. 1999;10:925–931. doi: 10.1021/bc9801023. [DOI] [PubMed] [Google Scholar]; b) Tatarets AL, Fedyunyayeva IA, Dybko TS, Povrozin YA, Doroshenko AO, Terpetschnig EA, Patsenker LD. Anal. Chim. Acta. 2006;570:214–223. doi: 10.1016/j.aca.2006.04.019. [DOI] [PubMed] [Google Scholar]
- [18].Arunkumar E, Forbes CC, Smith BD. Eur. J. Org. Chem. 2005:4051–4059. [Google Scholar]
- [19].Arunkumar E, Forbes CC, Noll BC, Smith BD. J. Am. Chem. Soc. 2005;127:3288–3289. doi: 10.1021/ja042404n. [DOI] [PubMed] [Google Scholar]
- [20].Arunkumar E, Fu N, Smith BD. Chem.-Eur. J. 2006;12:4684–4690. doi: 10.1002/chem.200501541. [DOI] [PubMed] [Google Scholar]
- [21].a) Kamat PV, Das S, Thomas KG, George MV. J. Phys. Chem. 1992;96:195–199. [Google Scholar]; b) Ramaiah D, Eckert I, Arun KT, Weidenfeller L, Epe B. Photochem. Photobiol. 2002;76:672–677. doi: 10.1562/0031-8655(2002)076<0672:sdfpts>2.0.co;2. [DOI] [PubMed] [Google Scholar]; c) Arunkumar E, Sudeep PK, Kamat PV, Noll BC, Smith BD. New J. Chem. 2007;31:677–683. doi: 10.1039/b616224j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Greenspan P, Mayer EP, Fowler SD. J. Cell. Biol. 1985;100:965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cole L, Davies D, Hyde GJ, Ashford AE. J. Microsc. 2000;197:239–249. doi: 10.1046/j.1365-2818.2000.00664.x. [DOI] [PubMed] [Google Scholar]
- [24].a) Leevy WM, Johnson JR, Lakshmi C, Morris J, Marquez M, Smith BD. Chem. Commun. 2006;15:1595–1597. doi: 10.1039/b517519d. [DOI] [PubMed] [Google Scholar]; b) Leevy WM, Gammon ST, Jiang H, Johnson JR, Maxwell DJ, Jackson EN, Marquez M, Piwnica-Worms D, Smith BD. J. Am. Chem. Soc. 2006;128:16476–16477. doi: 10.1021/ja0665592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.