Abstract
BACKGROUND
Adenomyosis is rarely diagnosed before hysterectomy and commonly coexists with uterine leiomyomas. The objective of this study was to identify distinct features of a concurrent diagnosis of adenomyosis in women with uterine leiomyomas.
METHODS
We conducted a case–control study of women undergoing hysterectomy with a histologic diagnosis of both adenomyosis and leiomyomas and women with uterine leiomyomas but no adenomyosis. A retrospective medical record review of hospital and ambulatory records was performed to ascertain sociodemographic and anthropometric variables, as well as to confirm intraoperative and pathologic findings.
RESULTS
Our study sample comprised 255 patients, 85 women with adenomyosis and leiomyomas and 170 women with only leiomyomas. In multivariable logistic regression analyses, women with adenomyosis and leiomyomas were more likely to have more pelvic pain [odds ratio (OR) 3.4, 95% confidence interval (CI) 1.8–6.4], have less fibroid burden (OR per doubling in fibroid size 0.6, 95% CI 0.5–0.8), were more likely to be parous (OR 3.8, 95% CI 1.4–10.5) and have lower body mass index (OR per 5 unit increase in BMI 0.8, 95% CI 0.6–1.0) when compared with women with leiomyomas alone.
CONCLUSIONS
Women undergoing hysterectomy with both adenomyosis and leiomyomas have a number of different clinical features compared with women with only leiomyomas at the time of hysterectomy. Women with substantial pain despite a smaller fibroid burden may be more likely to have concomitant adenomyosis.
Keywords: adenomyosis, uterine leiomyomas, hysterectomy, epidemiology, pelvic pain
Introduction
Uterine leiomyomas (fibroids or myomas) are benign myometrial neoplasms and represent the primary indication for hysterectomy in the USA (Walker and Stewart, 2005). Adenomyosis is a myometrial lesion characterized by the presence of ectopic endometrium with or without hyperplasia of the surrounding myometrium. Furthermore, both adenomyosis and leiomyomas commonly coexist; concomitant adenomyosis in hysterectomy specimens of women with leiomyomas ranges from 15 to 57% (Shaikh and Khan, 1990; Vercellini et al., 1995; Parazzini et al., 1997; Vavilis et al., 1997; Bergholt et al., 2001; Weiss et al., 2009). Risk factors for adenomyosis are age, multiparity, surgical disruptions of the endometrial–myometrial border, elevated levels of both FSH and prolactin (PRL), smoking habits and history of depression (Parazzini et al., 1997, 2009; Taran et al., 2009).
Leiomyomas are reported to cause a variety of symptoms including heavy menses, painful menses, pelvic pressure and bowel and urinary tract complaints (Stewart, 2001). Similarly, symptoms of adenomyosis are commonly reported as abnormal uterine bleeding, chronic pelvic pain and painful menses (Bergholt et al., 2001; Weiss et al., 2009). However, since both conditions frequently coexist in the same uterus, attributing symptoms to either condition can be problematic. Moreover, adenomyosis is typically diagnosed only at the time of hysterectomy and so contribution this disease to the symptoms is only understood retrospectively (Weiss et al., 2009).
Alternatives to hysterectomy, including uterine artery embolization (UAE) and magnetic resonance-guided focused ultrasound (MRgFUS), are reported as safe and effective minimally invasive procedures for symptomatic uterine leiomyomas (Spies et al., 2005; Gupta et al., 2006; Stewart et al., 2006; Edwards et al., 2007; Rabinovici et al., 2007; Goodwin et al., 2008). When concomitant adenomyosis is present the risk of treatment failure seems to be increased for both methods (Goodwin et al., 1999; Doyle et al., 2007).
The design of the current study aims to compare women undergoing hysterectomy with a pathological finding of both leiomyomas and adenomyosis to women with leiomyomas alone using a multivariable model. Identifying adenomyosis in women with leiomyomas will allow improved clinical decision-making regarding alternatives to hysterectomy and, likely, a decreased risk of treatment failure.
Materials and Methods
This retrospective matched case–control study was conducted at the Mayo Clinic, Rochester, MN, USA, and approved by the appropriate institutional review board (IRB). All study procedures are in accordance with ethical standards set forth in the revised Declaration of Helsinki.
The study group comprised women determined to have both adenomyosis and uterine leiomyomas following hysterectomy; the control group comprised women with a histologic diagnosis of leiomyomas but no adenomyosis. At Mayo Clinic, all surgical specimens are examined at the time of surgery so that the examining pathologist has the opportunity to examine the whole uterus not just representative slides.
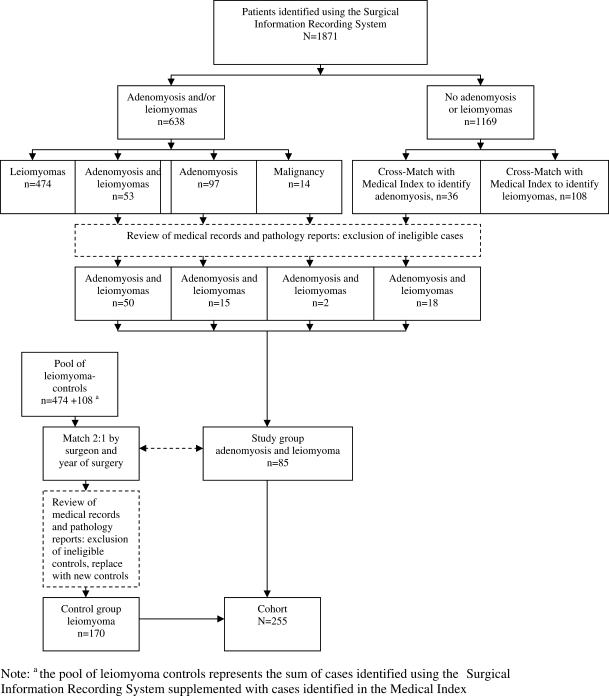
Using the Surgical Information Recording System (SIRS), a Mayo Clinic institutional database, we identified all women who underwent hysterectomy, either with a single procedure or with pelvic floor reconstruction procedures, between 1 January 2000 and 31 December 2007 at Rochester Methodist Hospital, Rochester, MN, USA (Fig. 1). Inclusion criteria for the study were residency in Olmsted County, MN, USA, authorization of use of medical records for research, premenopausal status and age <55 years at the time of surgery. Premenopausal status was defined as occurrence of at least one menstrual period within 12 months before surgery. Presence of gynecologic cancers on pathologic examination was an exclusion criterion. The 1169 records where neither disease was noted in SIRS were then searched, using the keyword ‘adenomyosis’ from the Medical Index, another Mayo Clinic institutional database, to identify misclassified records (Fig. 1). There were 200 women identified with a reported diagnosis of either adenomyosis or adenomyosis and leiomyomas, including 36 cases of pathology-confirmed adenomyosis and leiomyomas from the Medical Index. The diagnoses reported in SIRS and Medical Index were manually compared with the pathology note and only pathologically confirmed cases were analyzed, so that the study group comprised 85 patients (Fig. 1).
Figure 1.
Ascertainment of cases and controls.
The 582 patients with a diagnosis of only leiomyomas served as a pool of potential control subjects. Controls were matched in a 2:1 ratio to cases from the study group on the basis of the surgeon and surgical date (±1 year) using an optimal matching algorithm applied to the values of the matching factors. Matching on the basis of surgeon was done to eliminate confounders of referral patterns and bias of concomitant procedures based on practice style. Records were reviewed to confirm correct coding, and new controls were selected to replace ineligible controls. The control group thus comprised a total of 170 women (Fig. 1).
We were able to utilize the integrated electronic medical records system to perform a medical record review of both hospital (Rochester Methodist Hospital, Rochester, MN, USA) and complete ambulatory records (Mayo Clinic, Rochester, MN, USA) to ascertain sociodemographic and anthropometric variables, as well as intraoperative and pathologic findings. A history of endometriosis was recorded on the basis of pathological diagnosis. We considered heavy menses, metrorrhagia, painful menses, dyspareunia, pelvic pain (acyclic recurrent pain not linked to sexual intercourse) and pelvic pressure to be disease-specific symptoms for both adenomyosis and leiomyomas; if all symptoms were absent, the patient was considered to have no disease-specific symptoms.
For women with disease-specific symptoms (78 of 85, 91.8% of women in the adenomyosis and leiomyoma group and 157 of 169, 92.9% of women in the leiomyoma group), the indication of hysterectomy was either the preoperative diagnosis of adenomyosis or leiomyomas or the presence of one or more disease-specific symptoms. The remaining hysterectomies were performed for indications of uterine prolapse, cervical intraepithelial neoplasia, endometriosis or ovarian cancer prophylaxis.
Data were coded and entered into an Excel database (Microsoft, Redmond, Washington, DC, USA). Statistical analysis was carried out using JMP for Windows, 7.0.1 (SAS Institute, Cary, NC, USA). We report means and standard deviations or medians for continuous variables and frequency counts and percentages for nominal or categorical variables. To assess differences between groups of women, Pearson's χ2 or Fisher's exact test, as appropriate, were performed for nominal or categorical variables. Two-sample t-test and Wilcoxon rank sum tests were performed for normally and non-normally distributed continuous variables, respectively.
A multivariable stepwise logistic regression analysis was performed. Variables identified with a P-value <0.05 based on univariate analyses were considered for entry in the model and variables with a P-value <0.05 were retained in the final model. The c-index, equal to the area under a receiver operating characteristic curve, was used to summarize the overall predictive ability of the final model (Hanley and McNeil, 1982). Unadjusted and adjusted odds ratios (OR) were calculated with a 95% confidence interval (CI). All tests were two-tailed and P < 0.05 was considered statistically significant in all statistical analyses.
Results
Our study sample comprised 255 patients, 85 women with adenomyosis and leiomyomas and 170 women with leiomyomas. Surgeries in both groups were performed by nine surgeons, performing between 3 and 81 hysterectomies per surgeon over the study period (data not shown). The study population consisted mainly of Caucasian women, representing 94.1% of the cohort.
Characteristics of the study cohort are presented in Table I. Our study population was fairly typical for women undergoing hysterectomy for benign disease, with most women being in their fifth decade of life, multiparous and having an increased body mass index (BMI). Nevertheless, women with adenomyosis and leiomyomas had a significantly lower mean BMI (28.0 ± 6.0 versus 30.0 ± 6.8, P = 0.023) compared with women with leiomyomas (Table I).
Table I.
Characteristics of the cohort (n = 255).
| Adenomyosis and leiomyomas (n = 85) |
Leiomyomas alone (n = 170) |
P | |||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | ||
| Age (years) | 45.1 ± 4.4 | 45.0 | 44.5 ± 4.8 | 45.0 | NSa |
| BMI (kg/m2) | 28.0 ± 6.0 | 26.6 | 30.0 ± 6.8 | 28.9 | 0.023a |
| Menstrual bleeding (days) | 7.6 ± 3.4 | 7.0 | 7.7 ± 3.8 | 7.0 | NSb |
| Menstrual cycle length | 26.2 ± 4.8 | 28.0 | 27.6 ± 5.4 | 28.0 | 0.005b |
| Endometrial thickness (mm) | 9.2 ± 7.1 | 7.5 | 7.5 ± 3.8 | 7.0 | NSb |
| Preoperative hematocrit | 36.4 ± 5.4 | 37.4 | 37.0 ± 3.8 | 37.5 | NSb |
| Operative time (min) | 93.4 ± 38.0 | 92.0 | 99.0 ± 40.7 | 91.0 | NSb |
| Number of leiomyomas | 4.4 ± 5.4 | 2.0 | 6.6 ± 8.0 | 4.0 | 0.003b |
| Diameter of the largest leiomyoma (cm) | 2.4 ± 2.5 | 1.5 | 4.1 ± 3.8 | 3.0 | <0.001b |
| Uterine weight (g) | 228.5 ± 294.1 | 165.0 | 308.6 ± 28.6 | 192.5 | 0.006b |
SD, standard deviation; BMI, body mass index.
aTwo-sample t-test.
bWilcoxon sum rank test.
Both groups had enlarged uteri; however, women with concomitant adenomyosis had a lower median uterine weight (165.0 versus 192.5 g, P = 0.006), fewer leiomyomas (median, 2 versus 4, P = 0.003) and smaller leiomyomas (median, 1.5 versus 3.0 cm, P < 0.001) compared with women with only leiomyomas. Duration of menstrual bleeding, endometrial thickness assessed by vaginal ultrasound, preoperative hematocrit and operative time did not differ between groups (Table I).
Women with concomitant adenomyosis were significantly more likely to report various types of pain compared with women with leiomyomas. More than half of the women with concomitant adenomyosis reported pain with menses compared with approximately one-third of the women with leiomyomas alone (P = 0.017, OR 1.9, 95% CI 1.1–3.2; Table II). Women with adenomyosis and leiomyomas also had an increased risk of pain with intercourse (P = 0.019, OR 3.0, 95% CI 1.2–7.7) and non-cyclic pelvic pain (P<0.001, OR 2.8, 95% CI 1.6–4.9). Furthermore, women with adenomyosis and leiomyomas had a decreased risk of pelvic pressure (P = 0.017, OR 0.2, 95% CI 0.1–0.8) compared with women with only leiomyomas. No difference was observed between the patient groups in the occurrence of any aspect of abnormal uterine bleeding (Table II). We observed no differences between the two groups in medication history and history of previous surgical interventions. Furthermore, there was no difference regarding history of endometriosis or endometriosis found at the time of hysterectomy between the two groups (data not shown).
Table II.
Clinical symptoms and reproductive characteristics of the cohort.
| Variable | Adenomyosis and leiomyoma(s) (n = 85) | Leiomyoma(s) alone (n = 170)a | P value | OR (95%CI) |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Painful menses | 45 (53.0) | 63 (37.3) | 0.017b | 1.9 (1.1–3.2) |
| Dyspareunia | 11 (12.4) | 8 (4.7) | 0.019b | 3.0 (1.2–7.7) |
| Pelvic pain | 38 (44.7) | 38 (22.5) | <0.001b | 2.8 (1.6–4.9) |
| Heavy menses | 43 (50.6) | 86 (50.9) | NSb | 1.0 (0.6–1.7) |
| Menometrorrhagia | 27 (31.8) | 54 (32.0) | NSb | 1.0 (0.6–1.7) |
| Pelvic pressure | 3 (3.5) | 22 (13.0) | 0.017b | 0.2 (0.1–0.8) |
| Disease-specific symptoms | 78 (91.8) | 157 (92.9) | NSb | 0.9 (0.3–2.2) |
| Parity >0 | 78 (91.8) | 133 (78.2) | 0.007b | 3.1 (1.3–7.3) |
| Parity >1 | 65 (76.5) | 109 (64.1) | 0.046b | 1.8 (1.01–3.3) |
| Term delivery >0 | 76 (89.4) | 133 (78.2) | 0.029b | 2.3 (1.1–5.1) |
| Term delivery >1 | 64 (75.3) | 109 (64.1) | NSb | 1.7 (1.0–3.1) |
| History preterm delivery | 3 (3.5) | 0 (0.0) | 0.036c | 14.5 (0.7–283.3) |
| History spontaneous miscarriage | 24 (28.2) | 31 (18.2) | NSb | 1.8 (1.0–3.3) |
| History therapeutic abortion | 1 (1.2) | 4 (2.4) | NSc | 0.5 (0.1–4.5) |
| History Cesarean section | 13 (15.3) | 30 (17.6) | NSb | 0.8 (0.4–1.7) |
aOne patient in the leiomyoma(s) alone group lacked documented information on symptoms; OR, odds ratio, odds of adenomyosis and leiomyoma(s) group relative to the odds of leiomyoma(s) alone group; CI, confidence interval.
bPearson's χ2 test.
cFisher's exact test; the sum of numbers for each variable exceeds the total number of patients because some patients had multiple conditions that apply.
Reproductive characteristics of the cohort are also presented in Table II. Interestingly, women with both diseases were more likely to have at least one delivery (P = 0.007, OR 3.1, 95% CI 1.3–7.3) or more than one delivery (P = 0.046, OR 1.8, 95% CI 1.01–3.3) compared with women with leiomyomas. Furthermore, women with a concurrent diagnosis of adenomyosis were significantly more likely to have at least one term delivery compared with women with only leiomyomas (89.4 versus 78.2%, P = 0.029). A history of preterm delivery was noted only among women with adenomyosis and leiomyomas [3.5 (3 of 85) versus 0.0% (none in 170), P = 0.036; Table II).
In order to eliminate the possibility of adenomyosis being an incidental pathologic finding in women with leiomyomas, we performed an unconditional multivariable logistic regression analysis to identify the set of independent variables that best discriminated between the two groups of patients. The final multivariable model included the variables of parity, pelvic pain, size of leiomyomas and BMI (Table III). Parous women with leiomyomas were almost four times more likely to have a concomitant diagnosis of adenomyosis compared with women undergoing hysterectomy with only leiomyomas identified (OR 3.8, 95% CI 1.4–10.5). Women who had both adenomyosis and leiomyoma were more likely to have pelvic pain than women with leiomyoma alone (OR 3.4, CI 95% 1.8–6.4). Increasing measures for fibroid burden and BMI were both protective (OR per doubling in fibroid size 0.6, 95% CI 0.5–0.8; OR per 5 unit increase in BMI 0.8, 95% CI 0.6–1.0). The overall predictive ability of the variables included in this model was 0.75, as determined by the c-index.
Table III.
Summary of factors identified based on stepwise multivariable logistic regression analyses that are associated with having adenomyosis and leiomyomas.
| Variable | Pvalue | ORa (95% CI) |
|---|---|---|
| Diameter largest leiomyoma (>2 cm, log2) | <0.001 | 0.61 (0.48–0.77)b |
| Pelvic pain | <0.001 | 3.37 (1.79–6.35) |
| Parity >0 | 0.012 | 3.76 (1.35–10.53) |
| BMI | 0.039 | 0.78 (0.61–0.99)b |
aOR, adjusted odds ratio, odds of adenomyosis and leiomyoma(s) group relative to the odds of leiomyoma(s) alone group; CI, confidence interval.
bOdds per a doubling in diameter and 5 unit increase in BMI.
Discussion
The current study suggests a number of features that distinguish women with adenomyosis and leiomyomas from women with only leiomyomas at the time of hysterectomy. The finding that women with adenomyosis and leiomyomas undergoing hysterectomy have fewer and smaller leiomyomas suggests that adenomyosis is contributing to symptomatology which leads to hysterectomy. Consequently, in women with symptoms that seem disproportionate to the level of leiomyoma disease, clinicians may consider the presence of adenomyosis in the differential diagnosis.
In concordance with previous clinical evidence, we found that a high percentage of women with concurrent adenomyosis were multiparous (Parazzini et al., 1997; Levgur et al., 2000; Bergholt et al., 2001; Templeman et al., 2008). Since the control group was also undergoing hysterectomy, this suggests a relationship with the disease process rather than just an increased acceptance of hysterectomy in parous women. Furthermore, women with adenomyosis and leiomyomas had a decreased BMI, smaller leiomyomas and decreased uterine weight on pathologic examination compared with women with leiomyomas. These findings can be explained when our data are analyzed along with previous studies on adenomyosis or leiomyomas.
First, pregnancy might facilitate formation of adenomyosis by allowing adenomyotic foci to be included in the myometrium due to the invasive nature of the trophoblast on the extension of myometrial fibers. Second, the possibility of Cesarean section may lead to iatrogenic adenomyosis (Levgur et al., 2000; Panganamamula et al., 2004). However, we observed no difference regarding rates of Cesarean section or any other surgical procedure between women with adenomyosis and leiomyomas and women with only leiomyomas in this study. Third, the hormonal milieu of pregnancy may favor the development of islands of ectopic endometrium (Vercellini et al., 2006).
Finally, epidemiologic evidence indicates a decreased risk of leiomyomata for parous women compared with nulliparous women due to hormonal and non-hormonal mechanisms (Marshall et al., 1998; Walker et al., 2001; Parazzini, 2006). However, one study showed that excess body weight appears to weaken this protective effect (Wise et al., 2004). The effects are hypothesized to occur as results of a decrease in menstrual cycling, changes in levels of ovarian hormones and growth factors, a reduction in estrogen receptor levels in myometrial tissue and uterine remodeling clearing nascent fibroids following pregnancies (Walker et al., 2001; Baird and Dunson, 2003; Wise et al., 2004).
It has been suggested that pelvic pain not associated with menstruation is a rare presenting symptom in women with leiomyomas and should prompt a search for other diseases (Stewart and Strauss, 2004). Nevertheless, UAE studies reported pelvic pain in up to 20% of the participants (Pron et al., 2003; Edwards et al., 2007). Furthermore, a previous population-based study showed that pelvic pain and dyspareunia increased in severity in women with leiomyomas compared with women without leiomyomas; however, since the leiomyomas were diagnosed by transvaginal ultrasound, the presence of concurrent adenomyosis or other conditions could not be entirely ruled out (Lippman et al., 2003). Magnetic resonance imaging (MRI) seems to be a highly accurate tool in the preoperative diagnosis of adenomyosis; however, the combination of transvaginal ultrasound and MRI offers the highest sensitivity for preoperative diagnosis of adenomyosis (Kunz et al., 2005; Dueholm and Lundorf, 2007).
Consistent with these reports, we found, in the group of women with only leiomyomas, approximately one-fifth of women reporting non-cyclical pain. The proportion is, however, doubled in the group with adenomyosis and leiomyomas and the difference is significant in the multivariable model. These findings suggest that chronic pain is present in some women with leiomyomas but increased in women with both diseases.
The diagnosis of adenomyosis is related to the pathologist's awareness of the condition and the number and site of analyzed myometrial samples as well as the used histological criteria (Parazzini et al., 2009). Thus, the diagnosis of focal adenomyotic nodules can, in some cases, be missed. Furthermore, it has been demonstrated previously that frequency and severity of adenomyosis-associated symptoms are directly related to the degree of myometrial penetration. A major limitation of this study is therefore the absence of a standard protocol to identify adenomyosis.
Another limitation of this study was its retrospective design which precluded objective measures of symptom severity, although the fact that full ambulatory records were reviewed augments the assessment of symptoms. Furthermore, racial diversity is underrepresented in our study. Overall, there are little data on the epidemiology of adenomyosis and leiomyomas in different ethnic groups (Thomas and Clark, 1989). However, black women have been shown to have an increased incidence and prevalence of leiomyomas and to have more severe disease at the time of hysterectomy (Marshall et al., 1997; Wise et al., 2004; Peddada et al., 2008). While the characteristics of our study population are similar to those of Caucasian women in midwestern USA, validation of the model in prospective studies with larger cohorts, using standardized protocols to identify adenomyosis and to assess high-risk populations, would be important.
Funding
This work was supported by grant number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
References
- Baird DD, Dunson DB. Why is parity protective for uterine fibroids? Epidemiology. 2003;14:247–250. doi: 10.1097/01.EDE.0000054360.61254.27. [DOI] [PubMed] [Google Scholar]
- Bergholt T, Eriksen L, Berendt N, Jacobsen M, Hertz JB. Prevalence and risk factors of adenomyosis at hysterectomy. Hum Reprod. 2001;16:2418–2421. doi: 10.1093/humrep/16.11.2418. [DOI] [PubMed] [Google Scholar]
- Doyle JO, Betjes H, Missmer SA, Fennessy FM, Tempany CMC, Stewart EA. MRI-guided focused ultrasound ablation (MRgFUS) of uterine fibroids: clinical predictors of successful outcome at three years. Abstract. Fertil Steril. 2007;88:S82. [Google Scholar]
- Dueholm M, Lundorf E. Transvaginal ultrasound or MRI for diagnosis of adenomyosis. Curr Opin Obstet Gynecol. 2007;19:505–512. doi: 10.1097/GCO.0b013e3282f1bf00. [DOI] [PubMed] [Google Scholar]
- Edwards RD, Moss JG, Lumsden MA, Wu O, Murray LS, Twaddle S, Murray GD. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360–370. doi: 10.1056/NEJMoa062003. [DOI] [PubMed] [Google Scholar]
- Goodwin SC, McLucas B, Lee M, Chen G, Perrella R, Vedantham S, Muir S, Lai A, Sayre JW, DeLeon M. Uterine artery embolization for the treatment of uterine leiomyomata midterm results. J Vasc Interv Radiol. 1999;10:1159–1165. doi: 10.1016/s1051-0443(99)70213-7. [DOI] [PubMed] [Google Scholar]
- Goodwin SC, Spies JB, Worthington-Kirsch R, Peterson E, Pron G, Li S, Myers ER. Uterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID Registry. Obstet Gynecol. 2008;111:22–33. doi: 10.1097/01.AOG.0000296526.71749.c9. [DOI] [PubMed] [Google Scholar]
- Gupta J, Sinha A, Lumsden M, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2006;1:CD005073. doi: 10.1002/14651858.CD005073.pub2. [DOI] [PubMed] [Google Scholar]
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- Kunz G, Beil D, Huppert P, Noe M, Kissler S, Leyendecker G. Adenomyosis in endometriosis–prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum Reprod. 2005;20:2309–2316. doi: 10.1093/humrep/dei021. [DOI] [PubMed] [Google Scholar]
- Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688–691. doi: 10.1016/s0029-7844(99)00659-6. [DOI] [PubMed] [Google Scholar]
- Lippman SA, Warner M, Samuels S, Olive D, Vercellini P, Eskenazi B. Uterine fibroids and gynecologic pain symptoms in a population-based study. Fertil Steril. 2003;80:1488–1494. doi: 10.1016/s0015-0282(03)02207-6. [DOI] [PubMed] [Google Scholar]
- Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, Willet WC, Hunter DJ. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- Marshall LM, Spiegelman D, Goldman MB, Manson JE, Colditz GA, Barbieri RL, Stampfer MJ, Hunter DJ. A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril. 1998;70:432–439. doi: 10.1016/s0015-0282(98)00208-8. [DOI] [PubMed] [Google Scholar]
- Panganamamula UR, Harmanli OH, Isik-Akbay EF, Grotegut CA, Dandolu V, Gaughan JP. Is prior uterine surgery a risk factor for adenomyosis? Obstet Gynecol. 2004;104:1034–1038. doi: 10.1097/01.AOG.0000143264.59822.73. [DOI] [PubMed] [Google Scholar]
- Parazzini F. Risk factors for clinically diagnosed uterine fibroids in women around menopause. Maturitas. 2006;55:174–179. doi: 10.1016/j.maturitas.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Panazza S, Chatenoud L, Oldani S, Crosignani PG. Risk factors for adenomyosis. Hum Reprod. 1997;12:1275–1279. doi: 10.1093/humrep/12.6.1275. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Mais V, Cipriani S, Busacca M, Venturini P. GISE. Determinants of adenomyosis in women who underwent hysterectomy for benign gynecological conditions: results from a prospective multicentric study in Italy. Eur J Obstet Gynecol Reprod Biol. 2009;143:103–106. doi: 10.1016/j.ejogrb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, Semelka RC, Kowalik A, Armao D, Davis B, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:19887–19892. doi: 10.1073/pnas.0808188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pron G, Cohen M, Soucie J, Garvin G, Vanderburgh L, Bell S. The Ontario Uterine Fibroid Embolization Trial. Part 1. Baseline patient characteristics, fibroid burden, and impact on life. Fertil Steril. 2003;79:112–119. doi: 10.1016/s0015-0282(02)04539-9. [DOI] [PubMed] [Google Scholar]
- Rabinovici J, Inbar Y, Revel A, Zalel Y, Gomori JM, Itzchak Y, Schiff E, Yagel S. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771–777. doi: 10.1002/uog.4099. [DOI] [PubMed] [Google Scholar]
- Shaikh H, Khan KS. Adenomyosis in Pakistani women: four year experience at the Aga Khan University Medical Centre, Karachi. J Clin Pathol. 1990;43:817–819. doi: 10.1136/jcp.43.10.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies JB, Bruno J, Czeyda-Pommersheim F, Magee ST, Ascher SA, Jha RC. Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol. 2005;106:933–939. doi: 10.1097/01.AOG.0000182582.64088.84. [DOI] [PubMed] [Google Scholar]
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- Stewart EA, Strauss JF. Disorders of the uterus: leiomyomas, adenomyosis, endometrial polyps, abnormal uterine bleeding, intrauterine adhesions and painful menses. In: Barbieri RL, Strauss JF, editors. Yen and Jaffe's Reproductive Endocrinology. 5th edn. Philadelphia: Elsevier; 2004. pp. 713–734. [Google Scholar]
- Stewart EA, Rabinovici J, Tempany CM, Inbar Y, Regan L, Gastout B, Hesley G, Kim HS, Hengst S, Gedroyc WM. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006;85:22–29. doi: 10.1016/j.fertnstert.2005.04.072. [DOI] [PubMed] [Google Scholar]
- Taran FA, Weaver AL, Coddington CC, Stewart EA. Understanding adenomyosis: a case–control study. Fertil Steril. 2009 doi: 10.1016/j.fertnstert.2009.06.049. Jul 29 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeman C, Marshall SF, Ursin G, Horn-Ross PL, Clarke CA, Allen M, Deapen D, Ziogas A, Reynolds P, Cress R, et al. Adenomyosis and endometriosis in the California Teachers Study. Fertil Steril. 2008;90:415–424. doi: 10.1016/j.fertnstert.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JS, Jr, Clark JF. Adenomyosis: a retrospective view. J Natl Med Assoc. 1989;81:969–972. [PMC free article] [PubMed] [Google Scholar]
- Vavilis D, Agorastos T, Tzafetas J, Loufopoulos A, Vakiani M, Constantinidis T, Patsiaoura K, Bontis J. Adenomyosis at hysterectomy: prevalence and relationship to operative findings and reproductive and menstrual factors. Clin Exp Obstet Gynecol. 1997;24:36–38. [PubMed] [Google Scholar]
- Vercellini P, Parazzini F, Oldani S, Panazza S, Bramante T, Crosignani PG. Adenomyosis at hysterectomy: a study on frequency distribution and patient characteristics. Hum Reprod. 1995;10:1160–1162. doi: 10.1093/oxfordjournals.humrep.a136111. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Vigano P, Somigliana E, Daguati R, Abbiati A, Fedele L. Adenomyosis: epidemiological factors. Best Pract Res Clin Obstet Gynaecol. 2006;20:465–477. doi: 10.1016/j.bpobgyn.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- Walker CL, Cesen-Cummings K, Houle C, Baird D, Barrett JC, Davis B. Protective effect of pregnancy for development of uterine leiomyoma. Carcinogenesis. 2001;22:2049–2052. doi: 10.1093/carcin/22.12.2049. [DOI] [PubMed] [Google Scholar]
- Wise LA, Palmer JR, Harlow BL, Spiegelman D, Stewart EA, Adams-Campbell LL, Rosenberg L. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol. 2004;159:113–123. doi: 10.1093/aje/kwh016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Maseelall P, Schott LL, Brockwell SE, Schocken M, Johnston JM. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women's Health Across the Nation (SWAN) Fertil Steril. 2009;91:201–206. doi: 10.1016/j.fertnstert.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]