Abstract
BACKGROUND
Both human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) bear a great potential in regenerative medicine. In addition to optimized clinical grade culture conditions, efficient clinical grade cryopreservation methods for these cells are needed. Obtaining good survival after thawing has been problematic.
METHODS
We used a novel, chemically defined effective xeno-free cryopreservation system for cryostorage and banking of hESCs and iPSCs. The earlier established slow freezing protocols have, even after recent improvements, resulted in low viability and thawed cells had a high tendency to differentiate. The medium is a completely serum and animal substance free product containing dimethylsulfoxide, anhydrous dextrose and a polymer as cryoprotectants. The cells were directly frozen at −70°C, without a programmed freezer.
RESULTS
The number of frozen colonies versus the number of surviving colonies differed significantly for both HS293 (χ2 = 9.616 with one degree of freedom and two-tailed P = 0.0019) and HS306 (χ2 = 8.801 with one degree of freedom and two-tailed P = 0.0030). After thawing, the cells had a high viability (90–96%) without any impact on proliferation and differentiation, compared with the standard freezing procedure where viability was much lower (49%). The frozen–thawed hESCs and iPSCs had normal karyotype and maintained properties of pluripotent cells with corresponding morphological characteristics, and expressed pluripotency markers after 10 passages in culture. They formed teratomas containing tissue components of the three germ layers.
CONCLUSION
The defined freezing–thawing system described here offers an excellent simple option for banking of hESCs and iPSCs.
Keywords: human embryonic stem cells, defined, cryopreservation, survival, differentiation
Introduction
Human embryonic stem cells (hESCs) have generated much interest due to their great potential for cell therapy and regenerative medicine (Thomson et al., 1998; Trounson and Pera 2001; Klimanskaya et al., 2008). In order to fully exploit the remarkable potential of hESC, optimal cryopreservation methods of the pluripotent cells for transfer between research centers, promotion of scientific collaborations and facilitation of widespread usage of these cells has been lacking (Gearhart, 1998; Pera and Trounson, 2004). The sensitivity of hESCs to cryopreservation, in spite of clear improvements recently, has severely limited their utility (Martin-Ibanez et al., 2008). As hESCs are highly sensitive to cryo-injury, different cryopreservation methods have been extensively studied recent years (Reubinoff et al., 2001; Richards et al., 2004; Zhou et al., 2004; Ha et al., 2005; Heng et al., 2006; Yang et al., 2006). Traditionally, cryopreservation of stem cells has involved a medium containing a cryoprotectant agent, 10% dimethylsulfoxide (DMSO), allowing large cell numbers to be frozen in one vial. Upon thawing the survival of undifferentiated hESCs is poor and most cells either die or differentiate (Reubinoff et al., 2001), thus making traditional protocols sub-optimal. Vitrification in open straws has been effectively and widely used (Reubinoff et al., 2001), but it is difficult to keep the liquid nitrogen and the cells in open straws sterile. Security straws that are needed for clinical grade vitrification, allow cryopreservation of only small numbers of cells per unit. Clearly, novel freezing methods are needed for easy handling of bulk quantities of hESCs which will be required for various clinical and research applications (Martin-Ibanez et al., 2008).
hESCs have generally been cryopreserved as small aggregates in culture media containing serum replacement (SR) and 10% DMSO. Our recent results using a Rho kinase inhibitor allowed the freezing of single cell suspensions, but better survival would still be desired (Martin-Ibanez et al., 2008). DMSO, which has mainly been used for hESC freezing, is a permeable cryoprotectant. Use of combinations of cryoprotectants in hESC banking has not been reported. We have now evaluated the use of a new chemically defined cryopreservation medium and cell wash solution, which are completely free of animal substances, STEM-CELLBANKER and CELLOTION™. The freezing procedure can be conducted without dedicated instrumentation. We have now evaluated the freezing–thawing media for both hESC and human induced pluripotent stem cells (iPSCs).
Materials and Methods
hESCs and iPSCs maintenance and culture
We used two hESC lines, HS293 and HS306, both derived in our laboratory (Strom et al., 2007) and one iPSC line, CHiPS-A (see below). They were cultured and maintained on a feeder layer of human foreskin fibroblasts (CRL-2429; ATCC, Manassas, VA, USA) mitotically inactivated by irradiation (40 Gy). The culture medium was knockout Dulbecco's modified Eagle's medium (DMEM) supplemented with: 20% knockout SR, 2 mM glutamax, 0.5% penicillin–streptomycin, 1% non-essential amino acids (all from Gibco Invitrogen Corporation, Paisley, UK), 0.5 mM 2-mercaptoethanol (Sigma-Aldrich Co, St Luis, USA) and 8 ng/ml basic fibroblast growth factor (R&D Systems, Oxon, UK) (Hovatta et al., 2003; Inzunza et al., 2005). We also cryopreserved one human iPSC line, CHiPS-A, that we had established in collaboration with University of Geneva (Unger et al., 2009) by transducing human skin fibroblasts with Oct-4, Nanog, Sox2 and Lin28.
Cryopreservation and thawing
STEM-CELLBANKER contains 10% DMSO, glucose and a high polymer described in the Japanese Pharmacopeia (JP) as a second cryoprotectant, plus NaCl, KCl, Na2HPO4, HK2PO4, NaHCO3 as pH adjustors for maintenance of the cell function, all dissolved in phosphate-buffered saline (PBS). For optimal dehydration and minimal ice crystal formation, the medium contains both permeating and non-permeating cryoprotectants. All compounds are of Pharmacopoeia of the United States of America, European Pharmacopea or JP grade.
hESC colonies were harvested by mechanically removing undifferentiated colonies from their feeder layer with a surgical scalpel. Cell aggregates containing about 20 000 cells, equaling 10–30 colonies, were transferred into cryo vials. When the cells had settled to the bottom of the vial, excess medium was removed. Cold (4°C) freezing medium, 500 µl, (STEM-CELLBANKER™) was added directly into the cryo vials, with no other supplements. The vials were either placed in a Nalgene Cryo 1°C Freezing Container ‘Mr. Frosty’ (5100-0001) (Nalge Nunc International, Rochester, USA) containing isopropanol and immediately stored at −70°C, or frozen directly at −70°C without using the ‘Mr Frosty’, both procedures yielding similar results. CHiPS-A cells were frozen as a single-cell suspension with the Rho-associated kinase (ROCK) inhibitor, Y-27 632 as previously described (Watanabe et al., 2007; Martin-Ibanez et al., 2008) with STEM-CELLBANKER™ instead of SR-medium and 10% DMSO (Sigma-Aldrich Co, St Luis, USA), which at present is the most often used slow freezing system for hESC. A programmed freezer was not used in our cryopreservation method. The next day, cryo vials were removed from −70°C and stored in a liquid nitrogen tank.
To study thawed cells, culture plates were prepared 30 min prior to thawing. Vials were removed from the liquid nitrogen tanks and quickly placed in a 37°C water bath. When only a small amount of ice remained, the vials were sterilized with 70% ethanol; any remaining ethanol was allowed to evaporate before the vial was opened. The cell suspension was pipetted and placed into a 14 ml conical centrifuge tube and 8 ml of thawing solution (CELLOTION™) was added. CELLOTION™ is a washing solution containing NaCl. The tube was centrifuged at 300g for 7 min. The supernatant was discarded and the pellet with the cells was re-suspended with 1 ml SR culture medium that had been on a feeder plate for at least 30 min before cell thawing. The CHiPS-A cells were re-suspended with 1 ml SR-containing culture medium with ROCK-inhibitor (Merck Chemicals, Ltd, Nottingham, UK) diluted 1:500, a selective ROCK inhibitor which has been described to increase survival of dissociated hESCs, increase their cloning efficiency and diminish their dissociation-induced apoptosis (Watanabe et al., 2007). Control freezing was carried out using stem cell culture medium (DMEM/F12 1:1) supplemented by SR and 10% DMSO which is the medium regularly used for the time being.
Assessment of hESC viability after cryopreservation
The percentage of surviving cells was counted as a ratio between live hESCs after thawing and total number of initially frozen cells counted using a haemocytometer chamber and the trypan blue exclusion method. This counting was carried out in triplicate. The cell numbers were monitored for three passages by cell counts to assess the survival rate, and after the third passage, the cultures were considered to be stable with normal growth and expansion (Fig. 1A). Also the percentage of frozen–thawed hESCs was determined by colony formation after thawing, expressed as number of colonies after thawing and total number of initially frozen hESCs. We also used the calcein-esterase based live–dead assay (Molecular Probes, Eugene, USA) to show the percentage of live cells after thawing.
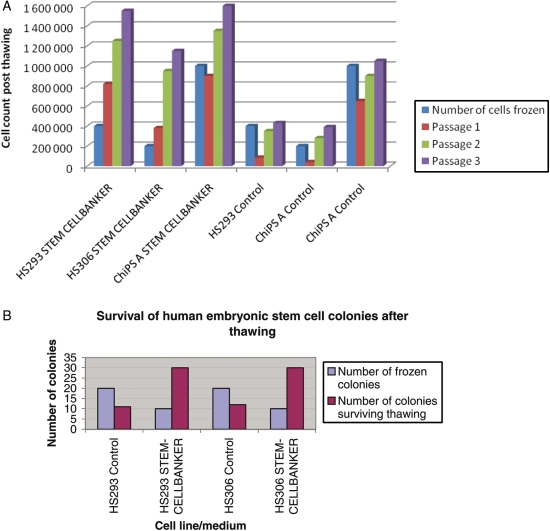
Figure 1.
Survival of stem cells after thawing. (A) Growth rates of HS293, HS306 and CHiPS-A post-thawing for the first three passages. (B) The growth rates of both HS293 and HS306 frozen with STEM-CELLBANKER compared with cells frozen with 10% DMSO in SR medium.
The level of differentiation was determined using inverted phase contrast microscopy based on morphological appearance: colonies with small cells, a large nucleus and sharp edges were regarded as undifferentiated (Fig. 2A and B), whereas colonies with large cells with abundant cytoplasm were regarded as differentiated (Fig. 2C). The morphological appearance was further assessed by Oct-4 immunostaining (Fig. 3) as described previously (Martin-Ibanez et al., 2008).
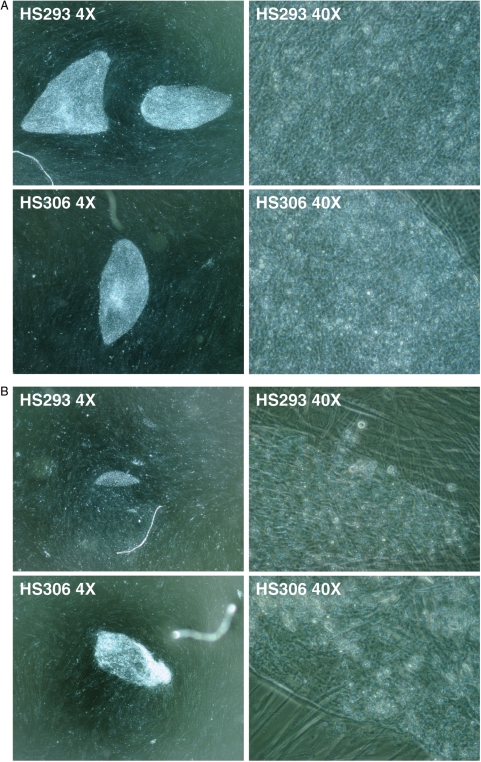
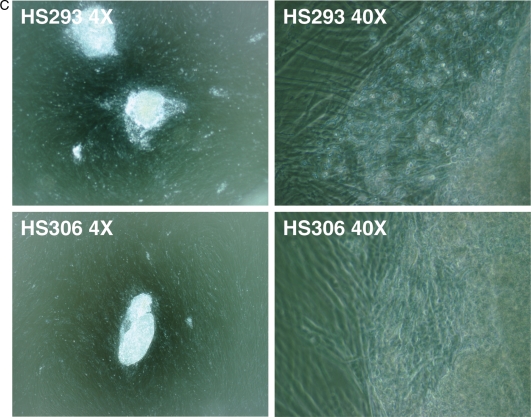
Figure 2.
Morphology of hESCs HS293 and HS306 colonies. (A) Typical colonies of cell lines HS293 and HS306 in magnification ×40 and ×400 using phase contrast objective (Ph) showed normal morphology with typical characteristics of human embryonic stem cells (hESCs) with large nuclei with surrounding cytoplasm, the cells being packed tightly together. (B) Colony formation of cell line HS293 and HS306 (×40 and ×400 Ph) after thawing cells which had been frozen with STEM-CELLBANKER and thawed with CELLOTION, 3 days after thawing, showing normal hESC morphology. (C) Colony formation (×40 and ×400 Ph) after thawing hESCs which had been frozen in 10% DMSO in serum replacement (SR)-containing medium. Colonies are growing in clumps, with a very small outgrowth of normal hESCs by the boarders of the cell clump.
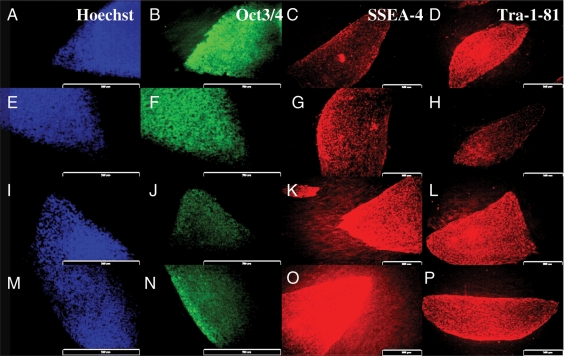
Figure 3.
Immunocytochemical detection of Hoechst, OCT3/4, stage-specific embryonic antigen (SSEA)-4 and Tra-1-81. (A–D) HS293 cells frozen using 10% DMSO in SR. (E–H) HS293 cells frozen with STEM-CELLBANKER. (I–L) HS306 cells frozen using 10% DMSO in SR. (M–P) HS306 cells frozen with STEM-CELLBANKER.
Immunocytochemical characterization
Immunocytochemical characterization was carried out on hESC colonies obtained at passage 10 after thawing. The primary antibodies were specific for Oct-4 (Chemicon, Temecula, CA, USA), Nanog, TRA-1-81 and stage-specific embryonic antigen (SSEA)-4 (all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and were all diluted at 1:200. Human foreskin fibroblasts were used as negative control cells. Immunostaining was performed by fixation of the cells with 4% paraformaldehyde in PBS for 20 min at room temperature followed by rinsing with PBS three times followed by blocking with 5% fetal bovine serum for 30 min. Permeabilization was carried out using 5% blocking buffer supplemented with 0.02% Triton X-100. The primary antibodies were added in blocking buffer overnight at 4°C. To remove any unbound antibodies, the samples were washed three times with PBS. Binding of the primary antibodies was detected with fluorochrome-conjugated secondary antibodies, diluted 1:200 in 5% blocking buffer and added to cells for 60 min at room temperature in the dark. After three washes with PBS, the cell nuclei were counterstained with Hoechst B2261 (Sigma-Aldrich) for 10 min.
In addition to negative control cells, hESC were also incubated without primary antibodies. Stained cells were analyzed using an Olympus IX71 inverted microscope (Olympus Sverige AB, Solna, Sweden) and images were acquired with Olympus DP71 camera and the Soft Imagine Cell F version 2.6 Software (Olympus Sverige AB).
Real-time quantitative PCR
The hESC colonies obtained after freezing and thawing were studied by analyzing the expression levels of Oct-4 and Nanog mRNA. As a house keeping gene, glyceraldehyde-3-phosphate dehydrogenase was used as an internal control, to normalize Oct-4 and Nanog.
In brief, total RNA from hESCs was extracted (QIAGEN, RNeasy Mini kit) according to the manufacturers recommended protocol, and converted to cDNA (Invitrogen, SuperScript™ III First-Strand Synthesis System for RT–PCR, Cat. No. 18 080-051), also according to the manufacturers recommended protocol. The cDNA was then analyzed using SYBR gene expression assay.
The data were analyzed using MxPro™ QPCR analysis software version 3.0 (STRATAGENE®). The assays were performed in triplicate. The sequences of primers used were as follows: Oct-4 (sense, 5′-GACAACAATGAAAATCTTCAGGAGA-3′; antisense, 5′-TTCTGGCGCCGGTTACAGAACCA-3′) and Nanog (sense, 5′-AAGACAAGGTCCCGGTCAAG-3′; antisense, 5′-CCTAGTGGTCTGCTGTATTAC-3′).
Karyotyping
Karyotyping of the cell lines HS293, HS306 and CHiPS-A was carried out using the standard G-banding technique at passage 10 post-recovery. The original passage number was for cell line HS293 passage 57, cell line HS306 passage 66 and CHiPS-A passage 16. Samples of cells were treated with colcemid KaryoMAX (0.1 µg/ml; GIBCO) for 5 h. The medium was removed into a centrifugation tube, and centrifuged at 50g, for 7 min. In the meantime, the cell culture was treated with trypsin for 5 min. Supernatants were removed after centrifugation and the trypsin-treated cells were added to the tube and re-suspended. Pre-warmed 0.0375 M KCl hypotonic solution was added to the tube and then incubated for 10 min. After further centrifugation, the cells were re-suspended in fixative (3:1 methanol:acetic acid). Metaphase spreads were prepared on glass microscope slides and G-banded by brief exposure to trypsin and stained with 4:1 Gurŕs/Leishmanńs stain (Sigma). A minimum of 10 metaphases was analyzed and an additional 20 counted and scored for a total of 30 cells for each cell line.
Teratomas
The pluripotency of these two lines with STEM-CELL BANKER and the same two cell lines in control condition using SR containing medium was tested in vivo as described previously (Inzunza et al., 2005; Li et al., 2008). In brief, exponentially growing cells from passage 10 were harvested from the culture plate using mechanical splitting. Five colonies (103–104 hESCs) were washed twice in PBS and put in a 1.5 ml collection tube with 80 µl SR culture media, and subsequently implanted beneath the testicular capsule of a young (7-week-old) severe combined immunodeficiency/beige mouse (C.B.-17/GbmsTac-scid-bgDF N7, M&B, Ry, Denmark). Teratoma growth was determined by palpation every week, and the mice were sacrificed (cervical dislocation) 8 weeks after implantation. The teratoma was fixed, and sections were stained with hematoxylin and eosin. The presence of tissue components of the three embryonic germ cell layers was analyzed in the stained sections (Inzunza et al., 2005). All animal experiments were performed at the specific pathogen free animal facility of Karolinska University Hospital in accordance with Ethics Committee approval.
Statistics
χ2 test and Student's t-test were used for the differences between the number of surviving colonies. A P < 0.05 was considered significant.
Results
Characterization of hESCs after cryopreservation
The survival of the cells as determined by cell count, colony formation and live/dead assay is shown in Table I. The proportion of surviving hESC in the lines HS293 and HS306 were 93 and 96%, respectively, and that of the iPSC line CHiPS-A 90% when using STEM-CELLBANKER together with the recovery solution CELLOTION. When we used the conventional slow freezing with the hESC culture medium (DMEM/F12 supplemented by SR), only about 50% of the cells survived. The ratio of numbers of frozen/thawed colonies differed widely between the two freezing methods (Table I).
Table I.
Colony formation and survival of frozen–thawed human embryonic stem cells in the presence or absence of STEM-CELLBANKER.
| STEM-CELLBANKER |
10% DMSO in SR |
|||||
|---|---|---|---|---|---|---|
| HS293* | HS306* | CHiPS-A | HS293 | HS306 | CHiPS-A | |
| Colony formation (frozen colonies/colonies formed post-thaw) | 10/>30 | 10/>30 | 500 000/500 000 (Single cells with ROCK) | 20/11 | 20/12 | 500 000/500 000 (Single cells with ROCK) |
| % Live cells after thawing | 93 | 96 | 90 | 49 | 49 | 50 |
Both human embryonic stem cell lines HS293 and HS306 formed three times more colonies after freezing with STEM-CELLBANKER than after the standard procedure of 10% DMSO in SR. Cell lines frozen using the standard procedure had almost 50% loss in colony formation after thawing. The human induced pluripotent stem cell line (CHiPS-A) showed good colony formation (100%) with both freezing procedures. Live–dead assay showed a higher survival of cells frozen with STEM-CELL BANKER than DMSO in SR (90 versus 50%, respectively). ROCK: the Rho-associated kinase inhibitor, Y-27 632.
*Significance STEM-CELLBANKER versus 10% dimethylsulfoxide in serum replacement (DMSO in SR, P < 0.05).
The number of surviving hESCs and colonies post-thawing was much higher than the number originally frozen (Fig. 1B), The explanation is that the colonies break during freezing–thawing, and this results in small pieces from the original colonies surviving as independent colonies, which gives the higher colony formation post-thawing. This does not seem to occur with the standard procedure using 10% DMSO in SR medium. By counting the cells directly after thawing before plating them onto fresh feeder cells, using eosin red, we saw that cells originally frozen had survived the freezing procedure. Adding a ROCK-inhibitor Y-27 632 to the freezing medium and to the plating medium after cryopreservation did not change the survival of the CHiPS-A cells. The morphological differences of the colonies frozen with the different methods are shown in (Fig. 2A–C). Frozen hESCs, using STEM-CELLBANKER, had a more typical stem cell morphology than those frozen using the standard method (SR medium supplemented with DMSO). Immunocytochemistry showed that colonies obtained after cryopreservation with STEM-CELLBANKER expressed the surface markers SSEA-4, TRA-1-81 and the nuclear marker Nanog, as did the cells frozen using the standard protocol (Fig. 3).
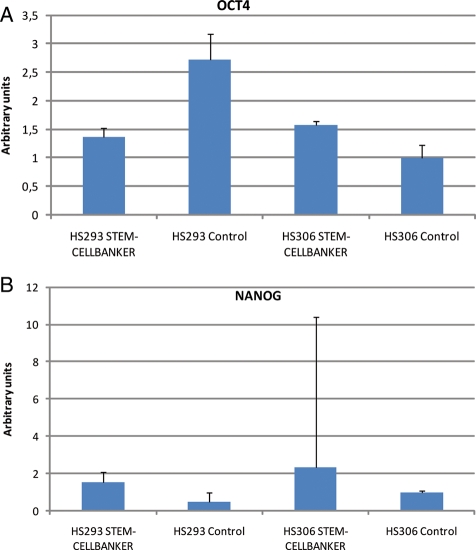
The mRNA expression of Oct-4 and Nanog of the cryopreserved hESCs, as determined by real-time quantitative PCR, confirmed maintenance of the undifferentiated state of the frozen–thawed cells (Fig. 4A and B).
Figure 4.
Expression of OCT-4 and Nanog with real-time quantitative PCR. Error bars = standard derivation. (A) Relative concentration OCT-4. HS293 and HS306 frozen with STEM-CELLBANKER have an almost equal expression of OCT-4, whereas HS293 cells frozen with 10% DMSO have an OCT-4 expression that is twice as high as HS306 frozen with 10% DMSO. (B) Both HS293 and HS306 with STEM-CELLBANKER have a clear Nanog expression, which is significantly stronger in HS306. The controls had a lower expression of Nanog.
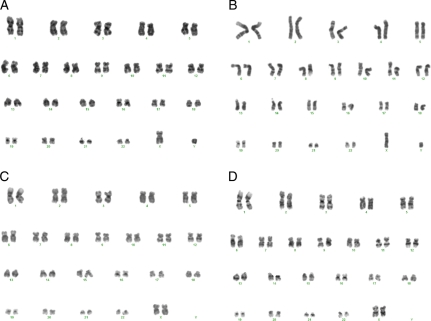
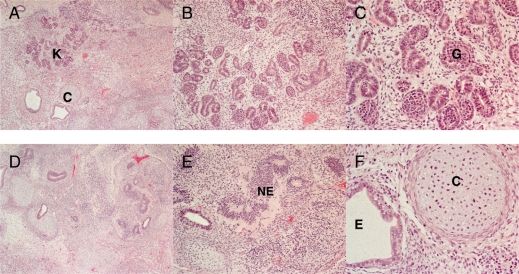
The analysis of the karyotypes of the hESC lines after 10 passages showed, for both hESC lines, normal karyotypes, male 46 XY (HS293) and female 46 XX (HS306) (Fig. 5), and 46 XY (CHiPS-A). The cell line CHiPS-A was also karyotyped at passage 38 and was 46 XY, indicating karyotypic stability. Both hESC lines formed teratomas with components of the three germ layers (Fig. 6).
Figure 5.
Karyotype. (A) Control line HS293 frozen with 10% DMSO in SR medium show a normal male karyotype 46 XY. (B) HS293 frozen with STEM-CELLBANKER and thawed with CELLOTION has a normal male karyotype 46 XY. (C) HS306 frozen with 10% DMSO in SR medium has a normal female karyotype 46 XX. (D) HS306 frozen with STEM-CELLBANKER and thawed with CELLOTION has a normal female karyotype 46 XX.
Figure 6.
Histological analysis of teratoma formed after implanting hESCs into a severe combined immunodeficiency mouse. The teratomas was fixed, and sections were stained with hematoxylin and eosin (A–C) Area of a teratoma with prominent formation of primitive renal components. In (A), the renal component is marked with K and small cartilaginous island with C. (B and C) Higher magnifications of kidney formation. Primitive glomeruli G are seen in (C). In (D), a low power overview illustrates a cell dense area of a teratoma containing diverse structures, among them neuroepithelium NE which is highlighted in (E). (F) A high power view of the small cartilage island seen in (A) with an adjoining epithelial lined lumen compatible with a structure derived from endoderm.
The number of frozen colonies versus the number of surviving colonies differed significantly for both HS293 (χ2 = 9.616 with one degree of freedom and two-tailed P = 0.0019) and HS306 (χ2 = 8.801 with one degree of freedom and two-tailed P = 0.0030).
Discussion
We have successfully applied a novel freezing and thawing medium to cryopreserve and thaw hESCs and iPSCs. The easy use of this cryopreservation system enables freezing of large quantities of cells for both research purposes and clinical long-term storage. It does not require a specific freezing apparatus, and it is easy to handle. Even though these media have not yet undergone the good manufacturing practice (GMP) validations, all the components are principally GMP compatible, making clinical grade achievable. Reubinoff et al. had good success with their open pulled straw vitrification protocol, which has been widely used for years (Reubinoff et al., 2001). We have also used the same technique for small cell samples from early hESC lines. However, on a larger scale, this method is too time consuming because of the small number of cell aggregates that can be frozen in straws. In 2008, a new bulk vitrification system was described for the first time (Li et al., 2008). In addition to the technical difficulties, another major problem with these vitrification methods is that they are open systems, and for clinical grade cryostorage, sterile liquid nitrogen would be needed, requiring a specific vacuum filtration system. The straws can of course be stored in closed vials in the gas phase of liquid nitrogen but this does not prevent the original contact during the vitrification procedure. Also, handling of different vitrification solutions, together with several incubation steps, is more likely to have technical drawbacks with the bulk vitrification method, rather than with this new easy and safe method that has fewer crucial steps.
Previously, freezing stem cells with only permeating cryoprotectants have been used. For other cell types, such as red blood cells (Meryman 2007), embryos (Saha and Suzuki, 1997; Kuleshova et al., 2001) and human oocytes (Liebermann et al., 2003), a combination of both permeating and non-permeating cryoprotectants have been widely and successfully used. We recently used polyvinylpyrrolidone (PVP) in combination with permeating cryoprotectants in vitrification of human ovarian tissue with good success (Keros et al., 2009). Many polymers, such as Ficoll, polyvinyl alcohol, PVP, dextran and hydroxyethyl starch (Franks et al., 1977; Wowk et al., 2000), have been used for many cell types. We have now demonstrated the efficacy of a freezing solution containing both non-permeating and permeating cryoprotectants in freezing and thawing of hESC and iPSC. The system is a fully defined, easy-to-use and xeno-free protocol for cryopreservation that is GMP compatible. The method resulted in significantly higher survival rates of the cells, for both hESCs and iPSCs than the previously reported slow freezing or vitrification systems, which are based on permeating cryoprotectants only. With this new freezing–thawing procedure, more than 90% of the cells survived, maintained their pluripotency, had normal karyotype and showed a good capacity to grow, without delays, after thawing. When compared with the freezing procedure using 10% DMSO in SR medium, where only 50% of the cells survived, this easy to use efficient freezing system offers an excellent option for banking of human pluripotent cell lines.
Authors' roles
F.H. and O.H. conceived the project, designed experiments, performed experiments, analyzed data and wrote the manuscript; R.B. co-supervised the project and participated in writing the manuscript; J.I. performed teratoma experiments; D.B. performed the karyotyping; B.R. analyzed the teratomas; A.F. derived and characterized together with O.H. the iPS cells; S.S., A.-M.S. participated in hESC culture work.
Funding
This study was supported by EU:eSTOOLS, the Swedish Research Council and the R&D Funds of Karolinska Institutet and Stockholm County (ALF).
Acknowledgements
We thank ZENOAQ for generous donation of the freezing and thawing media.
References
- Franks F, Asquith MH, Hammond CC, Skaer HB, Echlin P. Polymer cryoprotectants in the preservation of biological ultrastructure. I. Low temperature states of aqueous solutions of hydrophilic polymers. J Microsc. 1977;110:223–228. doi: 10.1111/j.1365-2818.1977.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Gearhart J. New potential for human embryonic stem cells. Science. 1998;282:1061–1062. doi: 10.1126/science.282.5391.1061. [DOI] [PubMed] [Google Scholar]
- Ha SY, Jee BC, Suh CS, Kim HS, Oh SK, Kim SH, Moon SY. Cryopreservation of human embryonic stem cells without the use of a programmable freezer. Hum Reprod. 2005;20:1779–1785. doi: 10.1093/humrep/deh854. [DOI] [PubMed] [Google Scholar]
- Heng BC, Ye CP, Liu H, Toh WS, Rufaihah AJ, Cao T. Kinetics of cell death of frozen-thawed human embryonic stem cell colonies is reversibly slowed down by exposure to low temperature. Zygote. 2006;14:341–348. doi: 10.1017/S0967199406003893. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Mikkola M, Gertow K, Stromberg AM, Inzunza J, Hreinsson J, Rozell B, Blennow E, Andang M, Ahrlund-Richter L. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum Reprod. 2003;18:1404–1409. doi: 10.1093/humrep/deg290. [DOI] [PubMed] [Google Scholar]
- Inzunza J, Gertow K, Stromberg MA, Matilainen E, Blennow E, Skottman H, Wolbank S, Ahrlund-Richter L, Hovatta O. Derivation of human embryonic stem cell lines in serum replacement medium using postnatal human fibroblasts as feeder cells. Stem Cells. 2005;23:544–549. doi: 10.1634/stemcells.2004-0201. [DOI] [PubMed] [Google Scholar]
- Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, Hreinsson J, Hovatta O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24:1670–1683. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- Klimanskaya I, Rosenthal N, Lanza R. Derive and conquer: sourcing and differentiating stem cells for therapeutic applications. Nat Rev Drug Discov. 2008;7:131–142. doi: 10.1038/nrd2403. [DOI] [PubMed] [Google Scholar]
- Kuleshova LL, Shaw JM, Trounson AO. Studies on replacing most of the penetrating cryoprotectant by polymers for embryo cryopreservation. Cryobiology. 2001;43:21–31. doi: 10.1006/cryo.2001.2335. [DOI] [PubMed] [Google Scholar]
- Li T, Zhou C, Liu C, Mai Q, Zhuang G. Bulk vitrification of human embryonic stem cells. Hum Reprod. 2008;23:358–364. doi: 10.1093/humrep/dem386. [DOI] [PubMed] [Google Scholar]
- Liebermann J, Dietl J, Vanderzwalmen P, Tucker MJ. Recent developments in human oocyte, embryo and blastocyst vitrification: where are we now? Reprod Biomed Online. 2003;7:623–633. doi: 10.1016/s1472-6483(10)62084-6. [DOI] [PubMed] [Google Scholar]
- Martin-Ibanez R, Unger C, Stromberg A, Baker D, Canals JM, Hovatta O. Novel cryopreservation method for dissociated human embryonic stem cells in the presence of a ROCK inhibitor. Hum Reprod. 2008;23:2744–2754. doi: 10.1093/humrep/den316. [DOI] [PubMed] [Google Scholar]
- Meryman HT. Cryopreservation of living cells: principles and practice. Transfusion. 2007;47:935–945. doi: 10.1111/j.1537-2995.2007.01212.x. [DOI] [PubMed] [Google Scholar]
- Pera MF, Trounson AO. Human embryonic stem cells: prospects for development. Development. 2004;131:5515–5525. doi: 10.1242/dev.01451. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Vajta G, Trounson AO. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum Reprod. 2001;16:2187–2194. doi: 10.1093/humrep/16.10.2187. [DOI] [PubMed] [Google Scholar]
- Richards M, Fong CY, Tan S, Chan WK, Bongso A. An efficient and safe xeno-free cryopreservation method for the storage of human embryonic stem cells. Stem Cells. 2004;22:779–789. doi: 10.1634/stemcells.22-5-779. [DOI] [PubMed] [Google Scholar]
- Saha S, Suzuki T. Vitrification of in vitro produced bovine embryos at different ages using one- and three-step addition of cryoprotective additives. Reprod Fertil Dev. 1997;9:741–746. doi: 10.1071/r97024. [DOI] [PubMed] [Google Scholar]
- Strom S, Inzunza J, Grinnemo KH, Holmberg K, Matilainen E, Stromberg AM, Blennow E, Hovatta O. Mechanical isolation of the inner cell mass is effective in derivation of new human embryonic stem cell lines. Hum Reprod. 2007;22:3051–3058. doi: 10.1093/humrep/dem335. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Trounson A, Pera M. Human embryonic stem cells. Fertil Steril. 2001;76:660–661. doi: 10.1016/s0015-0282(01)02880-1. [DOI] [PubMed] [Google Scholar]
- Unger C, Gao S, Cohen M, Jaconi M, Bergstrom R, Holm F, Galan A, Sanchez E, Irion O, Dubuisson JB, et al. Immortalized human skin fibroblast feeder cells support growth and maintenance of both human embryonic and induced pluripotent stem cells. Hum Reprod. 2009;24:2567–2581. doi: 10.1093/humrep/dep232. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Wowk B, Leitl E, Rasch CM, Mesbah-Karimi N, Harris SB, Fahy GM. Vitrification enhancement by synthetic ice blocking agents. Cryobiology. 2000;40:228–236. doi: 10.1006/cryo.2000.2243. [DOI] [PubMed] [Google Scholar]
- Yang PF, Hua TC, Wu J, Chang ZH, Tsung HC, Cao YL. Cryopreservation of human embryonic stem cells: a protocol by programmed cooling. Cryo Letters. 2006;27:361–368. [PubMed] [Google Scholar]
- Zhou CQ, Mai QY, Li T, Zhuang GL. Cryopreservation of human embryonic stem cells by vitrification. Chin Med J (Engl) 2004;117:1050–1055. [PubMed] [Google Scholar]