Abstract
Neuronal mechanisms underlying alcohol intoxication are unclear. We find that alcohol impairs motor coordination by enhancing tonic inhibition mediated by a specific subtype of extrasynaptic GABAA receptor (GABAR), α6β3δ, expressed exclusively in cerebellar granule cells. In recombinant studies, we characterize a naturally occurring single-nucleotide polymorphism that causes a single amino acid change (R100Q) in α6 (encoded in rats by the Gabra6 gene). We show that this change selectively increases alcohol sensitivity of α6β3δ GABARs. Behavioral and electrophysiological comparisons of Gabra6100R/100R and Gabra6100Q/100Q rats strongly suggest that alcohol impairs motor coordination by enhancing granule cell tonic inhibition. These findings identify extrasynaptic GABARs as critical targets underlying low-dose alcohol intoxication and demonstrate that subtle changes in tonic inhibition in one class of neurons can alter behavior.
Humans have been consuming alcohol for thousands of years, and the use of alcoholic beverages pervades human culture and society and can have substantial health effects1. Different mechanisms by which ethanol might depress brain function have been proposed based on ethanol’s ability to modulate a wide variety of ion channels2–4, neurotransmitter receptors5–10 and transporters11. Among these diverse targets, however, GABARs are arguably the most attractive candidates. This is in part because other classes of known GABAR modulators such as benzodiazepines, barbiturates and certain anesthetics lead to behavioral effects that closely resemble ethanol intoxication. Yet despite strong evidence implicating GABARs in ethanol’s action, critical details remain unclear. For instance, although it is known that native GABARs are heteropentamers assembled from 19 possible subunits12,13, it has not been possible to link the activity of particular GABAR subunits to changes in behavioral sensitivity to ethanol.
Recent studies suggest that specific combinations of GABAR subunits (those containing α4β3δ and α6β3δ) are uniquely sensitive to ethanol, showing dose dependencies that mirror blood alcohol levels associated with intoxication in humans9,10. GABARs containing α4 and δ subunits are expressed in many brain regions14,15, but α6 is found in only two types of neurons (cerebellar granule cells and granule cells in the cochlear nucleus) and is expressed together with δ only in cerebellar granule cells14,16,17. In granule cells α6 and δ combine with β subunits to give rise to high-affinity18 extrasynaptic GABARs19–21. These GABARs generate a tonic inhibitory conductance20–22 that exerts strong control over granule cell firing patterns in vivo23.
We set out to examine whether such extrasynaptic GABARs containing α6 and δ subunits account for behavioral effects of ethanol at moderately intoxicating doses. To link these particular GABARs to behavioral sensitivity, we first characterized a naturally occurring single-nucleotide polymorphism in the gene encoding rat α6 (Gabra6). This polymorphism is of interest because it has been reported to be present in alcohol-nontolerant (ANT) rats24 and enriched in Sardinian alcohol-nonpreferring rats25, two lines of animals selectively bred either for heightened ethanol-induced motor impairment (ANT rats) or for aversion to ethanol (Sardinian nonpreferring rats). A single nucleotide change (from a guanine to an adenine) leads to a single amino acid substitution, from arginine (R) to glutamine (Q), at amino acid position 100 in α6. The α6-R100Q polymorphism causes a marked increase in benzodiazepine impairment of motor coordination in vivo and has been shown to convert recombinant GABARs containing α6 and γ subunits from benzodiazepine insensitive to benzodiazepine sensitive24. However, because it has not been shown that this polymorphism affects ethanol sensitivity in recombinant or native GABARs, it has been suggested that other co-segregating polymorphisms might be responsible for the increased ethanol sensitivity in ANT rats26,27.
We report here that the α6-R100Q polymorphism further enhances the ethanol sensitivity of a specific subtype of GABAR composed of α6, β3 and δ subunits. Notably, we found that the α6-R100Q polymorphism is common in outbred strains of Sprague-Dawley rats. This allowed us to obtain rats homozygous for each of the two alleles and then to test ethanol sensitivity in situ. We showed that behaviorally-relevant concentrations of ethanol enhance tonic inhibition mediated by extrasynaptic GABARs in cerebellar granule cells from Gabra6100R/100R rats and cause an even larger increase in tonic inhibition in granule cells from Gabra6100Q/100Q rats. Finally, in a cerebellum-dependent behavioral task we found that ethanol more severely impairs coordination in Gabra6100Q/100Q rats. Together these findings imply that small increases in a tonic GABA conductance in a single class of cerebellar neurons can account for the adverse effects of ethanol on motor coordination. The results strongly imply that similar, more widely expressed isoforms of extrasynaptic GABARs are critical components of ethanol intoxication.
RESULTS
α 6-R100Q polymorphism enhances ethanol sensitivity
The ethanol sensitivities of heteromeric GABARs containing the α6-100R and α6-100Q polymorphisms were tested by expressing each variant in Xenopus laevis oocytes together with a β subunit and either δ or a γ2 subunit. We evaluated ethanol dose-response curves for several combinations of GABAR subunits thought to exist in granule cells (α6β2δ, α6β3δ, α6β2γ2 and α6β3γ2)16,28,29 as well as other combinations that may be present in these cells (Fig. 1 and Table 1). Consistent with published results24, we found that introduction of the α6-R100Q polymorphism into GABARs composed of α6, β3 and γ2 gave rise to benzodiazepine-sensitive receptors (data not shown). However, the low ethanol sensitivity of α6(100R)β3γ2 GABARs was unchanged in α6(100Q)β3γ2 receptors (Table 1).
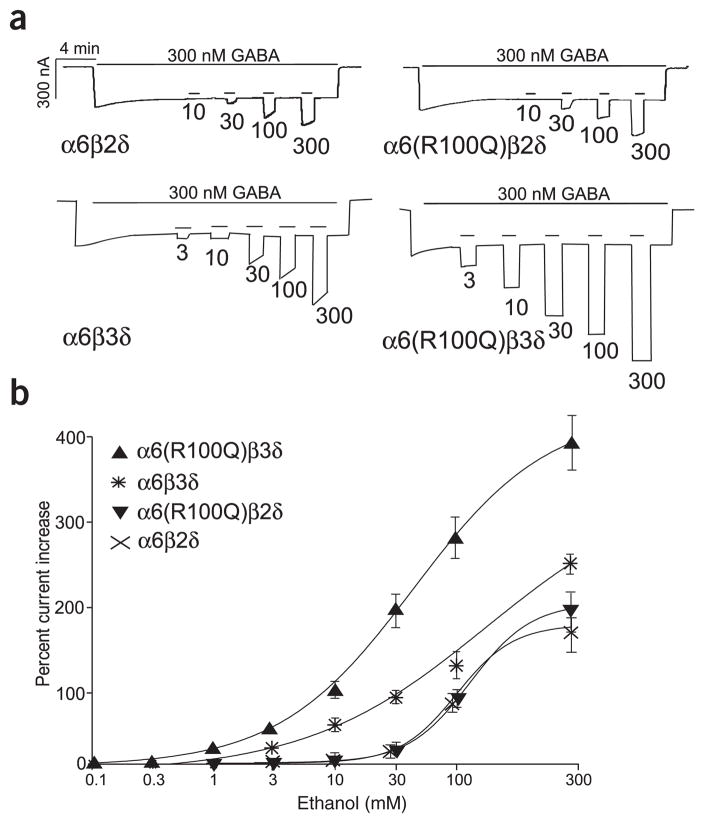
Figure 1.
The α6-R100Q polymorphism leads to a marked increase in ethanol sensitivity when expressed with β3 and δ subunits. (a) GABARs of the indicated subunit compositions were expressed in X. laevis oocytes and activated by steady-state GABA (300 nM ~EC30). Brief applications of ethanol at the indicated concentrations (in mM) enhance the current in a dose-dependent manner. (b) Dose-response curves for ethanol showing the peak enhancement of GABA current. Shown are wild-type and mutant versions of subunit combinations that are likely to be responsible for tonic GABA current in granule cells: α6β2δ (cross, n = 6), α6β3δ (asterisk, n = 10), α6(R100Q)β2δ (inverted triangles, n = 7) and α6(R100Q)β3δ (upright triangles, n = 8). Note that the β3 and δ subunits are required for the R100Q mutation to exert an effect and that replacement of β2 with β3 leads to an almost tenfold increase in ethanol sensitivity. Other combinations of subunits expressed in granule cells are summarized in Table 1.
Table 1.
Ethanol and GABA sensitivity for GABARs of different subunit combinationsa
| Percent enhancement by ethanol |
||||||
|---|---|---|---|---|---|---|
| Receptor | GABA EC50 (n) | n | 10 mM ethanol | 30 mM ethanol | 100 mM ethanol | 300 mM ethanol |
| α6(R100Q)β3δ | 0.68 ± 0.1 (5) | 8 | 99.3 ± 15.0 | 180.1 + 28.2 | 275.3 ± 32.4 | 389.2 ± 65.0 |
| α6β3δ | 0.70 ± 0.4 (6) | 10 | 41.2 ± 4.3 | 92.5 ± 9.0 | 125.3 ± 20.5 | 245.0 ± 33.6 |
| α6(R100Q)β2δ | 0.51 ± 0.09 (5) | 7 | 0 | 24.5 ± 10.7 | 97.0 ± 11.2 | 199.0 ± 38.1 |
| α6β2δ | 0.50 ± 0.03 (5) | 6 | 0 | 23.1 ± 7.9 | 88.4 ± 15.6 | 175.0 ± 35.8 |
| α6(R100Q)β1δ | 0.62 + 0.04 | 8 | 0 | 24.1 ± 4.0 | 50.3 ± 7.8 | 185.2 ± 9.4 |
| α6β1δ | 0.56 + 0.07 | 9 | 0 | 21.2 ± 3.3 | 52.0 ± 5.6 | 167.9 ± 10.0 |
| α6(R100Q)β2/3γ2L/S | 19 ± 3.5 (6) | 16 | 0 | 0 | 40.3 ± 7.8 | 167.5 ± 14.2 |
| α6β2/3γ2L/S | 19 ± 0.5 (6) | 10 | 0 | 0 | 39.0 ± 7.5 | 182.6 ± 11.3 |
| α1β3δ | 0.56 ± 0.05 (4) | 8 | 32.5 ± 5.4 | 88.2 ± 7.0 | 117.5 ± 10.6 | 295.3 ± 19.7 |
| α1β2/3γ2L | 6.8 ± 0.8 (5) | 9 | 0 | 0 | 34.4 ± 9.9 | 147.3 ± 12.9 |
| α1β2/3γ2S | 8.8 ± 1.1 (6) | |||||
For each indicated subunit combination, GABA dose-response relationships were determined by fitting the Hill equation (see Methods). Current enhancement by ethanol was measured by co-application of ethanol and GABA (~EC30). Reported values are mean (±s.d.) percent increases above responses to GABA alone, zeroes represent measurements of no change in current, and n indicates the number of oocytes used to determine the average ethanol dose response curves. In cases where the identities of β or γ2 subunits did not lead to differences, results have been pooled.
In contrast, for the highly ethanol-sensitive α6β3δ GABARs10, we found that changing amino acid position 100 in α6 from R to Q leads to a further increase in ethanol sensitivity (Fig. 1). Notably, α6β3δ receptors were the only GABARs that showed a significant change in ethanol sensitivity in response to the α6-R100Q polymorphism (Fig. 1 and Table 1). In particular, α6β2δ receptors, another species of GABAR that may contribute to tonic GABA currents in granule cells, were unaffected.
Ethanol enhances tonic inhibition mediated by α6β3δ GABARs
Based on these results, we hypothesized that the presence of this polymorphism in α6 should enhance ethanol sensitivity of tonic GABA conductances in cerebellar granule cells, the only class of neurons in the brain that express α6 together with β3 and δ subunits. We fortuitously discovered that the Gabra6100Q allele is present in outbred Sprague-Dawley rats obtained from Charles River (Fig. 2). Genotyping 35 rats showed that 10 were Gabra6100R/100R, 11 were Gabra6100Q/100Q and 14 were heterozygotes. Whole-cell recordings were made from granule cells in cerebellar slices prepared from rats of the two homozygous genotypes (Fig. 3). We tested ethanol concentrations that would result in moderate to severe intoxication (30 and 100 mM) and found that they enhanced tonic GABA currents in granule cells from rats of both genotypes. However, the enhancement was significantly larger in granule cells from Gabra6100Q/100Q rats (Fig. 3b,c). Consistent with a recent report30, ethanol also enhanced the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) in granule cells, and the mean increases in frequency were larger in recordings from the mutant rats (Fig. 3d). We did not find any ethanol-induced changes in the mean amplitudes of sIPSCs nor in their decay rates. These findings demonstrate that tonic GABA current is sensitive to low concentrations of ethanol in wild-type rats and that the presence of R100Q in α6 subunits renders tonic GABA current even more sensitive to ethanol. Furthermore, the increased sIPSC frequency in Gabra6100Q/100Q compared with Gabra6100R/100R rats is likely to result indirectly from changes in granule cell excitability, as α6 is not expressed in any other cerebellar cell type14,16.
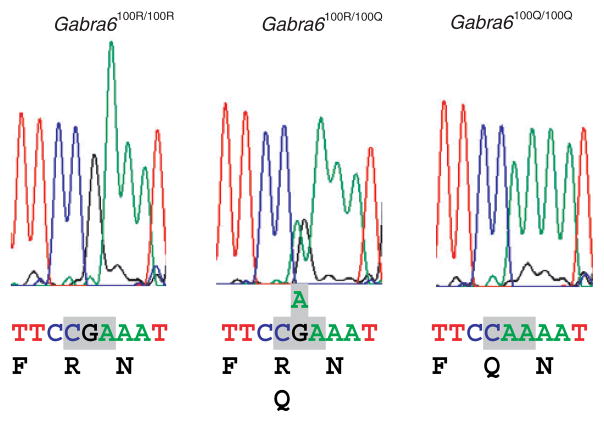
Figure 2.
A single-nucleotide polymorphism (a guanine-to-adenine substitution) in the gene encoding the rat GABAA receptor α6 subunit (Gabra6) is common in Sprague-Dawley rats obtained from Charles River Laboratories. Shown from left to right are the sequence chromatograms from a homozygous Gabra6100R/100R, a heterozygous Gabra6100R/100Q and a homozygous Gabra6100Q/100Q rat. The lower panel shows the sequence of each Gabra6 genotype along with the corresponding amino acid translation (bottom). Gray boxes denote the codon for amino acid position 100.
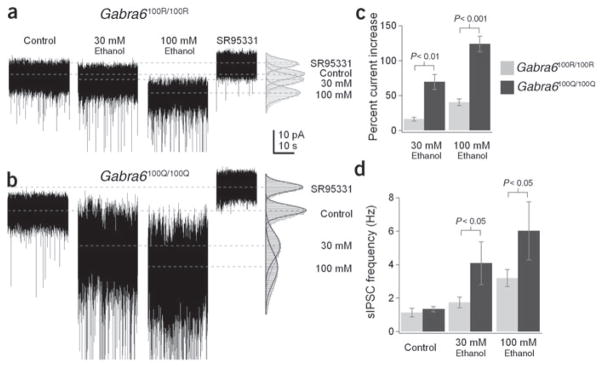
Figure 3.
Ethanol enhances granule cell tonic GABA current, and the enhancement is larger in Gabra6100Q/100Q rats. (a,b) Tonic GABA currents in granule cells recorded from Gabra6100R/100R (a) or Gabra6100Q/100Q (b) slices in the presence of indicated concentrations of ethanol or in a saturating concentration (10μM) of the GABAR antagonist SR95531. To the right are histograms of all points in each segment. Gaussian functions have been fit to each condition and are superimposed. The dashed lines indicate the mean current from these fits. (c) Summary of the mean ± s.e.m. percentage change in tonic current amplitude caused by 30 or 100 mM ethanol in the two genotypes (wild type, n = 5; mutant, n = 7 granule cells). Ethanol caused a significantly larger enhancement of mutant versus wild-type currents. Mean tonic GABA current under control conditions was −9.7 ± 1.7 pA and did not differ significantly between the two genotypes (P > 0.4). (d) Ethanol-induced increases in sIPSC frequency are significantly larger in mutant granule cells (Gabra6100R/100R, n = 5; Gabra6100Q/100Q, n = 7 granule cells). No significant changes were observed in either the amplitude (Gabra6100R/100R, 112.43 ± 12.57% of control, P > 0.49, n = 5; Gabra6100Q/100Q, 101.55 ± 2.42% of control, P > 0.7, n = 7) or the decay of sIPSCs (Gabra6100R/100R, 90 ± 12% of control, P > 0.42, n = 4; Gabra6100Q/100Q, 96 ± 18% of control, P > 0.77, n = 6) in ethanol (data not shown).
Low ethanol doses act directly on extrasynaptic GABARs
In recombinant studies, we have shown that wild-type GABARs containing α4 or α6 along with β3 and δ subunits are enhanced by 10 mM ethanol, a concentration below the legal maximally permitted blood alcohol level for motor vehicle operation in many countries (typically 0.08% (wt/vol) or 17.4 mM). To examine postsynaptic effects of such low doses of ethanol on tonic GABA currents, we performed experiments in the presence of 0.5 μM tetrodotoxin, 2 μM NBQX, 300 nM added GABA and 10 μM NO-711, a GABA transporter antagonist. Significant enhancement by 10 mM ethanol was observed for Gabra6100R/100R granule cells, and even larger increases were observed for Gabra6100Q/100Q granule cells (Fig. 4). Together, these results indicate that increases in tonic GABA current could be detected independent of changes in GABA release and in response to concentrations of ethanol associated with minimal intoxication.
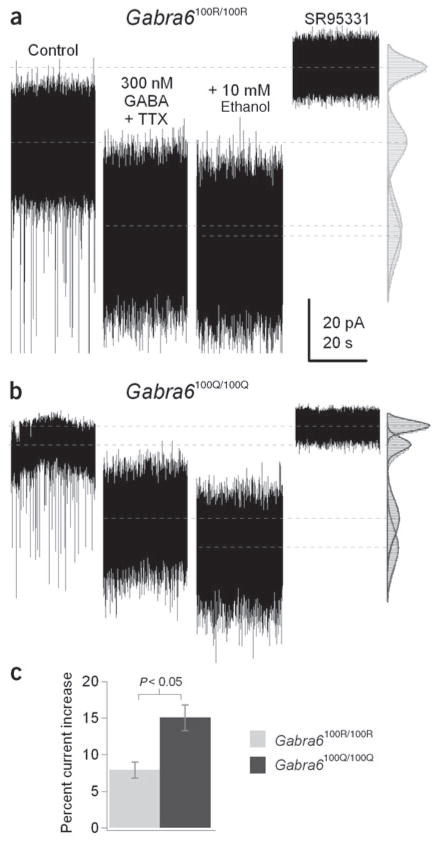
Figure 4.
Tonic GABA current in granule cells is enhanced by low concentrations of ethanol in Gabra6100R/100R and Gabra6100Q/100Q rats. (a,b) Tonic currents recorded from Gabra6100R/100R (a) or Gabra6100Q/100Q (b) granule cells showing that 10 mM ethanol enhances the tonic current in the continuous presence of 300 nM GABA, TTX, NBQX and NO-711. (c) Summary data indicating that in response to 10 mM ethanol, significant increases in tonic current are observed in Gabra6100R/100R granule cells (P < 0.05, n = 4) and larger enhancements are found in Gabra6100Q/100Q granule cells (P < 0.05, n = 4).
Ethanol more severely impairs behavior in Gabra6100Q/100Q rats
To explore whether increases in granule cell tonic inhibition in response to alcohol could account for motor behaviors associated with intoxication, we compared Gabra6100R/100R and Gabra6100Q/100Q rats in cerebellar and non-cerebellar behavioral tests. In the accelerating rotarod test, a cerebellum-dependent behavioral assay, we found that low ethanol concentrations impaired Gabra6100R/100R rats in a dose- and time-dependent manner (Fig. 5a–d). Administration of identical doses of ethanol to Gabra6100Q/100Q rats led to significantly larger impairment on the rotarod at the three doses shown (0.75, 1 and 1.25 g kg−1). Blood ethanol concentrations obtained in these behavioral tests 25–30 min after administration of 1g kg−1 ethanol (15.2 ± 2 mM in Gabra6100R/100R, 16.4 ± 2 mM in Gabra6100Q/100Q, P = 0.34, n = 4 and 5, respectively) confirmed that plasma concentrations of ethanol were not affected by genotype and showed that the concentrations achieved were the equivalent of mildly intoxicating doses in humans. In contrast, hypersensitivity to ethanol was not found in the loss of righting reflex (LORR) assay, a common behavioral test for sedative effects that arise principally from non-cerebellar brain areas (Fig. 5e,f).
Figure 5.
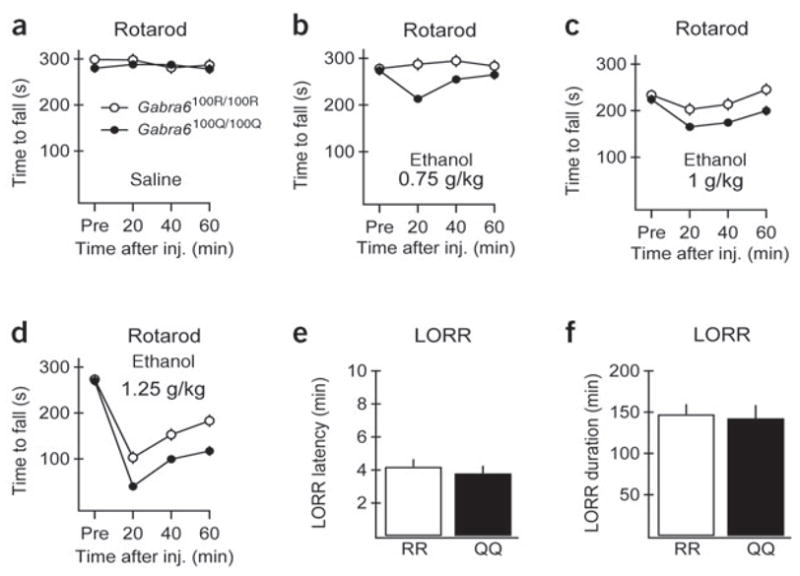
Rats homozygous for the α6-100Q polymorphism show increased alcohol-induced motor impairment as compared with Gabra6100R/100R rats. Motor coordination was assessed by testing Gabra6100R/100R (open circles) and Gabra6100Q/100Q (filled circles) rats in an accelerating rotarod test. (a–d) Performance was measured as the latency to fall from the rotating rod before (‘Pre’) and 20, 40 and 60 min after intraperitoneal injection of saline (a; n = 5 rats each group), 0.75 g kg−1 (b, n = 7 and 8 rats for Gabra6100R/100R and Gabra6100Q/100Q, respectively), 1 g kg−1 (c; n = 6 and 7 rats for Gabra6100R/100R and Gabra6100Q/100Q, respectively) or 1.25 g kg−1 ethanol (d; n = 8 rats each group). Tests on the three postinjection data points yielded the following statistics: saline controls, Gabra6100R/100R versus Gabra6100Q/100Q: F2,7 = 0.50, P = 0.63; 0.75 g kg−1 ethanol, Gabra6100R/100R versus Gabra6100Q/100Q: F2,12 = 6.66, P = 0.011; 1 g kg−1 ethanol, Gabra6100R/100R versus Gabra6100Q/100Q: F2,10 = 5.41, P = 0.026; 1.25 g kg−1 ethanol, Gabra6100R/100R versus Gabra6100Q/100Q: F2,13 = 135.6, P < 0.001. (e,f) Latency (e) and duration (f) of LORR was determined after intraperitoneal injection of 3 g kg−1 ethanol (n = 9 for each group) and did not differ in Gabra6100Q/100Q versus Gabra6100R/100R rats (P = 0.51 for LORR latency, P = 0.81 for LORR duration).
Our finding that this single amino acid difference is sufficient for increased ethanol sensitivity of motor coordination suggests that it makes an important contribution to the ANT phenotype. Moreover, the α6-R100Q polymorphism enhances ethanol sensitivity only in GABARs composed of α6, β3 and δ subunits. As receptors of this molecular makeup are found only in extrasynaptic membrane of granule cells, the tonic GABA current carried they carry must reduce the excitability of this specific class of cerebellar neurons to account for the hypersensitive phenotype.
DISCUSSION
Mechanisms underlying the effects of ethanol
Two general hypotheses have been put forward to explain how ethanol depresses neuronal activity. One proposes an indirect influence on ion channels and receptors through nonspecific interactions with membrane lipids. An alternative hypothesis is that ethanol interacts with specific, saturable binding sites on receptor or ion channel proteins. Many targets have been proposed including potassium channels2–4, glutamate-gated channels6, glycine-gated7 channels and GABAA receptors5,8–10. However, not all of these channels respond to the low concentrations of ethanol associated with mild intoxication, and it is unclear how ethanol acting at these various sites alters behavior. Our results suggest that extrasynaptic GABARs are important targets for mildly intoxicating concentrations of ethanol. This is dramatically demonstrated by the Gabra6100Q/100Q rats, in which a single amino acid change in an extrasynaptic GABA receptor subtype expressed in one class of cerebellar neurons (granule cells) leads to the predicted enhancement of ethanol sensitivity of granule cell tonic inhibition and of cerebellum-dependent behavior.
Link between tonic inhibition and behavior
Tonic inhibition mediated by extrasynaptic, δ subunit–containing GABARs has been identified in a number of neurons31–35 and is thought to regulate neuronal excitability; however, the role of tonic inhibition in shaping behavior is unclear. The results reported here were obtained from cerebellar granule cells, a cell type for which there is strong evidence that receptors containing α6 and δ subunits are extrasynaptic16,19,36 and are required for tonic GABA current20,21. We demonstrate that tonic current is facilitated by low ethanol concentrations and, in addition, identify a variant of α6 (α6-100Q) that renders tonic current carried by α6β3δ GABARs much more sensitive to ethanol. Behavioral analysis suggests that enhancement of this specific extrasynaptic GABAR subtype in granule cells results in motor impairment. How do modest changes in tonic inhibition of these neurons lead to such pronounced behavioral differences? First, granule cells have extremely high input resistance; only small changes in conductance are required to affect excitability. Second, tonic inhibition seems to influence the gain of the input-output relationship in addition to shifting the amount of excitation required for granule cell firing37. Ethanol is not the only compound reported to selectively increase tonic inhibition in granule cells. Tonic currents have also been shown to be highly sensitive to endogenous neuroactive steroids21, raising the possibility that these compounds could similarly affect motor behavior. Future studies using such specific endogenous and exogenous modulators should provide insight into the role of extrasynaptic GABARs in influencing information processing in the granule cell layer.
Gabra6100Q allele and behavioral sensitivity to ethanol
Through selective breeding, several lines of rodents that show ethanol hypersensitivity have been generated. In two such lines, the ANT and the Sardinian nonpreferring rats, the Gabra6100R and Gabra6100Q alleles have been found to segregate into ethanol-hyposensitive and -hypersensitive groups, respectively. For the Sardinian nonpreferring rats, it is a partial segregation25, whereas for the alcohol tolerant (AT)/alcohol non-tolerant (ANT) line, almost all of the ANT rats are homozygous for the Gabra6100Q allele24. However, because the α6-R100Q polymorphism had not been shown to affect ethanol sensitivity of recombinant receptors24 and because backcrosses of ANT/AT rats had led researchers to question the correlation between this polymorphism and the ethanol-hypersensitive phenotype, it has been concluded that genetic differences other than the one causing the α6-R100Q polymorphism were important27,38.
It remains to be determined whether the α6 polymorphism accounts for all aspects of the ANT phenotype and whether it contributes to complex behaviors that do not obviously involve the cerebellum (such as alcohol preference in the Sardinian line or anxiety in ANT rats39). In these selectively bred lines, control and experimental groups of rats are typically isolated by more than 40 generations of breeding, a strategy likely to segregate multiple genetic loci. In the present study, control (Gabra6100R/100R) and experimental (Gabra6100Q/100Q) groups were identified only by genotype with respect to this one gene. As is the case for all knockout and ‘knock-in’ animal studies, we cannot completely exclude the possibility that other alleles near the genetic locus of interest cosegregate and contribute to cellular and behavioral phenotypes. However, considered with the positive evidence provided here that α6-100Q markedly increases ethanol sensitivity of recombinant α6β3δ receptors and tonic current in cerebellar granule cells, it seems very likely that the α6-100Q polymorphism is responsible for the additional ethanol-induced motor impairment.
Our findings suggest that mice deficient in the Gabra6 gene product might show less ethanol-induced motor impairment. High doses of ethanol (2 g kg−1) have been reported to similarly impair wild-type and Gabra6−/− mice in rotarod tests40, and at ethanol doses of 3.5 g kg−1, no significant differences have been observed between wild type and Gabra6−/− in a LORR test41,42. At such high ethanol doses, we also find no change for Gabra6100R/100R and Gabra6100Q/100Q rats in rotarod performance (data not shown) or in LORR (Fig 5e,f). It is possible that Gabra6−/− mice will show less impairment on the rotarod in a lower range of ethanol doses, but there are reasons to believe that ethanol sensitivity of knockout mice may be complicated. First, the expression of GABAR subunits α1, β2, γ2 and δ is changed markedly in Gabra6−/− mice19,36,43, and other potential ethanol targets such as two-pore domain K+ channels20 show increased function in granule cells, making it difficult to rule out compensatory changes in Gabra6−/− mice. Second, differences in cerebellum-dependent behavior at moderate ethanol doses may be obscured in the Gabra6−/− mouse by other more abundant and widely expressed ethanol targets. These and other high-affinity ethanol targets may make it more difficult to detect behavioral changes in animals lacking the α6 gene than in animals having the ethanol-hypersensitive polymorphism, in which very low doses of ethanol selectively impair motor behavior (as in Fig. 5b).
The observation that a specific combination of GABAR subunits forms an important ethanol target in cerebellum is similar to recent results showing that certain anesthetics and benzodiazepines act on heteromeric GABARs composed of particular subunits. Some of these studies make use of mice engineered to carry single amino acid changes in positions homologous to the R100Q site in α6. Point mutations at these sites, which are known to form part of a high-affinity binding pocket for benzodiazepines at the α-γ2 interface in the pentameric receptor, render GABARs containing those particular subunits insensitive to benzodiazepines. The resulting ‘knock-in’ mice show specific behavioral insensitivities in response to diazepam13, implying that important clinical properties of diazepam such as sedation44,45, amnesia44 and anxiolysis46 are attributable to GABARs containing particular α subunits. A similar strategy involving mice carrying a point mutation in β3 shows that this subunit is necessary for the anesthetic actions of etomidate47. The naturally occurring α6-R100Q mutation (polymorphism) in rats allowed us to examine the roles of α6 subunits and of granule cells in ethanol-induced impairment of cerebellar function. Like the mice with experimentally introduced (‘knock-in’) point mutations discussed above, these rats should prove to be useful tools for examining the role of α6 in certain features of ethanol and benzodiazepine intoxication.
Ethanol sensitivity of other extrasynaptic GABARs
Extrasynaptic GABARs composed of α4, β3 and δ subunits are likely to be found in neurons located in the thalamus3, dentate gyrus21, striatum14,15 and cerebral cortex15. As such GABARs are also sensitive to low ethanol concentrations9,10,48, we predict that ethanol-induced increases in tonic inhibition in these neuronal populations may contribute to sedative-hypnotic and anxiolytic effects and to the depressant actions of this drug on higher cognitive functions.
METHODS
Electrophysiology
Standard methods were used for isolation, injection and recordings from X. laevis oocytes and for preparation of cRNA10. For brain slice experiments, 300 μm parasagittal slices of cerebellum were prepared from 24- to 42-day-old Sprague-Dawley rats using standard techniques20,21 with the exceptions that slicing solution consisted of (in mM) 250 sucrose, 26 NaHCO3, 10 glucose, 4 MgCl2, 3 myoinositol, 2.5 KCl, 2 sodium pyruvate, 1.25 NaH2PO4, 0.5 ascorbic acid, 0.1 CaCl2, and 0.001 D,L-APV. Slice storage and recording solutions were saturated with 95% O2/5% CO2 and consisted of (in mM) 119 NaCl, 26 NaHCO3, 11 glucose, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, and 1 NaH2PO4; in addition, storage solution contained 0.001 D,L-APV. All procedures were in accordance with a protocol approved by the University of California at Los Angeles (UCLA) Chancellor’s Animal Research Committee. For voltage-clamp recordings (holding potential −70 mV, 20–23 °C), whole-cell pipettes contained (in mM) 140 CsCl, 10 HEPES, 1 EGTA, 4 magnesium ATP, 0.4 GTP, titrated to pH 7.3 with CsOH. Recording pipettes had a bath resistance of 6–10 MΩ.
Whole-cell data were filtered at 5 kHz and acquired at a sampling rate of 20 kHz. Analysis was conducted using customized routines written in IGOR Pro 4.0 (Wavemetrics). Tonic GABAR-mediated current was defined as the steady-state current blocked by 10 μM SR95531; its magnitude was calculated by plotting all-point histograms of relevant 30-s segments of data (as in Figs. 3,4). These data were fit to the Gaussian equation, constraining fits to values 2 bins more negative than the peak. This ensured that the tail of higher amplitude values (representing sIPSCs) did not influence the fit. The effects of 10 mM ethanol on tonic current (Fig. 4) were compared with changes in tonic current observed over otherwise identical sham perfusion periods.
Genotyping
After isolation of genomic DNA from ear snips, the exon coding for the α6-100 position was amplified with primers designed to be located in introns flanking the region of interest (to avoid amplification of mRNA). The PCR fragment was sequenced using standard fluorescent dye sequencing.
Behavior
Rats were housed with food and water ad libitum in a 12 h/12 h light/dark cycle. Homozygous male and female rats (Gabra6100R/100R and Gabra6100Q/100Q, > P55) were used for the rotarod (MedAssociates) and sleep time (LORR) studies. These rats were either obtained directly from a breeding colony at Charles River Laboratories or bred at UCLA. In the accelerating rotarod test, the speed of rotation increases at a constant rate from 4 to 40 r.p.m. over 5 min. All rats used in the rotarod tests were naive to ethanol and were used to test only one condition. Blood samples (20–50 μl) were taken from the tail and serum ethanol concentration was determined with an Analox enzymatic blood alcohol analyzer.
Statistics
Values are reported as mean ± s.e.m. unless noted otherwise. To evaluate the effect of ethanol dose and time in the rotarod experiments, we used a general linear model (GLM) with repeated measures. The Wilk’s lambda multivariate test was used to test the effect of the ethanol dose–time interaction. These statistical analyses were conducted using SPSS for Windows version 12. All other statistical comparisons were conducted using paired and unpaired Student’s t-tests as appropriate.
Acknowledgments
We thank C. Gundersen and the UCLA Anesthesiology Department for providing X. laevis oocytes, A. Taylor and D. Tio for help with blood alcohol analysis, and K. Olofsdotter-Otis for helpful comments on the manuscript. The work was supported by a Human Frontiers Science Program Long Term Fellowship to P.D.D. and by US National Institutes of Health grants AA015460 to H.J.H, NS41651 to T.S.O., and NS35985 and AA07680 to R.W.O.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Vallee BL. Alcohol in the western world. Sci Am. 1998;278:80–85. doi: 10.1038/scientificamerican0698-80. [DOI] [PubMed] [Google Scholar]
- 2.Davies AG, et al. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi T, et al. Ethanol opens G-protein-activated inwardly rectifying K+ channels. Nat Neurosci. 1999;2:1091–1097. doi: 10.1038/16019. [DOI] [PubMed] [Google Scholar]
- 4.Lewohl JM, et al. G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci. 1999;2:1084–1090. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- 5.Hodge CW, et al. Supersensitivity to allosteric GABAA receptor modulators and alcohol in mice lacking PKCε. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- 6.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- 7.Mihic SJ, et al. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 8.Suzdak PD, et al. A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science. 1986;234:1243–1247. doi: 10.1126/science.3022383. [DOI] [PubMed] [Google Scholar]
- 9.Sundstrom-Poromaa I, et al. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ GABAA receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi DS, et al. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci. 2004;7:855–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 13.Mohler H, Crestani F, Rudolph U. GABAA-receptor subtypes: a new pharmacology. Curr Opin Pharmacol. 2001;1:22–25. doi: 10.1016/s1471-4892(01)00008-x. [DOI] [PubMed] [Google Scholar]
- 14.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 15.Peng Z, et al. GABAA receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- 16.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campos ML, de Cabo C, Wisden W, Juiz JM, Merlo D. Expression of GABAA receptor subunits in rat brainstem auditory pathways: cochlear nuclei, superior olivary complex and nucleus of the lateral lemniscus. Neuroscience. 2001;102:625–638. doi: 10.1016/s0306-4522(00)00525-x. [DOI] [PubMed] [Google Scholar]
- 18.Saxena NC, Macdonald RL. Properties of putative cerebellar GABAA receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- 19.Nusser Z, et al. Alterations in the expression of GABAA receptor subunits in cerebellar granule cells after the disruption of the α6 subunit gene. Eur J Neurosci. 1999;11:1685–1697. doi: 10.1046/j.1460-9568.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- 20.Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- 21.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi DJ, Hamann M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity α6 subunit GABAA receptors and glomerular geometry. Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- 23.Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- 24.Korpi ER, Kleingoor C, Kettenmann H, Seeburg PH. Benzodiazepine-induced motor impairment linked to point mutation in cerebellar GABAA receptor. Nature. 1993;361:356–359. doi: 10.1038/361356a0. [DOI] [PubMed] [Google Scholar]
- 25.Saba L, et al. The R100Q mutation of the GABAA α6 receptor subunit may contribute to voluntary aversion to ethanol in the sNP rat line. Brain Res Mol Brain Res. 2001;87:263–270. doi: 10.1016/s0169-328x(01)00003-1. [DOI] [PubMed] [Google Scholar]
- 26.Farrant M, Cull-Candy S. GABA receptors, granule cells and genes. Nature. 1993;361:302–303. doi: 10.1038/361302a0. [DOI] [PubMed] [Google Scholar]
- 27.Radcliffe RA, et al. Behavioral characterization of alcohol-tolerant and alcohol-non-tolerant rat lines and an f(2) generation. Behav Genet. 2004;34:453–463. doi: 10.1023/B:BEGE.0000023650.32243.39. [DOI] [PubMed] [Google Scholar]
- 28.Wisden W, Korpi ER, Bahn S. The cerebellum: a model system for studying GABAA receptor diversity. Neuropharmacology. 1996;35:1139–1160. doi: 10.1016/s0028-3908(96)00076-7. [DOI] [PubMed] [Google Scholar]
- 29.Poltl A, Hauer B, Fuchs K, Tretter V, Sieghart W. Subunit composition and quantitative importance of GABAA receptor subtypes in the cerebellum of mouse and rat. J Neurochem. 2003;87:1444–1455. doi: 10.1046/j.1471-4159.2003.02135.x. [DOI] [PubMed] [Google Scholar]
- 30.Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J Physiol (Lond) 1995;485:419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Overstreet LS, Westbrook GL. Paradoxical reduction of synaptic inhibition by vigabatrin. J Neurophysiol. 2001;86:596–603. doi: 10.1152/jn.2001.86.2.596. [DOI] [PubMed] [Google Scholar]
- 33.Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- 34.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- 36.Jones A, et al. Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- 38.Korpi ER, et al. Cerebellar GABAA receptors in two rat lines selected for high and low sensitivity to moderate alcohol doses: pharmacological and genetic studies. Alcohol. 1992;9:225–231. doi: 10.1016/0741-8329(92)90058-i. [DOI] [PubMed] [Google Scholar]
- 39.Vekovischeva OY, Haapalinna A, Sarviharju M, Honkanen A, Korpi ER. Cerebellar GABAA receptors and anxiolytic action of diazepam. Brain Res Mol Brain Res. 1999;837:184–187. doi: 10.1016/s0006-8993(99)01691-1. [DOI] [PubMed] [Google Scholar]
- 40.Korpi ER, et al. Cerebellar granule-cell-specific GABAA receptors attenuate benzodiazepine-induced ataxia: evidence from α6-subunit-deficient mice. Eur J Neurosci. 1999;11:233–240. doi: 10.1046/j.1460-9568.1999.00421.x. [DOI] [PubMed] [Google Scholar]
- 41.Homanics GE, et al. Mice devoid of GABAA receptor β3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc Natl Acad Sci USA. 1997;94:4143–4148. doi: 10.1073/pnas.94.8.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Homanics GE, et al. Gene knockout of the α6 subunit of the GABAA receptor: lack of effect on responses to ethanol, pentobarbital, and general anesthetics. Mol Pharmacol. 1997;51:588–596. doi: 10.1124/mol.51.4.588. [DOI] [PubMed] [Google Scholar]
- 43.Uusi-Oukari M, et al. Long-range interactions in neuronal gene expression: evidence from gene targeting in the GABAA receptor β2-α6-α1-γ2 subunit gene cluster. Mol Cell Neurosci. 2000;16:34–41. doi: 10.1006/mcne.2000.0856. [DOI] [PubMed] [Google Scholar]
- 44.Rudolph U, et al. Benzodiazepine actions mediated by specific GABAA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 45.McKernan RM, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 46.Low K, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 47.Jurd R, et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor β3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 48.Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]