This work shows a novel role of PTP1B in the regulation of N-cadherin trafficking. PTP1B is required for the association of p120 to the N-cadherin precursor and this event is crucial for trafficking of the complex through the early stages of the secretory pathway.
Abstract
PTP1B bound to mature N-cadherin promotes the association of β-catenin into the complex, the stable expression of the complex at cell surface, and cadherin-mediated adhesion. Here we show that PTP1B is also required for N-cadherin precursor trafficking through early stages of the secretory pathway. This function does not require association of PTP1B with the precursor. In PTP1B null cells, the N-cadherin precursor showed higher sensitivity to endoglycosidase H than in cells reconstituted with the wild-type enzyme. It also showed slower kinetics of ER-to-Golgi translocation and processing. Trafficking of the viral stomatitis vesicular glycoprotein, VSV-G, however, revealed no differences between PTP1B null and reconstituted cells. N-cadherin precursor complexes contained similar levels of α- and β-catenin regardless of PTP1B expression. In contrast, the associated p120 catenin (p120) was significantly reduced in absence of PTP1B expression. An N-cadherin precursor construct defective in p120 binding, and expressed in PTP1B reconstituted cells, showed higher sensitivity to endoglycosidase H and slower kinetics of processing than the wild-type precursor. Our results suggest that PTP1B promotes the association of p120 to the N-cadherin precursor, facilitating the trafficking of the complex from the ER to the Golgi complex.
INTRODUCTION
N-cadherin is a calcium-dependent cell–cell adhesion molecule expressed at the surface of several neuronal and nonneuronal cells (Derycke and Bracke, 2004). N-cadherin function depends on interaction of its cytoplasmic domain with catenins (α-, β-, and p120-catenin), a process modulated by tyrosine phosphorylation (Lilien and Balsamo, 2005; Alema and Salvatore, 2007). Although the binding of β-catenin and p120 is direct, that of α-catenin is indirect (Ozawa and Kemler, 1992; Hinck et al., 1994, Pokutta and Weiss, 2007).
Protein tyrosine kinases of the Src family, EGFR and c-Met phosphorylate β-catenin decreasing its binding to cadherin (Kinch et al., 1995; Roura et al., 1999; Piedra et al., 2003; Lilien and Balsamo, 2005). In contrast, phosphorylation of p120 by Fer, Src, and Fyn increases the binding to cadherin (Roura et al., 1999; Piedra et al., 2003; Castano et al., 2007).
PTP1B is a nonreceptor protein tyrosine phosphatase associated with the cytosolic face of endoplasmic reticulum (ER) membranes by a hydrophobic amino acid sequence of the C-terminus (Frangioni et al., 1992). The catalytic domain of PTP1B is oriented to the cytosol where it can potentially interact with proteins at this compartment. Indeed, ER-bound PTP1B dephosphorylates several growth factor receptors, either during their biosynthetic route to the cell surface or after being endocytosed (Haj et al., 2002; Boute et al., 2003; Romsicki et al., 2004; Cohen et al., 2004). In addition, PTP1B binds directly to the cytosolic domain of mature N-cadherin and dephosphorylates β-catenin in the complex, increasing the stability of the N-cadherin–actin linkage, and promoting N-cadherin–mediated adhesion (Balsamo et al., 1998; Rhee et al., 2001; Xu et al., 2002, 2004; Lilien and Balsamo, 2005). Similar function of PTP1B was observed in epithelial cells expressing E- or VE-cadherin (Sheth et al., 2007; Winter et al., 2008; Nakamura et al., 2008). A role of PTP1B in the dephosphorylation of p120 was recently suggested. Knockdown of PTP1B in Colo 205 cells increases the phosphorylation state of p120 (Ezaki et al., 2007). In addition, a comparative phospho-proteomic analysis between wild-type (WT) and PTP1B-deficient fibroblasts identified peptides derived from p120 that were hyperphosphorylated in PTP1B-deficient cells (Mertins et al., 2008). However, the functional consequences of this regulation were not assessed.
Cadherins are synthesized in ER-bound ribosomes as precursor proteins, with a prodomain at the N-terminus that must be removed to generate mature and adhesive competent proteins (Ozawa, 2002; Koch et al., 2004). Prodomain removal occurs as a post-Golgi event, by a furin subgroup of proprotein convertases (Posthaus et al., 1998; Wahl et al., 2003). Early after biosynthesis, cadherin precursors associate with β-catenin and p120 (Ozawa and Kemler, 1992; Hinck et al., 1994; Wahl et al., 2003; Curtis et al., 2008). Mutations impairing the binding of β-catenin led to retention of E-cadherin in the ER and to an inefficient expression at the cell surface (Chen et al., 1999). On the other hand, mutations impairing the binding of p120 led to delayed recruitment of N-cadherin at cell–cell contacts during calcium-initiated junction reassembly (Chen et al., 2003). This effect was attributed to inefficient coupling of the N-cadherin complex with kinesins, which are required for transport of N-cadherin from Golgi to the cell surface (Mary et al., 2002; Chen et al., 2003; Yanagisawa et al., 2004; Teng et al., 2005). At the cell surface, p120 bound to cadherin prevents its endocytosis and degradation (Davis et al., 2003; Xiao et al., 2003, 2007; Kowalczyc and Reynolds, 2004).
When N-cadherin constructs deleted in the PTP1B binding site are transfected in L-cells, which do not express endogenous cadherins, they are not expressed efficiently at the cell surface and accumulate intracellularly (Xu et al., 2002). This prompted us to assess the role of PTP1B in N-cadherin trafficking. Using PTP1B null cells, and derivative lines reconstituted with WT PTP1B (Haj et al., 2002), we have defined a novel regulatory role of PTP1B that promotes trafficking of the N-cadherin precursor through early stages of the secretory pathway. This function of PTP1B does not require its association with the cadherin precursor. We found that PTP1B is required for association of p120 to the N-cadherin precursor, an event that is crucial for progression of the complex through the early stages of the secretory pathway. This function of PTP1B differs from its previously described effect on β-catenin regulation at cell surface N-cadherin complexes (Lilien and Balsamo, 2005).
MATERIALS AND METHODS
Antibodies and Reagents
Monoclonal antibodies against PTP1B, β-catenin, phosphotyrosine (clone PY20), and N-cadherin were from BD Transduction Laboratories (Lexington, KY). Polyclonal anti-β-catenin was from W. J. Nelson (Stanford University). Monoclonal anti-p120 (clone 6H11) was from A. B. Reynolds (Vanderbilt University). High-affinity monoclonal anti-hemagglutinin (HA; clone 3F10) was from Roche (Penzberg, Germany). Affinity-purified biotin conjugated goat anti-mouse, streptavidin-agarose and monoclonal antibodies against HA (clone HA-7), c-myc (clone 9E10), α-catenin, and the ectodomain of N-cadherin (clone GC-4) were from Sigma-Aldrich (St. Louis, MO). mAb specific for chicken N-cadherin (NCD-2; Balsamo et al., 1998) was a gift from Jack Lilien (University of Iowa). Monoclonal anti-Xpress was from Invitrogen (Carlsbad, CA). Rhodamine-phalloidin, Alexa-488– and Alexa-568–conjugated fluorescent goat secondary antibodies were from Molecular Probes (Eugene, OR). Protein G-Sepharose was from GE Healthcare (Uppsala, Sweden). Horseradish peroxidase (HRP)-conjugated goat anti-mouse and aminomethylcoumarin acetate (AMCA)-conjugated streptavidin were from Jackson ImmunoResearch (West Grove, PA).
DNA Constructs
PTP1B constructs used in this work were previously described (Hernandez et al., 2006). HA-N-cadherin-GFP was obtained by PCR, adding the HA epitope (YPYDVPDYA) into the propeptide (between Ala-30 and Thr-31) of chick N-cadherin fused at the C-terminus with the enhanced green fluorescent protein (pEGFP-N1, Clontech BD Biosciences, Mountain View, CA). To obtain N-cadherinΔ878-891-GFP, a fragment KpnI/ApaI containing the deletion was excised from the pCMV-N-cadherinΔ878-891 and replaced into the N-cadherin-GFP (Xu et al., 2002). To obtain the HA-N-cadherinΔ884-891-GFP, a fragment KpnI/ApaI containing the deletion was excised from pCMV-N-cadherinΔ884-891 (Xu et al., 2002) and replaced into the HA-N-cadherin-GFP construct. Murine N-cadherin-3A-yellow fluorescent protein (YFP; Chen et al., 2003) was a kind gift from K. Green (Northwestern University, Chicago, IL). HA-N-cadherin-3A-YFP was prepared by PCR insertion of HA epitope between Ala-25 and Ser-26 of the propeptide. The cDNAs encoding JMP and JMP-3A were amplified by PCR using chick N-cadherin-GFP and mouse N-cadherin-3A-YFP as templates, respectively. PCR fragments were subcloned into EcoRI and BamHI sites of pcDNA 3.1. Both peptides contain the X-press epitope at the N-terminus and have 88 residues of the juxtamembrane domain (JMP starts at Lys-757 and ends at Glu-844, and JMP-3A starts at Lys-751 and ends at Glu-838). All constructs were confirmed by DNA sequencing. Vesicular stomatitis virus glycoprotein (VSV-G) tsO45–3X myc was kindly provided by J. C. Hay (University of Montana, Missoula, MT). β-Catenin constructs were a kind gift of M. Duñach (Universidad Autónoma de Barcelona, Barcelona, Spain). Sial-T2-HA and GalNacT-DsRed were provided by J. L. Daniotti and H.J.F. Maccioni (Centro de Investigaciones en Química Biológica de Córdoba, Universidad Nacional de Córdoba, Argentina).
Cell Culture, DNA Transfection, and Drug Incubations
PTP1B null cells and derivative lines reconstituted with WT or the substrate trapping PTP1B DA was previously described (Haj et al., 2002) and was provided by B. G. Neel (Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA). Cells were cultured in high-glucose DMEM containing l-glutamine, supplemented with 10% fetal bovine serum, penicillin, and streptomycin (Invitrogen, Carlsbad, CA). Transient transfections were performed in 24-well tissue-culture plates (1.5 μg DNA/plasmid/well) or in 60-mm dishes (10 μg/plasmid/dish) using Lipofectamine 2000 (Invitrogen). Brefeldin A (BFA, Sigma-Aldrich) was used at 5 μg/ml and incubated (30 min) with cells in complete DMEM. For traffic recovery, BFA was washed with three changes of medium, and then cells were incubated for the times indicated. When indicated, cells were preincubated for 30 min with 0.1 mM sodium pervanadate before processing for immunofluorescence or immunoprecipitation. Sodium pervanadate was prepared fresh by incubating 50 mM sodium orthovanadate with 50 mM H2O2 in 20 mM HEPES buffer, pH 7.3, for 30 min at room temperature.
Pulse Chase and Biotinylation
Cells (5 × 105) were seeded in 60-mm dishes and were grown for 48 h. Thirty minutes before labeling, the medium was replaced by serum-free DMEM without l-methionine and l-cysteine, and then cells were labeled (20 min) with 250 μCi/dish of [35S]methionine (1175 Ci/mmol; NEG-772 EasyTag express, PerkinElmer, Boston, MA). After washing three times with regular culture medium cells were incubated for different times. At the end of each chasing period cells were chilled on ice, and cell surface proteins were labeled for 30 min with 0.8 mg/ml EZ-Link sulfo-NHS-LC-Biotin (Pierce Chemical, Rockford, IL). After washing with PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4, pH 7.4) containing 100 mM glycine, cells were processed for immunoprecipitation of N-cadherin complexes.
Immunoprecipitation and Western Blots
Soluble protein extracts were prepared by incubation (30 min) of cells on ice with lysis buffer (20 mM Tris-HCl, pH 7.4, 137 mM NaCl, 1% Triton X-100, 1 mM PMSF, 10 μg/ml leupeptin, 5 μg/ml aprotinin, 2.5 mM NaVO3, and 10 mM NaF). Dishes were scrapped, and the cell suspension was centrifuged at 13,000 × g for 10 min at 4°C. About ∼1 mg of supernatant protein was sequentially incubated at 4°C with 2 μg/ml primary monoclonal antibodies (3 h), and protein G-Sepharose (1.5 h). Immunocomplexes were washed with lysis buffer and boiled in SDS-PAGE sample buffer. Supernatants were fractionated by SDS-PAGE and transferred to polyvinyl difluoride membranes (Millipore, Bedford, MA). Blots were probed with primary antibodies followed by HRP-conjugated second antibodies and revealed by enhanced chemiluminescence. For stripping, blots were incubated (30 min, 55°C) with Tris-buffered saline (TBS) containing 5% 2-mercaptoethanol and 2% SDS, blocked, and reprobed. Soluble proteins from metabolically labeled and cell surface–biotinylated cells were immunoprecipitated with a monoclonal anti-N-cadherin. To isolate the fraction of cell surface N-cadherin, half of the immunoprecipitated beads were boiled 3 min in lysis buffer containing 1% SDS, the supernatant was diluted with 900 μl of TBS, and the biotinylated N-cadherin was pulled down using streptavidin-agarose. Total and cell surface N-cadherin was analyzed by SDS-PAGE followed by fluorography using DMSO-PPO (2,5-diphenyloxazole). Semiquantitative analysis of the signal intensity of the bands was performed after scanning Rx films. Integrated optical densities of bands were determined using the routine to analyze one-dimensional electrophoretic gels from ImageJ (http://rsb.info.nih.gov/ij/; Wayne Rasband, NIH, Bethesda, MD).
Endoglycosidase-H Treatments
Forty hours after transfection, cells expressing HA-tagged N-cadherin constructs were processed for immunoprecipitation with anti-HA antibodies. Immunoprecipitates were resuspended in endoglycosidase-H (endo-H) denaturing buffer (0.5% SDS, 40 mM DTT) and heated at 100°C for 10 min. Then, 1/10 volume of 0.5 M sodium citrate, pH 5.5, was added. Samples were split into halves and incubated with/without 500 U of endo-H according to the manufacturer's instructions (New England Biolabs, Beverly, MA). Cells transfected with VSV-G tsO45-myc were incubated for 16 h at 40°C. Then, temperature was shifted to 32°C, and the cells were incubated for the times indicated. VSV-G tsO45-myc was immunoprecipitated and processed as described previously.
Immunofluorescence
Cells grown on fibronectin-coated coverslips (20 μg/ml) were fixed with 4% paraformaldehyde in PBS for 20 min, permeabilized with 0.5% Triton X-100 in PBS for 10 min, and blocked with 3% BSA in PBS for 60 min. Primary antibodies were incubated overnight at 4°C, and fluorescently conjugated secondary antibodies 45 min at room temperature. Cells were mounted in Vectashield (Vector Laboratories, Burlingame, CA). For qualitative assessment cells were analyzed with a 100×/1.4 NA objective in a Nikon E600 microscope (Melville, NY) coupled to a Spot RT Slider CCD camera (Diagnostic Instruments, Sterling Heights, MI), or with a 60×/1.4 NA objective on a Bio-Rad MRC 1024 laser scanning confocal microscope (Hercules, CA). For quantitative analysis, cells were analyzed with a 60×/1.4 NA objective in a Nikon TE2000 coupled to a Hamamatsu Orca AG 12-bit camera (Hamamatsu Photonics, Hamamatsu, Japan).
Image Quantifications
Twelve-bit images were processed using ImageJ. Only images with the relevant fluorescence signal below the saturation level were used for quantification. Background fluorescence was subtracted from noncellular regions.
Analysis of ER–Golgi Transport after BFA Washout.
To quantify the redistribution of N-cadherin-GFP into the perinuclear Golgi location at different times after BFA washout, we followed the following procedure. At each time point, two regions of interest (ROIs) were drawn at peripheral locations and the corresponding mean fluorescence values were averaged. This value represents an estimation of the N-cadherin-GFP at non-Golgi locations (ER plus plasma membrane). Golgi-associated fluorescence was determined from ROIs drawn at perinuclear regions. Golgi formation was also monitored by cotransfection with Sial-T2, a sialyl transferase that localizes in Golgi (Daniotti et al., 2000).
Ratio Analysis of Trafficking.
To estimate trafficking of HA-N-cadherin-GFP and derivatives constructs we analyzed HA/GFP fluorescence ratios from intracellular puncta. Puncta represent trafficking carriers, as judged by time lapse and colocalization analysis (not shown). The HA epitope was detected by immunolabeling using Alexa 568 as fluorescent secondary antibodies (red channel). Because the HA-containing prodomain is removed as a single step at post-Golgi locations, we predicted that carriers originated before this location have higher HA/GFP fluorescence ratios than post-Golgi carriers. ROIs were manually drawn around puncta, and the mean intensity values were calculated for both the red (HA label) and green (GFP label) channels. Images with pixel misalignments were discarded. The fluorescence contribution of the N-cadherin-GFP located at the plasma membrane was estimated averaging the mean intensity values of two to three ROIs drawn at ER-free thin peripheral areas of the cell. This value was subtracted from all ROIsGFP. Values from all ROIHA and ROIGFP pairs were transferred to Excel sheets (Microsoft, Redmond, WA) for calculation of HA/GFP ratios. In the case of BFA-treated cells, in which puncta were not visible, ROIs were drawn over ER tubules at the cell periphery using the HA image. HA/GFP ratios were analyzed in frequency plots.
Analysis of Junctional Cadherin.
To evaluate the effect of JMP and JMP-3A constructs on N-cadherin-GFP distribution, pairs of transfected cells in contact with each other were analyzed. For each cell of the pair two nonoverlapping ROIs were drawn; one that enclosed the intercellular junction (junctional fluorescence) and the other included the remaining area of the cell (intracellular fluorescence). Mean fluorescence values were used to calculate junctional/intracellular ratios.
Aggregation Assays
Cells were washed with HBSGK (20 mM HEPES, pH 7.4, 150 mM NaCl, 2 mM glucose, and 3 mM KCl) and resuspended with 0.01% trypsin in HBSGK containing 1 mM CaCl2. After trypsin neutralization with soybean trypsin inhibitor, cells were washed and collected by centrifugation. The cell pellet was resuspended with HBSGK containing 10 μg/ml DNAse. Dispersed cells were seeded in 24-well plates (1.5 × 105 cells/well) previously blocked with 1% BSA and incubated with or without additives (1 mM CaCl2 or 1 mM EDTA) for 30 min at 37°C with constant rotation at 90 rpm. Samples at the start and at the end of the incubation period were fixed, and the number of particles immediately was assessed by phase-contrast microscopy. The extent of aggregation was represented by the N30/N0 index (Takeichi, 1977), where N0 is the number of particles at t0 and N30 the number of particles after 30 min.
RESULTS
ER-bound PTP1B Associates with N-Cadherin at Cell–Cell Junctions But Not with the Intracellular Precursor
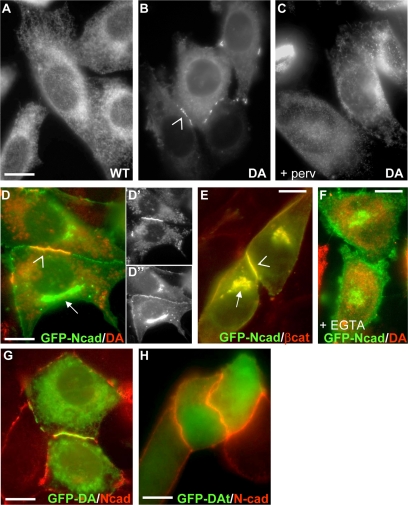
Previous studies have shown that PTP1B binds directly to the cytoplasmic domain of cell surface N-cadherin, ensuring β-catenin dephosphorylation and promoting N-cadherin–mediated adhesion (Balsamo et al., 1998; Rhee et al., 2001; Xu et al., 2002, 2004). Because PTP1B is tail-anchored to the ER, it may also potentially bind to the cytoplasmic domain of N-cadherin precursors while they travel from the ER to the cell surface. To investigate this possibility, we analyzed the colocalization of intracellular N-cadherin with either the WT PTP1B or the substrate trapping mutant PTP1B-D181A. Because of its capacity of forming long-lived complexes with substrates, PTP1B-D181A has proved to be crucial for detection, by FRET and BRET techniques, of direct interactions between PTP1B and different growth factor receptors at the surface of the ER (Haj et al., 2002; Boute et al., 2003; Romsicki et al., 2004). A PTP1B null cell line stably reconstituted with WT PTP1B (WT-cells) shows the typical distribution of PTP1B at the ER (Figure 1A), as previously reported (Haj et al., 2002; Hernandez et al., 2006). However, null cells stably reconstituted with PTP1B-D181A (DA-cells) show that in addition to its ER localization, the PTP1B-D181A also accumulates at cell–cell boundaries (Figure 1B, compare with A). This accumulation is prevented by preincubation with pervanadate, which inactivates the active site of the enzyme (Figure 1C). Because WT- and DA-cells express similar levels of PTP1B (Hernandez et al., 2006), our results suggest the particular distribution of PTP1B-D181A is due to its trapping by substrates at intercellular junctions.
Figure 1.
ER-bound PTP1B distributes with N-cadherin at cell–cell junctions and not at intracellular locations. Cells stably expressing (A) wild-type (WT) PTP1B or (B and C) substrate trapping mutant D181A PTP1B (DA) were fixed and immunostained for PTP1B. DA accumulates at cell–cell junctions (B, arrowhead), and this effect is inhibited by preincubation of cells with 0.1 mM sodium pervanadate 30 min before fixation (C). DA-cells transfected with N-cadherin-GFP were immunostained for PTP1B (D, D′, D″, and F) or β-catenin (E). Note that N-cadherin colocalizes with PTP1B in junctions (arrowhead in D) but not in the Golgi complex (arrow in D). In contrast, N-cadherin colocalizes with β-catenin in junctions (arrowhead in E) and in the Golgi complex (arrow in E). The colocalization of DA and N-cadherin-GFP at the cell periphery is lost by EGTA preincubation (F). KO-cells transfected with GFP fusions of DA (G), or DA truncated at the ER targeting sequence (DAt; H) were immunostained for N-cadherin. Note the loss of accumulation of the trap mutant at junctions when its targeting to the ER is prevented. Bars, 20 μm.
To examine whether PTP1B interacts with intracellular N-cadherin in transit to the cell surface, we analyzed their colocalization in DA-cells transfected with N-cadherin-GFP. When overexpressed, newly synthesized N-cadherin-GFP accumulates to some extent at the Golgi complex (Figure 2, H-K). This intracellular pool of N-cadherin-GFP never colocalized with PTP1B-D181A, in clear contrast to the tight overlap of both proteins at cell–cell boundaries (Figure 1, D–D″). As expected, immunolabeling of β-catenin, which associates with the cadherin precursor shortly after its biosynthesis (Ozawa and Kemler, 1992; Hinck et al., 1994; Chen et al., 1999; Wahl et al., 2003; Curtis et al., 2008), tightly colocalizes with N-cadherin-GFP at both Golgi and cell–cell junctions (Figure 1E). These results suggest that PTP1B does not bind to the N-cadherin precursor, but it can be recruited to junctional N-cadherin complexes. Indeed, disruption of junctional N-cadherin with EGTA leads to the dispersal of PTP1B-D181A from cell–cell boundaries (Figure 1F). The ER anchor is also required for the accumulation of the trap at cell–cell boundaries because the distribution of GFP-PTP1B-D181A/t381, a truncation mutant that terminates at glutamic acid 381, removing just the ER-targeting sequence (Hernandez et al., 2006), becomes uniform and did not accumulate at intercellular junctions, as it did the full-length counterpart (Figure 1, G and H). Thus, ER anchor serves as a platform to position PTP1B at peripheral regions of the cell (Hernandez et al., 2006; Fuentes and Arregui, 2009).
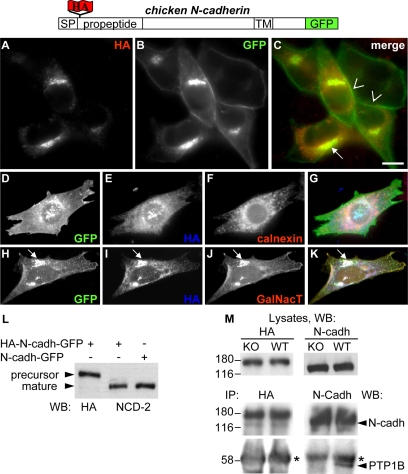
Figure 2.
PTP1B does not bind to the N-cadherin precursor. HA-N-cadherin-GFP was transfected in WT-cells, fixed and immunolabeled with anti-HA (A–C). Note that HA and GFP label codistribute at intracellular locations but not at cell–cell junctions where only GFP fluorescence is visible. The intracellular accumulation of HA and GFP label colocalize with the ER marker calnexin (D–G) and cotransfected GalNacT-DsRed at the Golgi complex (H–K, arrow). (L) Whole protein extracts from WT cells transfected with HA-N-cadherin-GFP or N-cadherin-GFP were analyzed by Western blots using anti-HA and an mAb specific for chicken N-cadherin (NCD-2). Note that HA specifically labels the precursor HA-N-cadherin-GFP at ∼174 kDa. The NCD-2 detects equivalent levels of mature, processed N-cadherin-GFP at ∼159 kDa. (M) KO- and WT-cells transfected with HA-N-cadherin-GFP were lysed, and the precursor was immunoprecipitated with anti-HA. Alternatively, cell lysates from nontransfected KO- and WT-cells were immunoprecipitated with anti-N-cadherin to isolate the mature N-cadherin (N-cadh). The presence of PTP1B in the complexes was analyzed in Western blots. Note that at equivalent levels of immunoprecipitated HA-containing precursor (left panels) and mature N-cadherin (right panels) the band of PTP1B is only present in complexes of mature N-cadherin at WT-cells. Asterisks mark the IgG heavy chain. Numbers indicate kDa. Blot panels are representative of more than three independent experiments. Bar, 20 μm.
To confirm the lack of association between PTP1B and the N-cadherin precursor in transit to the cell surface, we prepared a construct with the hemagglutinin (HA) epitope inserted into the N-terminal propeptide of chicken N-cadherin-GFP (HA-N-cadherin-GFP; Figure 2). This construct allows for the selective isolation and detection of the N-cadherin precursor using the HA tag, and of the total pool (precursor plus mature) using the C-terminus GFP tag. Expression of HA-N-cadherin-GFP in WT-cells shows that the GFP fluorescence and the HA immunolabel codistribute in intracellular locations, whereas only the GFP fluorescence is detected at cell–cell boundaries (Figure 2, A–C). The intracellular pool of HA-N-cadherin-GFP colocalizes with the ER marker calnexin and with the Golgi marker GalNacT (Giraudo and Maccioni, 2003; Figure 2, D–K). These results indicate that the HA-containing prodomain is efficiently removed from N-cadherin before arrival to the cell surface, in agreement with previous findings (Wahl et al., 2003; Koch et al., 2004). Western blot analysis shows that the anti-HA antibody selectively detects a band of ∼174 kDa corresponding to the HA-N-cadherin-GFP precursor (147-kDa N-cadherin precursor + 27-kDa GFP) and that equivalent levels of processed chicken N-cadherin-GFP is obtained regardless of the presence of the HA tag (Figure 2L). Immunoprecipitation of HA from HA-N-cadherin-GFP transfected KO- and WT-cells does not show PTP1B associated in the complexes (Figure 2M, left panels). In contrast, equivalent amounts of immunoprecipitated mature N-cadherin reveals a consistent amount of PTP1B in the complexes of WT-cells (Figure 2M, right panels). Taken collectively, the colocalization and coimmunoprecipitation analysis show that ER-bound PTP1B associates preferentially to the mature, junctional form of N-cadherin and not to the trafficking precursor.
PTP1B Is Required for Traffic and Function of N-Cadherin
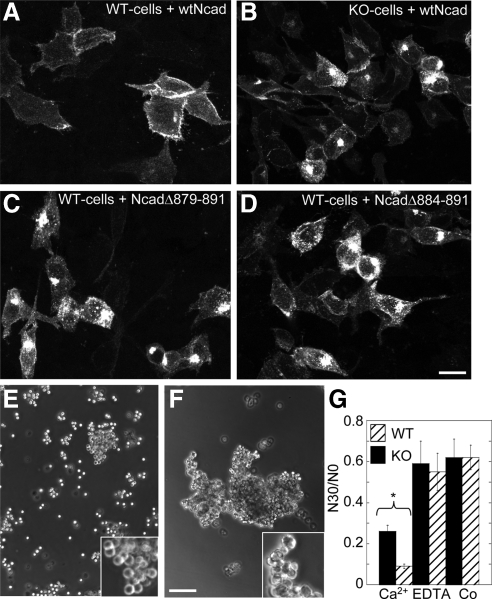
Cadherin-deficient L-cells transfected with N-cadherin constructs that bear deletions in the PTP1B binding site showed impaired localization of the constructs at the cell surface and enhanced accumulation at intracellular pools (Xu et al., 2002). To determine whether PTP1B expression affects the distribution of N-cadherin, KO- and WT-cells were transfected with N-cadherin-GFP, and the fluorescence distribution was analyzed by confocal microscopy. In WT-cells, N-cadherin-GFP localizes most prominently at cell–cell boundaries, with rare intracellular puncta and occasional accumulation in the Golgi apparatus (Figure 3A). In contrast, KO-cells expressing N-cadherin-GFP at equivalent expression levels show frequent accumulation of fluorescence in the Golgi apparatus and in intracellular puncta, in addition to the typical fluorescence in cell–cell boundaries (Figure 3B). A similar fluorescence distribution is observed in WT-cells transfected with two different N-cadherin-GFP constructs deleted in the PTP1B-binding site (Figure 3, C and D). These results suggest that PTP1B is required for traffic and surface expression of N-cadherin-GFP (see below). To evaluate the cell–cell adhesive capacity of WT- and KO-cells, we tested their ability to aggregate in suspension. Although both cell lines form aggregates, those of KO-cells are significantly smaller and less tight than those of WT-cells (Figure 3, E–G, and insets). Thus, expression and binding of PTP1B to the cytoplasmic domain of cadherin promotes the localization and function of N-cadherin at cell–cell junctions.
Figure 3.
PTP1B expression and binding to N-cadherin cytoplasmic domain are required for N-cadherin targeting and function. WT-cells (A, C, and D) and KO-cells (B) transfected with GFP fusions of WT N-cadherin (A and B) or two deletion mutants which cannot bind PTP1B (C and D) were fixed and analyzed by fluorescence confocal microscopy. Note the similar phenotype between KO-cells (B) and WT-cells expressing the deletion mutants (C and D). Both display enhanced accumulation of fluorescence in Golgi and in scattered puncta. (E–G) Aggregation assays. Representative phase-contrast fields showing cell suspensions of KO-cells (E) and WT-cells (F) after 30-min incubation in HBSGK-1 mM CaCl2. Note that KO-cells form smaller and looser aggregates than WT-cells (E, F, and insets). Quantification of the aggregation in HBSGK alone (Co), or in the presence of 1 mM CaCl2 or 1 mM EDTA (see Materials and Methods) is shown in the graph (G). Data are means ± SEM of four experiments. Asterisk denotes significant difference (Student's t, p = 0.001). Bars, (A–D) 60 μm; (E and F) 150 μm.
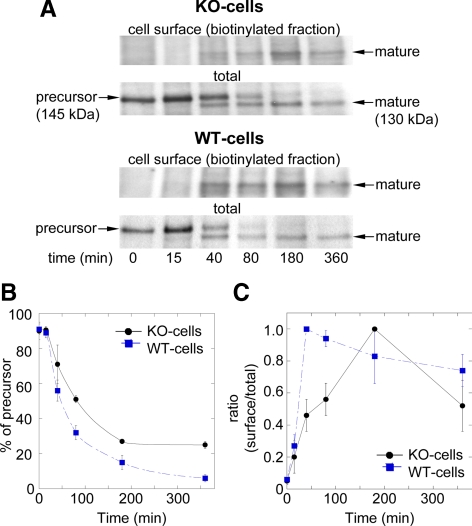
To determine the effect of PTP1B in traffic and surface expression of N-cadherin, WT- and KO-cells were pulse-labeled for 20 min with [35S]methionine and then chased for different times. At the end of each chase point, cell surface proteins were labeled with membrane-impermeable sulfo-NHS-LC-biotin. Total N-cadherin was immunoprecipitated and split into halves; one was further reprecipitated with streptavidin-agarose to isolate the surface-labeled N-cadherin pool. Both halves were analyzed by SDS-PAGE followed by fluorography. Immediately after labeling (t0), a band of ∼145 kDa corresponding to the N-cadherin precursor was visible in both KO- and WT-cells, and as expected, was not detected in the biotinylated fraction (Figure 4A). After 15 min of chase the precursor band was gradually converted to a lower molecular weight band of ∼130 kDa, corresponding to the mature N-cadherin. Average values from three to five experiments show that the precursor is processed with a t1/2 of 45 min in WT-cells and 80 min in KO-cells (Figure 4, A and B). In WT-cells, mature N-cadherin is first detected at cell surface after 15 min of chase and reaches 100% at ∼40 min (Figure 4, A and C). In contrast, in KO-cells ∼50% of the total mature N-cadherin reached the cell surface by 40 min, and the 100% was reached at 180 min (Figure 4, A and C). These results show that PTP1B expression is required for the anterograde traffic of the N-cadherin precursor.
Figure 4.
Processing and surface expression of N-cadherin is impaired in KO-cells. (A) KO- and WT-cells were labeled with [35S]methionine and then chased for the indicated times (minutes). At the end of each period, cells were surface biotinylated, lysed, and immunoprecipitated with anti-N-cadherin. Samples were divided in halves with one representing the sum of intracellular and surface pools of N-cadherin (total); the other half was incubated with streptavidin-agarose to isolate biotinylated N-cadherin (surface). All samples were fractionated by SDS-PAGE followed by fluorography. Bands in the films were scanned and processed with ImageJ. (B) The integrated intensity of the signal associated with the band of the precursor (145 kDa) at each time point is expressed as percentage of the total N-cadherin signal (precursor + mature). (C) The integrated intensity of the signal corresponding to mature N-cadherin (130 kDa band) present at the biotinylated fraction (cell surface) is expressed as fraction of the total mature N-cadherin signal (biotinylated + nonbiotinylated). Shown are mean ± SD of three to five independent experiments.
ER-to-Golgi Traffic of N-Cadherin Precursors Requires PTP1B Expression But Not Binding to Their Cytoplasmic Domains
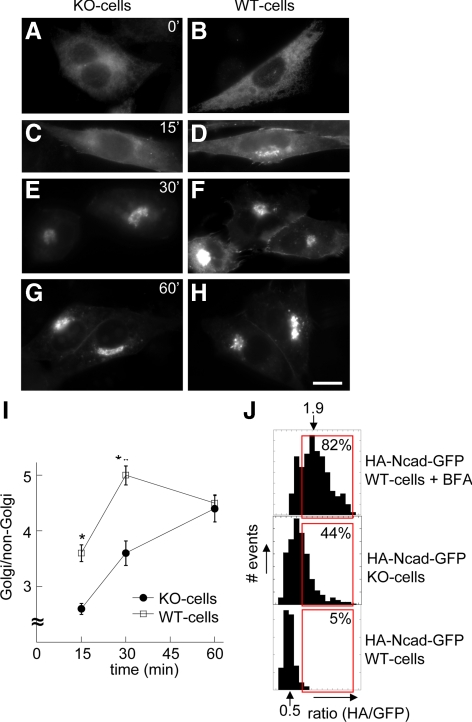
The prodomain removal from N-cadherin precursors is a post-Golgi event (Wahl et al., 2003; Koch et al., 2004; see Figure 2A). In this context, the slower kinetics of N-cadherin maturation occurring in KO-cells suggests that PTP1B regulates an early trafficking step. To examine this possibility, we analyzed the redistribution of N-cadherin-GFP into the Golgi region after BFA recovery. BFA causes redistribution of cis- and medial Golgi components into the ER (Klausner et al., 1992). KO- and WT-cells incubated with BFA shows most of the Golgi Sial-T2 (Supplementary Figure S1; Daniotti et al., 2000) and N-cadherin-GFP dispersed at the ER (Figure 5, A and B). After BFA washout, the kinetics of N-cadherin-GFP accumulation into the Golgi region of WT-cells is faster than that of KO-cells (Figure 5, C–F and I). This difference disappears by 60 min, probably because of the quick export of N-cadherin-GFP from the trans-Golgi in WT-cells (Figure 5, G–I). We confirmed these results by HA/GFP fluorescence ratio analysis of HA-N-cadherin-GFP transfected in KO- and WT-cells. We reasoned that at pre-Golgi locations (before prodomain cleavage), HA/GFP ratios should be higher than at post-Golgi locations (after prodomain cleavage; see Materials and Methods for details). The frequency plot of HA/GFP fluorescence ratios in WT-cells has a Gaussian shape with a mean of 0.5. Gating values higher than two SDs include ∼5% of the whole data set (Figure 5J, bottom plot). As expected, BFA treatment led to a shift of HA/GFP ratios to higher values, with a new mean of 1.9 (Figure 5J, top plot). KO-cells also showed a shift of HA/GFP ratios to higher values, as expected for an accumulation of noncleaved precursors (Figure 5J, middle plot). Applying the previous gate to these distributions includes 82 and 44% of data, respectively.
Figure 5.
BFA recovery kinetics and HA/GFP ratio analysis reveal impaired ER-to-Golgi traffic of N-cadherin precursor in KO-cells. KO- and WT-cells transfected with N-cadherin-GFP were incubated with BFA for 30 min. Then, cells were washed and fixed immediately (A and B) or allowed to recover in medium without BFA for different times (C–H). All samples were analyzed by fluorescence microscopy. At each time point, the mean fluorescence intensity of perinuclear (Golgi) and nonperinuclear (non-Golgi) ROIs were expressed as ratios in the graph (I). Data represent means ± SEM of 70–120 cells from three independent experiments. Asterisks at 15 and 30 min denote p < 0.0001 (Student's t test). (J) HA/GFP fluorescence ratios corresponding to the HA-N-cadherin-GFP construct were expressed in frequency plots (see Materials and Methods for details). Red boxes represent the percentage of values higher than the mean plus two SDs in control WT-cells (bottom plot). This gate was applied to the other datasets. Note a shift of data to higher HA/GFP ratios in WT-cells treated with BFA (top plot) and in KO-cells (middle). Bar in A–H, 20 μm.
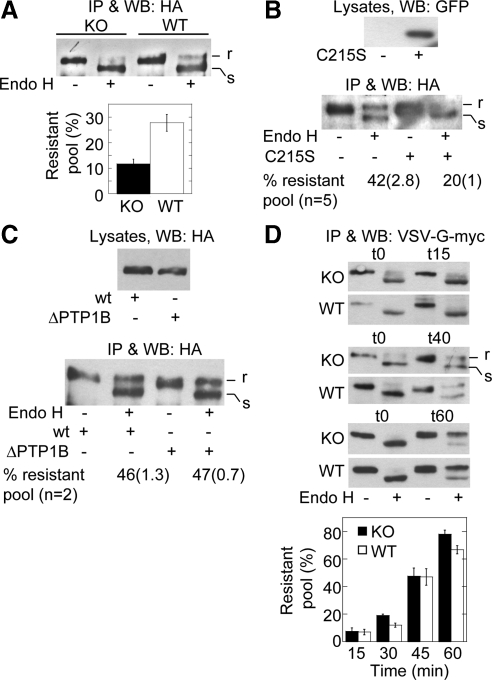
To confirm by biochemical means that PTP1B is required for trafficking of N-cadherin precursors at pre-Golgi stages, we performed endo-H digestion of the HA-N-cadherin-GFP precursor in KO- and WT-cells. N-linked oligosaccharides added in the ER are sensitive to cleavage by endo-H; however, subsequent modifications occurring in the Golgi complex confer resistance to cleavage (Hubbard and Ivatt, 1981). HA-N-cadherin-GFP transfected in KO- and WT-cells was isolated by HA immunoprecipitation and analyzed before/after endo-H digestion. The fraction of HA-N-cadherin-GFP resistant to endo-H in KO-cells was half of that in KO-cells (Figure 6A). Similar reduction of the endo-H–resistant fraction was observed in WT-cells expressing GFP-PTP1B-C215S (Figure 6B, compare with 6A). PTP1B-C215S is a dominant negative mutant that efficiently impairs the function of WT PTP1B in a variety of cell types (Arregui et al., 1998; Balsamo et al., 1998; Pathre et al., 2001; Rhee et al., 2001; Yigzaw et al., 2003; Chang et al., 2006).
Figure 6.
Expression but not binding of PTP1B to the N-cadherin precursor is selectively required for its passage to the Golgi complex. (A) WT- and KO-cells transfected with HA-N-cadherin-GFP were lysed, and the precursor was isolated by immunoprecipitation with-anti-HA. Immunoprecipitated samples were split in two equal aliquots, and one was digested with endo-H. Proteins were fractionated by SDS-PAGE and immunoblotted with anti-HA. Top, a representative blot is shown indicating endo-H–resistant (r) and –sensitive (s) fractions. Bottom, graph of the quantifications of the endo-H–resistant fractions (expressed as percentages of total). Columns represent means ± SEM (n = 7; Student's t, p = 0.002). (B) WT-cells were cotransfected with HA-N-cadherin-GFP and either empty vector or the dominant negative GFP-PTP1B C215S. Cells were processed for endo-H analysis of the HA-N-cadherin-GFP precursor as before. A representative blot and quantitative data of several experiments are shown. Data represent the mean and SEM in parentheses. Note the reduction of the endo-H–resistant fraction in cells expressing the dominant negative C215S. (C) WT-cells were transfected with HA-N-cadherin-GFP (wt) or HA-N-cadherin-Δ884-891-GFP (ΔPTP1B), a deletion mutant of N-cadherin that cannot bind PTP1B. The precursor was isolated and subjected to endo-H analysis as before. A representative blot of two independent experiments is shown. Note that the precursor of both constructs has similar percentages of endo-H–resistant fractions. (D) WT- and KO-cells were transfected with VSV-G (tsO45)-myc and cultured for 18–20 h at 40°C to accumulate the construct in the ER. Afterward, the temperature was shifted to 32°C to allow trafficking; incubation time (in minutes) is given in parentheses. VSV-G was immunoprecipitated and processed for endo-H treatment as before. The graph below shows the quantification of three to five experiments. Note that endo-H–resistant fractions of VSV-G accumulate in a time-dependent manner and is similar in KO- and WT-cells.
Because our data indicate that PTP1B is required for pre-Golgi trafficking of the N-cadherin precursor without being part of the complex, we predicted that the precursor of the HA-N-cadherinΔ884-891-GFP construct, which cannot bind PTP1B because of the deletion of the binding site (Rhee et al., 2001; Xu et al., 2002), would traffic normally when transfected in WT-cells. As expected, the endo-H–resistant fraction of the HA-N-cadherinΔ884-891-GFP precursor was similar in magnitude to that of HA-N-cadherin-GFP (Figure 6C). The precursor of this construct binds β-catenin and p120 at equivalent levels as the HA-N-cadherin-GFP construct (Supplementary Figure S2). Collectively, our results indicate that PTP1B expression is required for trafficking of the N-cadherin precursor at pre-Golgi stages; however, association of PTP1B with the precursor is not essential for this function.
To determine whether the function of PTP1B in N-cadherin trafficking applies to other glycoproteins using the secretory pathway, we performed endo-H analysis of the temperature-sensitive mutant VSV-G tsO45 in KO- and WT-cells. At the restrictive temperature (40°C) VSV-G tsO45 is retained at the ER and is endo-H–sensitive. At the permissive temperature (32°C) VSV-G tsO45 is exported from the ER and become endo-H–resistant (Balch and Keller, 1986). At the restrictive temperature, most of the VSV-G tsO45 expressed in KO- and WT-cells was sensitive to endo-H (Figure 6D). After shifting to the permissive temperature, endo-H–resistant fractions of VSV-G accumulated progressively and at similar proportions in KO- and WT-cells (Figure 6D). These results indicate a specific regulation of N-cadherin precursor trafficking by PTP1B.
Association of Catenins to the N-Cadherin Precursor
Although our results do not reveal the presence of PTP1B in the N-cadherin precursor complexes, we still considered the possibility that PTP1B may stabilize the binding of β-catenin to the N-cadherin precursor, because it occurs in the mature N-cadherin complexes (Lilien and Balsamo, 2005). Stabilizing the binding of β-catenin to N-cadherin precursors may be required for their trafficking, as it has been shown for E-cadherin in Madin-Darby canine kidney (MDCK) cells (Chen et al., 1999). Isolation of the HA-N-cadherin-GFP precursor from transfected WT- and KO-cells, by HA immunoprecipitation, followed by Western blot analysis revealed similar levels of associated α- and β-catenins in both cell lines (Supplementary Figure S3A). Preincubation of KO-cells with pervanadate, a general inhibitor of tyrosine phosphatases, led to an overall increase of phosphotyrosine, as expected (Supplementary Figure S3B). However, this treatment did not change the levels of α- and β-catenin bound to the precursor. In addition, phenylalanine substitution of the critical tyrosine-654 in β-catenin did not affect the binding (Supplementary Figure S3C). These results indicate that in the context of the N-cadherin precursor, the association of β-catenin is not regulated by PTPs, as it happens in the cell surface complexes (Balsamo et al., 1998; Xu et al., 2002).
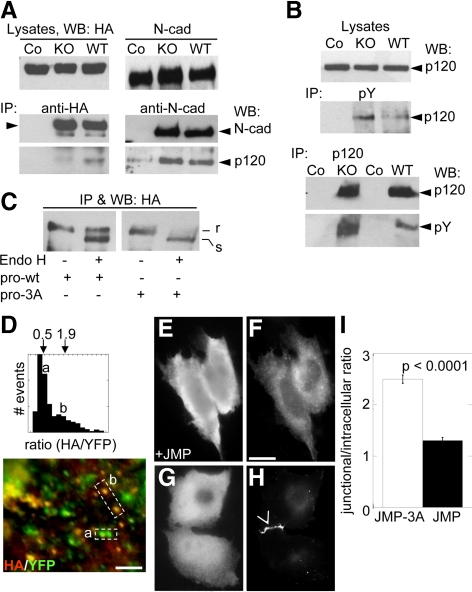
P120 binds to the N-cadherin precursor before the incorporation of α- and β-catenin into the complex (Wahl et al., 2003). P120 has been implied in post-Golgi trafficking of N-cadherin (Mary et al., 2002; Chen et al., 2003; Yanagisawa et al., 2004; Ichii and Takeichi, 2007). However, there is no evidence that P120 plays a role in ER–Golgi trafficking. Our examination of the levels of p120 associated to the HA-N-cadherin-GFP precursor in WT- and KO-cells reveals a significant reduction in KO-cells, which is about half of that found in WT-cells (Figure 7A). In contrast, p120 is present at equivalent levels in complexes of mature N-cadherin isolated from KO- and WT-cells. Because it has been shown that tyrosine phosphorylation of p120 modulates its binding to E-cadherin (Roura et al., 1999), we sought to determine whether the level of phosphotyrosine in p120 was altered in KO-cells. Immunoprecipitation of total tyrosine-phosphorylated proteins from equal amount of proteins of KO- and WT-cells reveals higher levels of p120 in KO-cells (Figure 7B). In agreement with this, the reciprocal immunoprecipitation of p120 from lysates of KO- and WT-cells shows higher levels of phosphotyrosine of p120 in KO-cells. These results suggest that PTP1B contributes to the association of p120 to the N-cadherin precursor likely through regulation of p120 phosphorylation. However, more complex mechanisms may operate to regulate the binding of p120 to mature N-cadherin complexes.
Figure 7.
P120 association with the N-cadherin precursor is required for ER-to-Golgi trafficking. (A) WT- and KO-cells were transfected with HA-N-cadherin-GFP or with a mock vector. Cell lysates were immunoprecipitated with anti-HA (left panels) and anti-N-cadherin (right panels), respectively. In control immunoprecipitations (Co) using WT-cells, the IP antibodies were omitted. The presence of p120 in the complexes was detected by immunoblotting. Note the reduction of p120 associated to the N-cadherin precursor in KO-cells. (B) Cell lysates from KO- and WT-cells were immunoprecipitated with anti-pY or anti-p120. The amount of p120 and phosphotyrosine was assessed by immunoblotting with anti-p120 and anti-pY, respectively. (C) Cell lysates from WT-cells transfected with HA-N-cadherin-GFP (prowt) or HA-N-cadherin-3A-YFP (pro3A) were immunoprecipitated with anti-HA and processed for endo-H digestion. Sensitive (s) and resistant (r) fractions of the precursor were detected in immunoblots with anti-HA. Note that the precursor of HA-N-cadherin-3A-YFP was mostly endo-H sensitive. (D) WT-cells transfected with HA-N-cadherin-3A-YFP were fixed and immunostained for HA. HA/GFP fluorescence ratios were obtained as described in Materials and Methods and expressed in a frequency plot. Means of populations with low and high HA/GFP ratios were indicated, and correspond to puncta labeled with (a) and (b), respectively, in the picture below. (E–H) CHO-K1 cells were cotransfected with N-cadherin-GFP and JMP (E and F) or JMP-3A (G and H). Note that N-cadherin-GFP accumulates at the cell–cell contact in the presence of JMP-3A (arrowhead) but not in the presence of JMP. Also note the enhanced intracellular localization of N-cadherin-GFP in the presence of JMP. The relative junctional/intracellular fluorescence was estimated for both conditions (I). Data represent means ± SEM of >60 cells for each condition. Bars, (D) 5 μm; (F) 25 μm.
Requirement of p120 Catenin in Early Stages of N-Cadherin Precursor Trafficking
Our findings that in KO-cells the N-cadherin precursor showed impaired ER–Golgi trafficking and reduced association with p120 suggest that both phenomena could be associated. To examine this possibility further, we inserted the HA tag in the prodomain of N-cadherin-3A-YFP, a construct bearing a triple alanine substitution in the juxtamembrane domain, which specifically disrupts its interaction with p120 (Thoreson et al., 2000; Chen et al., 2003), and analyzed the ER–Golgi trafficking by endo-H digestion analysis. This construct does not bind to p120 but recruits β-catenin similarly to the HA-N-cadherin-GFP (not shown). HA-N-cadherin-GFP and HA-N-cadherin-3A-YFP constructs were expressed in WT-cells, and the respective precursors were isolated by HA-immunoprecipitation. Half of each immunoprecipitate was kept as control, and the other half was treated with endo-H and analyzed in Western blots as described before. Although the endo-H–resistant fraction of HA-N-cadherin-GFP was ∼ 46%, that of HA-N-cadherin-3A-YFP was barely detectable (Figure 7C). These results were confirmed by microscopy analyzing the HA/YFP fluorescence ratios. When expressed in WT-cells, HA-N-cadherin-3A-YFP distributes in two distinct populations of fluorescent puncta, one with low HA/YFP fluorescence ratios (∼0.5) and one with high ratios (∼1.9; Figure 7D). High ratios suggest less processing of the precursor and resemble the condition observed for the HA-N-cadherin-GFP construct in KO-cells or in WT-cells treated with BFA (Figure 5J).
We additionally analyzed the effects of perturbing the association of p120 to N-cadherin by competition with small peptides encompassing the p120 binding site in the N-cadherin cytoplasmic domain. The cDNAs encoding a small competitor peptide of the juxtamembrane (JMP) region of N-cadherin, which sequesters endogenous p120, and a noncompetitive peptide (JMP-3A, control) containing the triple substitution EED by AAA, which disrupts the p120 binding site were prepared (Anastasiadis et al., 2000; Thoreson et al., 2000; Chen et al., 2003). Expressing the peptides in COS cells showed that JMP but not the JMP-3A led to a significant reduction of p120 and N-cadherin from intercellular junctions (Supplementary Figure S4, A–D). In addition, JMP expression led to an enhanced intracellular labeling of the N-cadherin (Supplementary Figure S4F). To examine the effect of peptides on a pool of recently synthesized N-cadherin, we coexpressed the peptides with N-cadherin-GFP in CHO-K1 cells, which do not express endogenous cadherins. In these cells, JMP inhibited the localization of N-cadherin-GFP in intercellular junctions and enhanced the accumulation of intracellular fluorescence (Figure 7, E, F, and I). In contrast, JMP-3A did not perturb the N-cadherin localization at cell–cell contacts, and the intracellular fluorescence was weak (Figure 7, G–I). Together, these results suggest that binding of p120 to the N-cadherin precursor is required for normal trafficking through early steps of the secretory pathway.
DISCUSSION
PTP1B bound to the cytoplasmic domain of surface N-cadherin has been implicated in the regulation of N-cadherin–dependent adhesion through dephosphorylation of β-catenin in the complex (Balsamo et al., 1998; Rhee et al., 2001; Lilien and Balsamo, 2005). In the present work we report a novel function of PTP1B in the regulation of anterograde trafficking of the N-cadherin precursor. This apparently does not require PTP1B binding to the precursor and does not involve β-catenin dephosphorylation and dissociation from the complex. Instead, PTP1B dephosphorylates and promotes the binding of p120 to the N-cadherin precursor complex, facilitating its movement through early stages of the secretory pathway.
PTP1B Regulates N-Cadherin Precursor Trafficking
We used several approaches to study the trafficking of N-cadherin in WT- and KO-cells. Metabolic labeling combined with cell surface biotinylation revealed that in KO-cells the proteolytic processing and arrival of mature N-cadherin at the cell surface occurred with slower kinetics than in WT-cells. We interpreted this result as a consequence of an impaired trafficking of the N-cadherin precursor from ER to the Golgi apparatus. Microscopic and further biochemical analyses support this view. Indeed, most of HA-N-cadherin-GFP precursor was endo-H–sensitive in KO-cells, whereas a fraction close to 40% was resistant in WT-cells. The redistribution of N-cadherin-GFP in the Golgi region after BFA recovery occurred with a slower kinetics in KO-cells than in WT-cells. Furthermore, ratio analysis of HA/GFP fluorescence puncta in KO- and WT-cells expressing HA-N-cadherin-GFP showed that the subset of higher ratios were 44 and 5%, respectively, suggesting that the arrival of the N-cadherin precursor to the post-Golgi compartment where the HA-containing propeptide is removed did not occur efficiently in KO-cells. The PTP1B function on trafficking reveals some degree of specificity for N-cadherin because endo-H analysis of another plasma membrane glycoprotein, the temperature-sensitive mutant of VSV-G, did not show differences between WT- and KO-cells.
PTP1B Regulates ER–Golgi Trafficking of N-Cadherin Precursor through a p120-dependent Mechanism
What could be the mechanism underlying the PTP1B function in N-cadherin precursor trafficking? The catalytic domain of the ER-bound PTP1B is in the right topological orientation for dephosphorylation of substrates at the cytosolic face of the ER, as recently demonstrated (Haj et al., 2002; Boute et al., 2003; Romsicki et al., 2004; Cohen et al., 2004; Hernandez et al., 2006; Anderie et al., 2007). We considered the possibility that PTP1B regulates the binding of catenins to the cytosolic tail of N-cadherin precursors. A candidate protein whose binding to the E-cadherin precursor is a condition for its export from the ER is β-catenin (Chen et al., 1999). Tyrosine phosphorylation of β-catenin negatively regulates its binding to mature N-cadherin, and PTP1B has been identified as a major phosphatase that dephosphorylates β-catenin and stabilizes the N-cadherin/β-catenin complex (Balsamo et al., 1998; Roura et al., 1999; Lilien and Balsamo, 2005). In KO-cells, however, we did not observe a reduction in the levels of β-catenin associated with the N-cadherin precursor. A possibility is that absence of overt tyrosine phosphorylation in the context of the N-cadherin precursor complexes at the ER turns unnecessary the role of tyrosine phosphatases. Indeed, preincubation of KO-cells with the general inhibitor of tyrosine phosphatases pervanadate did not alter the levels of β-catenin bound to the N-cadherin precursor, even when the phosphotyrosine levels of nonbound β-catenin increased (Supplementary Figure 3B). Nevertheless, we found a reduction of ∼50% in the amount of p120 associated to the N-cadherin precursor in KO-cells. P120 is the first catenin that associates with the N-cadherin precursor in the biosynthetic pathway (Wahl et al., 2003; Curtis et al., 2008). Because of its ability to interact with the conventional kinesin KIF5, a microtubule plus end–directed motor, p120 is thought to facilitate the post-Golgi traffic and peripheral location of N-cadherin (Mary et al., 2002; Chen et al., 2003; Yanagisawa et al., 2004; Ichii and Takeichi, 2007). Is p120 association to the N-cadherin precursor also required for movement of the complex through early stages of the biosynthetic pathway? We addressed this question using different approaches. We detected the accumulation of a population of puncta with high HA/YFP fluorescence ratios in WT-cells expressing HA-N-cadherin-3A-YFP, a construct that cannot bind p120, suggesting that HA-N-cadherin-3A-YFP accumulates at pre-Golgi stages. Further support to this view is the observation that the HA-N-cadherin-3A-YFP precursor was almost 100% sensitive to endo-H. Finally, overexpression of small juxtamembrane competitor peptides that sequester p120 and impair its association to full-length cadherin (Anastasiadis et al., 2000) led to visible intracellular accumulation of N-cadherin-GFP. P120 may be dephosphorylated by ER-bound PTP1B previous to its binding to the N-cadherin precursor. Indeed, we observed that p120 in KO-cells have higher levels of phosphotyrosine compared with WT-cells, as expected for a PTP1B substrate (Mertins et al., 2008).
The mechanism by which bound p120 affects N-cadherin precursor trafficking is presently unknown. A possibility is that p120 may contribute to link N-cadherin precursor complexes to the microtubule system, which ferries cargo between the ER and Golgi. P120 was reported to interact directly and indirectly with microtubules (Kogata et al., 2003; Piedra et al., 2003; Franz and Ridley, 2004; Xu et al., 2004; Yanagisawa et al., 2004; Ichii and Takeichi, 2007). The anterograde movement of cargo from the ER to Golgi requires, however, the engagement of the N-cadherin precursor complex to a microtubule minus end–directed motor, such as dynein or a minus-end–directed kinesin, such as the C-kinesin KIFC3 (Xu et al., 2002). Alternatively, p120 may play a role in the export of the N-cadherin precursor from the ER, facilitating the recognition by the COPII machinery. Regardless of the mechanism involved, PTP1B did not appear to be a necessary component of the N-cadherin precursor complex. Consistent with this view, the N-cadherin precursor deleted in the PTP1B-binding site, HA-N-cadherinΔ884-891-GFP, develop endo-H resistance similar to that of the WT construct, suggesting normal ER–Golgi traffic. Also, we were unable to detect a PTP1B/N-cadherin precursor association by microscopy and coimmunoprecipitation analyses. Binding of PTP1B likely appears to become necessary for post-Golgi trafficking of N-cadherin, as it is suggested by the enhanced intracellular puncta accumulated by N-cadherinΔ884-891-GFP, a mutant that cannot bind PTP1B. Thus, our results suggest that PTP1B has different targets and functions in N-cadherin complexes assembled at the ER and at the plasma membrane.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. Neel for providing the PTP1B knockout cells used in this work. We also want to acknowledge J. Daniotti, M. Duñach, K. Green, J. Hay, H. Maccioni, W.J. Nelson, and A. Reynolds for plasmids and antibodies. This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica (PICT 31939 and 332) to C.O.A. M.V.H. and D.P.W. were supported by predoctoral fellowships from the Consejo Nacional de Investigaciones Científicas y Técnicas and Agencia Nacional de Promoción Científica y Tecnológica, respectively.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-10-0880) on February 24, 2010.
REFERENCES
- Alema S., Salvatore A. M. p120 catenin and phosphorylation: mechanisms and traits of an unresolved issue. Biochim. Biophys. Acta. 2007;1773:47–58. doi: 10.1016/j.bbamcr.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Anastasiadis P. Z., Moon S. Y., Thoreson M. A., Mariner D. J., Crawford H. C., Zheng Y., Reynolds A. B. Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2000;2:637–644. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- Anderie I., Schulz I., Schmid A. Direct interaction between ER membrane-bound PTP1B and its plasma membrane-anchored targets. Cell Signal. 2007;19:582–592. doi: 10.1016/j.cellsig.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Arregui C. O., Balsamo J., Lilien J. Impaired integrin-mediated adhesion and signaling in fibroblasts expressing a dominant-negative mutant PTP1B. J. Cell Biol. 1998;143:861–873. doi: 10.1083/jcb.143.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo J., Arregui C., Leung T., Lilien J. The nonreceptor protein tyrosine phosphatase PTP1B binds to the cytoplasmic domain of N-cadherin and regulates the cadherin-actin linkage. J. Cell Biol. 1998;143:523–532. doi: 10.1083/jcb.143.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Keller D. S. ATP-coupled transport of vesicular stomatitis virus G protein. Functional boundaries of secretory compartments. J. Biol. Chem. 1986;261:14690–14696. [PubMed] [Google Scholar]
- Boute N., Boubekeur S., Lacasa D., Issad T. Dynamics of the interaction between the insulin receptor and protein tyrosine-phosphatase 1B in living cells. EMBO Rep. 2003;4:313–319. doi: 10.1038/sj.embor.embor767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano J., Solanas G., Casagolda D., Raurell I., Villagrasa P., Bustelo X. R., Garcia de Herreros A., Dunach M. Specific phosphorylation of p120-catenin regulatory domain differently modulates its binding to RhoA. Mol. Cell. Biol. 2007;27:1745–1757. doi: 10.1128/MCB.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y., Ceacareanu B., Zhuang D., Zhang C., Pu Q., Ceacareanu A. C., Hassid A. Counter-regulatory function of protein tyrosine phosphatase 1B in platelet-derived growth factor or fibroblast growth factor–induced motility and proliferation of cultured smooth muscle cells and in neointima formation. Arterioscler. Thromb. Vasc. Biol. 2006;26:501–507. doi: 10.1161/01.ATV.0000201070.71787.b8. [DOI] [PubMed] [Google Scholar]
- Chen Y. T., Stewart D. B., Nelson W. J. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J. Cell Biol. 1999;144:687–699. doi: 10.1083/jcb.144.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Kojima S., Borisy G. G., Green K. J. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J. Cell Biol. 2003;163:547–557. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Oren-Young L., Klingmuller U., Neumann D. Protein tyrosine phosphatase 1B participates in the down-regulation of erythropoietin receptor signalling. Biochem. J. 2004;377:517–524. doi: 10.1042/BJ20031420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M. W., Johnson K. R., Wheelock M. J. E-cadherin/catenin complexes are formed cotranslationally in the endoplasmic reticulum/Golgi compartments. Cell. Commun. Adhes. 2008;15:365–378. doi: 10.1080/15419060802460748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniotti J. L., Martina J. A., Giraudo C. G., Zurita A. R., Maccioni H. J. GM3 alpha 2,8-sialyltransferase (GD3 synthase): protein characterization and sub-Golgi location in CHO-K1 cells. J. Neurochem. 2000;74:1711–1720. doi: 10.1046/j.1471-4159.2000.0741711.x. [DOI] [PubMed] [Google Scholar]
- Davis M. A., Ireton R. C., Reynolds A. B. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derycke L. D., Bracke M. E. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int. J. Dev. Biol. 2004;48:463–476. doi: 10.1387/ijdb.041793ld. [DOI] [PubMed] [Google Scholar]
- Ezaki T., Guo R. J., Li H., Reynolds A. B., Lynch J. P. The homeodomain transcription factors Cdx1 and Cdx2 induce E-cadherin adhesion activity by reducing beta- and p120-catenin tyrosine phosphorylation. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G54–G65. doi: 10.1152/ajpgi.00533.2006. [DOI] [PubMed] [Google Scholar]
- Fuentes F., Arregui C. O. Microtubule and cell contact dependency of ER-bound PTP1B localization in growth cones. Mol. Biol. Cell, 2009;20:1878–1889. doi: 10.1091/mbc.E08-07-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangioni J. V., Beahm P. H., Shifrin V., Jost C. A., Neel B. G. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992;68:545–560. doi: 10.1016/0092-8674(92)90190-n. [DOI] [PubMed] [Google Scholar]
- Franz C. M., Ridley A. J. P120 catenin associates with microtubules: inverse relationship between microtubule binding and Rho GTPase regulation. J. Biol. Chem. 2004;279:6588–6594. doi: 10.1074/jbc.M312812200. [DOI] [PubMed] [Google Scholar]
- Giraudo C. G., Maccioni H.J.F. Ganglioside glycosiltransferases organize in distinct multienzyme complexes in CHO-K1 cells. J. Biol. Chem. 2003;278:40262–40271. doi: 10.1074/jbc.M305455200. [DOI] [PubMed] [Google Scholar]
- Haj F. G., Verveer P. J., Squire A., Neel B. G., Bastiaens P. I. Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science. 2002;295:1708–1711. doi: 10.1126/science.1067566. [DOI] [PubMed] [Google Scholar]
- Hernandez M. V., Sala M. G., Balsamo J., Lilien J., Arregui C. O. ER-bound PTP1B is targeted to newly forming cell-matrix adhesions. J. Cell Sci. 2006;119:1233–1243. doi: 10.1242/jcs.02846. [DOI] [PubMed] [Google Scholar]
- Hinck L., Nathke I. S., Papkoff J., Nelson W. J. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J. Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Ichii T., Takeichi M. P120-catenin regulates microtubule dynamics and cell migration in a cadherin-independent manner. Genes Cells. 2007;12:827–839. doi: 10.1111/j.1365-2443.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- Kinch M. S., Clark G. J., Der C. J., Burridge K. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J. Cell Biol. 1995;130:461–471. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. W., Farooq A., Shan W., Zeng L., Colman D. R., Zhou M. M. Structure of the neural (N-) cadherin prodomain reveals a cadherin extracellular domain-like fold without adhesive characteristics. Structure. 2004;12:793–805. doi: 10.1016/j.str.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Kogata N., Masuda M., Kamioka Y., Yamagishi A., Endo A., Okada M., Mochizuki N. Identification of Fer tyrosine kinase localized on microtubules as a platelet endothelial cell adhesion molecule-1 phosphorylating kinase in vascular endothelial cells. Mol. Biol. Cell. 2003;14:3553–3564. doi: 10.1091/mbc.E03-02-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk A. P., Reynolds A. B. Protecting your tail: regulation of cadherin degradation by p120-catenin. Curr. Opin. Cell Biol. 2004;16:522–527. doi: 10.1016/j.ceb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Lilien J., Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr. Opin. Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Mary S., Charrasse S., Meriane M., Comunale F., Travo P., Blangy A., Gauthier-Rouviere C. Biogenesis of N-cadherin-dependent cell-cell contacts in living fibroblasts is a microtubule-dependent kinesin-driven mechanism. Mol. Biol. Cell. 2002;13:285–301. doi: 10.1091/mbc.01-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertins P., Eberl H. C., Renkawitz J., Olsen J. V., Tremblay M. L., Mann M., Ullrich A., Daub H. Investigation of protein-tyrosine phosphatase 1B function by quantitative proteomics. Mol. Cell Proteom. 2008;7:1763–1777. doi: 10.1074/mcp.M800196-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, et al. Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ. Res. 2008;102:1182–1191. doi: 10.1161/CIRCRESAHA.107.167080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M. Lateral dimerization of the E-cadherin extracellular domain is necessary but not sufficient for adhesive activity. J. Biol. Chem. 2002;277:19600–19608. doi: 10.1074/jbc.M202029200. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Kemler R. Molecular organization of the uvomorulin-catenin complex. J. Cell Biol. 1992;116:989–996. doi: 10.1083/jcb.116.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathre P., Arregui C., Wampler T., Kue I., Leung T. C., Lilien J., Balsamo J. PTP1B regulates neurite extension mediated by cell-cell and cell-matrix adhesion molecules. J. Neurosci. Res. 2001;63:143–150. doi: 10.1002/1097-4547(20010115)63:2<143::AID-JNR1006>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Piedra J., Miravet S., Castano J., Palmer H. G., Heisterkamp N., Garcia de Herreros A., Dunach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin interaction. Mol. Cell. Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S., Weis W. I. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu. Rev. Cell Dev. Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- Posthaus H., Dubois C. M., Laprise M. H., Grondin F., Suter M. M., Muller E. Proprotein cleavage of E-cadherin by furin in baculovirus over-expression system: potential role of other convertases in mammalian cells. FEBS Lett. 1998;438:306–310. doi: 10.1016/s0014-5793(98)01330-1. [DOI] [PubMed] [Google Scholar]
- Rhee J., Lilien J., Balsamo J. Essential tyrosine residues for interaction of the non-receptor protein-tyrosine phosphatase PTP1B with N-cadherin. J. Biol. Chem. 2001;276:6640–6644. doi: 10.1074/jbc.M007656200. [DOI] [PubMed] [Google Scholar]
- Romsicki Y., Reece M., Gauthier J. Y., Asante-Appiah E., Kennedy B. P. Protein tyrosine phosphatase-1B dephosphorylation of the insulin receptor occurs in a perinuclear endosome compartment in human embryonic kidney 293 cells. J. Biol. Chem. 2004;279:12868–12875. doi: 10.1074/jbc.M309600200. [DOI] [PubMed] [Google Scholar]
- Roura S., Miravet S., Piedra J., Garcia de Herreros A., Dunach M. Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J. Biol. Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- Sheth P., Seth A., Atkinson K. J., Gheyi T., Kale G., Giorgianni F., Desiderio D. M., Li C., Naren A., Rao R. Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem. J. 2007;402:291–300. doi: 10.1042/BJ20060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J. Cell Biol. 1977;75:464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng J., Rai T., Tanaka Y., Takei Y., Nakata T., Hirasawa M., Kulkarni A. B., Hirokawa N. The KIF3 motor transports N-cadherin and organizes the developing neuroepithelium. Nat. Cell Biol. 2005;7:474–482. doi: 10.1038/ncb1249. [DOI] [PubMed] [Google Scholar]
- Thoreson M. A., Anastasiadis P. Z., Daniel J. M., Ireton R. C., Wheelock M. J., Johnson K. R., Hummingbird D. K., Reynolds A. B. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J. Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl J. K., 3rd, Kim Y. J., Cullen J. M., Johnson K. R., Wheelock M. J. N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. J. Biol. Chem. 2003;278:17269–17276. doi: 10.1074/jbc.M211452200. [DOI] [PubMed] [Google Scholar]
- Winter M. C., Shasby S., Shasby D. M. Compromised E-cadherin adhesion and epithelial barrier function with activation of G protein-coupled receptors is rescued by Y-to-F mutations in beta-catenin. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294:L442–L448. doi: 10.1152/ajplung.00404.2007. [DOI] [PubMed] [Google Scholar]
- Xiao K., Allison D. F., Buckley K. M., Kottke M. D., Vincent P. A., Faundez V., Kowalczyk A. P. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J. Cell Biol. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., Oas R. G., Chiasson C. M., Kowalczyk A. P. Role of p120-catenin in cadherin trafficking. Biochim. Biophys. Acta. 2007;1773:8–16. doi: 10.1016/j.bbamcr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Xu G., Arregui C., Lilien J., Balsamo J. PTP1B modulates the association of beta-catenin with N-cadherin through binding to an adjacent and partially overlapping target site. J. Biol. Chem. 2002;277:49989–49997. doi: 10.1074/jbc.M206454200. [DOI] [PubMed] [Google Scholar]
- Xu G., Craig A. W., Greer P., Miller M., Anastasiadis P. Z., Lilien J., Balsamo J. Continuous association of cadherin with beta-catenin requires the non-receptor tyrosine-kinase Fer. J. Cell Sci. 2004;117:3207–3219. doi: 10.1242/jcs.01174. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kaverina I. N., Wang A., Fujita Y., Reynolds A. B., Anastasiadis P. Z. A novel interaction between kinesin and p120 modulates p120 localization and function. J. Biol. Chem. 2004;279:9512–9521. doi: 10.1074/jbc.M310895200. [DOI] [PubMed] [Google Scholar]
- Yigzaw Y., Poppleton H. M., Sreejayan N., Hassid A., Patel T. B. Protein-tyrosine phosphatase-1B (PTP1B) mediates the anti-migratory actions of Sprouty. J. Biol. Chem. 2003;278:284–288. doi: 10.1074/jbc.M210359200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.