Atypical protein kinase Cζ (PKCζ) is emerging as a mediator of differentiation. Here, we describe a critical role for PKCζ during myogenic differentiation. Our results identify PKCζ as a controller of myogenic differentiation by its regulation of Cdk5.
Abstract
Atypical protein kinase Cζ (PKCζ) is emerging as a mediator of differentiation. Here, we describe a novel role for PKCζ in myogenic differentiation, demonstrating that PKCζ activity is indispensable for differentiation of both C2C12 and mouse primary myoblasts. PKCζ was found to be associated with and to regulate the Cdk5/p35 signaling complex, an essential factor for both neuronal and myogenic differentiation. Inhibition of PKCζ activity prevented both myotube formation and simultaneous reorganization of the nestin intermediate filament cytoskeleton, which is known to be regulated by Cdk5 during myogenesis. p35, the Cdk5 activator, was shown to be a specific phosphorylation target of PKCζ. PKCζ-mediated phosphorylation of Ser-33 on p35 promoted calpain-mediated cleavage of p35 to its more active and stable fragment, p25. Strikingly, both calpain activation and the calpain-mediated cleavage of p35 were shown to be PKCζ-dependent in differentiating myoblasts. Overall, our results identify PKCζ as a controller of myogenic differentiation by its regulation of the phosphorylation-dependent and calpain-mediated p35 cleavage, which is crucial for the amplification of the Cdk5 activity that is required during differentiation.
INTRODUCTION
Members of the protein kinase C (PKC) superfamily of phospholipid-dependent serine/threonine kinases are involved in a multitude of cellular processes (Nishizuka, 1995). The different isoforms are classified into three subfamilies according to their allosteric activators: classical PKCs (α, β, and γ; cPKCs) require both calcium and diacylglycerol (DAG) for activation, novel PKCs (δ, ε, θ, and η; nPKCs) are calcium-independent, and atypical PKCs (ι, λ, and ζ; aPKCs) are activated neither by calcium nor DAG. The aPKCs are activated by lipid components, such as phosphatidyl inositols (for instance PI-3,4,5 triphosphate), phosphatidic acid, ceramide, and arachidonic acid (Nishizuka, 1995). The activation mechanism has two essential steps: 1) release of a membrane-bound myristoylated pseudosubstrate from the substrate-binding cavity and 2) autophoshorylation of the kinase domain (reviewed in Hirai and Chida, 2003; Hofmann, 1997).
Ubiquitously expressed PKCζ has been implicated as a central regulator of many key intracellular signaling pathways (reviewed in Hirai and Chida, 2003) and is emerging as a mediator of differentiation. The role of PKCζ during development was first demonstrated in developing Xenopus oocytes (Berra et al., 1993) and PKCζ has subsequently been shown to be involved in monocytic (Ways et al., 1994; Liu et al., 1998), erythroid (Mansat de Mas et al., 2002), and nerve growth factor (NGF)-mediated neuronal differentiation (Wooten et al., 2000, 2001). Moreover, the importance of PKCζ upon differentiation becomes evident as its activity is necessary to control cell polarity (Etienne-Manneville and Hall, 2001; Suzuki et al., 2001; Gao et al., 2002) and migration (Etienne-Manneville and Hall, 2001; Guo et al., 2009), both processes important steps for differentiation.
Another differentiation-related kinase, cyclin-dependent kinase 5 (Cdk5), is a serine/threonine kinase first identified as a member of the cyclin-dependent kinase family, based on sequence comparisons (Lew et al., 1995). However, unlike other Cdks, Cdk5 is not involved in cell cycle progression and is not activated by cyclins but rather by specific activators, p35 or p39 (reviewed in Dhavan and Tsai, 2001). Cdk5 has been shown to be indispensable for neuronal differentiation, affecting the migration of neuronal progenitors, neurite outgrowth, and the cytoskeletal dynamics of developing neuronal cells (reviewed in Cicero and Herrup, 2005; Dhavan and Tsai, 2001; Cruz and Tsai, 2004; Miyamoto et al., 2007). Moreover, Cdk5 plays a crucial role in regulating both the early development of muscle tissue (Lazaro et al., 1997; Philpott et al., 1997) and the formation of neuromuscular junctions (Fu et al., 2001). We have recently shown that the intermediate filament (IF) protein nestin forms a regulatory scaffold for Cdk5, affecting targeting and activity of the Cdk5/p35 signaling complex determining the differentiation of myoblasts and myofibers (Sahlgren et al., 2003) and the survival of neuronal progenitor cells (Sahlgren et al., 2006). In addition, there appears to be a phosphorylation-dependent feedback mechanism between Cdk5 and nestin, because Cdk5, by phosphorylating nestin, can affect the reorganization and turnover of its own scaffold (Sahlgren et al., 2003, 2006). Although several Cdk5 substrates have been identified in both neuronal and myogenic tissue (reviewed in Cruz and Tsai, 2004; Dhavan and Tsai, 2001; Nguyen et al., 2007; Schnack et al., 2008; Seo et al., 2008), very little is known about the upstream regulators of Cdk5. C-Abl, Fyn, and Cdk7 have been implicated as facilitators of activity as they are involved in phosphorylation of Cdk5 (Zukerberg et al., 2000; Sasaki et al., 2002; Rosales et al., 2003).
Although our earlier work demonstrated that the Cdk5–nestin complex determines myogenic differentiation (Sahlgren et al., 2003), we wanted to establish what could be the upstream regulator of this signaling cascade. Because PKCζ has been shown to act as a mediator of differentiation processes (reviewed above), we wanted to know whether PKCζ could acts as an upstream regulator of Cdk5, affecting myoblast differentiation. Indeed we observed that PKCζ activity is necessary for differentiation and moreover, it directly regulates the activity of the Cdk5/p35 signaling complex by phosphorylating p35 and by facilitating calpain-dependent cleavage of p35 to the more stable p25 fragment. This new role for p25 in muscle differentiation represents a novel aspect of p25-mediated Cdk5 activation, an activation mode that has to date been primarily associated with neurodegenerative conditions (Cheung and Ip, 2004). Thus, our data reveal PKCζ as a novel key regulator of the Cdk5/p35 signaling complex during muscle differentiation.
MATERIALS AND METHODS
Cell Culture Reagents and Transfection Materials
Mouse C2C12 myoblasts and monkey Cos-7 kidney cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Gaithersburg, MD) supplemented with 10% (vol/vol) fetal bovine serum, 2 mM glutamine, and penicillin G (100 U/ml)/streptomycin (100 μg/ml; growth medium). C2C12 cells were grown to 80% confluence and induced to differentiate by replacing the growth medium with differentiation medium (DMEM supplemented with 2% [vol/vol] fetal bovine serum, 2 mM glutamine, and penicillin G (100 U/ml)/streptomycin (100 μg/ml). To inhibit PKCζ activity, myoblasts were induced to differentiate in the presence of 20 μM pseudosubstrate inhibitor peptide (PS; Myr-SIYRRGARRWRKL) or control scrambled peptide (Scr-P; Myr-RLYRKRIWRSAGR; MilleGen Prologue Biotech, Labege Cedex, France). Both of the peptides were linked to a myristoyl group to facilitate the transport through the cell membrane. The Cdk inhibitor roscovitine, the proteasomal inhibitor MG132, calpain inhibitor III, the PKC inhibitor chelerythrine (Calbiochem, San Diego, CA) and the calcium ionophore A23187 (Calbiochem, Darmstadt, Germany) were used at final concentrations of 10, 20, 15, 5, and 10 μM respectively. A luciferase-based assay (the calpain-Glo assay; Promega, Madison, WI) was used to monitor calpain activity.
For transfection, Cos-7 cells were pelleted and resuspended in OPTIMEM (Invitrogen, Invitrogen Foundation, Washington, DC) supplemented with 5% (vol/vol) fetal bovine serum (FBS) and electroporated at 220 V and 975 μF. Transfected cells were plated and harvested after 48 h for further analysis. A p35-encoding DNA plasmid, with p35 cloned into the pcDNA3.1-His vector, was kindly provided by Dr. Harish Pant (National Institutes of Health). The PKCζ-Flag plasmid was a kind gift of Dr. J. Blenis (Department of Cell Biology, Harvard Medical School, Boston, MA). Empty plasmids were used as transfection controls. C2C12 myoblasts were transfected using JetPEI transfection reagent (Polyplus-transfection, New York, NY) according to the manufacturer's protocol. Myc-tagged p35-encoding plasmid was ordered from Addgene (Cambridge, MA) and mutated to p35 S33A using Stratagene mutagenesis kit (La Jolla, CA). C2C12 myoblasts and mouse primary cells were transfected with 20 or 80 pmol of PKCζ small interfering RNA (siRNA; siPKCζ) or scrambled siRNA (Scr-R; Santa Cruz Biotechnology, Santa Cruz, CA) using the Lipofectamine-plus reagent, according to the manufacturer's (Invitrogen, Rockville, MD) instructions. Twenty hours after transfection, cells were switched to differentiation medium.
Primary Mouse Myoblast Culture
Cultures of primary myoblasts were established from the limb skeletal muscles of 2-d-old FVB-n mice. Muscle tissue was minced and enzymatically digested by incubation in 0.2% (wt/vol) type XI collagenase (Roche Diagnostics, Basel, Switzerland) and 0.1% (wt/vol) trypsin at 37°C for 45 min. The resulting slurry was filtered to remove large pieces of tissue and rinsed with growth medium as follows: (Ham's F-10 [Sigma-Aldrich, St. Louis, MO] supplemented with 15% (vol/vol) fetal bovine serum, 2 mM glutamine, penicillin G (100 U/ml)/streptomycin (100 μg/ml), and 2.5 ng/ml β-FGF (fibroblast growth factor). Cells were centrifuged at 1000 × g for 5 min, resuspended in growth medium, and seeded into tissue culture dishes. After attainment of 80% confluence, differentiation was induced by replacing growth medium with differentiation medium as follows: DMEM supplemented with 2% (vol/vol) FBS, 2 mM glutamine, and penicillin G (100U/ml)/streptomycin (100 μg/ml).
Immunofluorescence Labeling
For immunostaining, myoblasts grown on coverslips were fixed in 3% (vol/vol) paraformaldehyde and permeabilized with 0.1% (vol/vol) Triton X-100 in PBS for 10 min at room temperature (RT). Nonspecific binding sites were blocked by incubation in 2% (wt/vol) bovine serum albumin (BSA) in PBS (phosphate-buffered saline) with 0.05% (vol/vol) Triton X-100 for 30 min at RT. Cells were subsequently stained for 2 h with primary antibodies, after which coverslips were rinsed three times with PBS and stained for 40 min with fluorescence tag-labeled secondary antibodies (Alexa 488 goat anti-mouse or Alexa 568 goat anti-rabbit; Molecular Probes, Invitrogen, Rockville, MD). Cells were washed three times in PBS before mounting in DAPI/Vectashield (Vector Laboratories, Burlingame, CA). Images were collected using a Zeiss LSM confocal laser scanning microscope equipped with argon and helium-neon lasers (Thornwood, NY).
Immunoprecipitation
C2C12 cells or transfected Cos-7 cells were lysed in immunoprecipitation buffer (50 mM HEPES, pH 7.4, 140 mM NaCl, 5 mM MgCl2, 5 mM EGTA, 0.4% [vol/vol] NP40, 10 mM pyrophosphate, 5 mM sodium orthovanadate, and a protease inhibitor cocktail; Roche Diagnostics) for 30 min on ice followed by centrifugation at 12,000 × g for 10 min at 4°C. Protein concentration was measured by the Bradford assay, and 800 μg of each lysate was precleared using Sepharose beads, for 45 min, followed by immunoprecipitation of p35 (Santa Cruz Biotechnology), Cdk5 (Biosource Invitrogen, Washington, DC), calpain 3 (Abcam, Cambridge, United Kingdom), or PKCζ (Santa Cruz Biotechnology). Immunocomplexes were captured on protein G-Sepharose beads, washed four times in 20 mM HEPES, pH 7.4, 2 mM EGTA, 100 mM NaCl, 0.4% (vol/vol) NP40, and 1 mM dithiothreitol (DTT), and finally resuspended in Laemmli sample buffer.
Western Blotting and Antibodies
Proteins were separated on SDS-PAGE and transferred to nitrocellulose membranes using a wet transfer apparatus (Bio-Rad, Hercules, CA). After blocking of nonspecific binding with 5% (wt/vol) nonfat dry milk, membranes were first probed overnight using primary antibodies and next incubated for 1 h with appropriate secondary antibodies coupled to horseradish peroxidase. Proteins were visualized using an ECL Western blotting kit (GE Healthcare, Little Chalfont, United Kingdom).
The following primary antibodies were purchased from Santa Cruz Biotechnology: goat/rabbit anti-PKCζ (used at 1:500), rabbit anti-troponin (1:500), rabbit anti-myosin light chain (myosin heavy chain [MHC]; 1:500), rabbit anti-p35 (N20, C19; 1:200), rabbit anti-14-3-3tau (1:500), mouse anti-desmin (1:500), rabbit anti-RhoA (1:200), rat anti-Hsc70 (1:10,000), and rat anti-HSP90 (1:1000). In addition, the following antibodies were used: mouse anti-Cdk5 (used at 1:1000; Biosource, Invitrogen Foundation), mouse anti-nestin (1:1000; PharMingen, San Diego, CA), mouse anti-actin (1:1000; Sigma, St. Louis, MO), mouse anti-p35 (1:500; Sigma), mouse anti-calpain 1 (1:1000; Cell Signaling Technology, Danvers, MA), and mouse anti-calpain 3 (1:500; Abcam, Cambridge, United Kingdom). The secondary antibodies (used at 1/10,000–20,000) were purchased from Southern Biotechnology (Birmingham, AL). To block rabbit anti-p35 (C-19), a blocking peptide was used (1:1000, Santa Cruz Biotechnology).
In Vitro Phosphorylation and Kinase Activity Assays
In vitro phosphorylation assays were performed using immunoprecipitated p35, calpain 3, recombinant calpain 1 (Calbiochem), His-p35 (aa 1-120), glutathione S-transferase (GST)-p35 (aa 208-308), or the complex GSTp35/HisCdk5 (Abnova, Taipei City, Taiwan) as substrates. Cell lysates were incubated with protein G-Sepharose beads for 45 min, and immunoprecipitates were washed four times in 50 mM HEPES, pH 7.4, 5 mM EDTA, 125 mM NaCl, 0.2% (vol/vol) NP40, and 1 mM DTT and twice then with 50 mM HEPES, pH 7.4, 25 mM NaCl, and 1 mM DTT and resuspended in PKCζ kinase buffer (50 mM HEPES, pH 7.4, 1 mM EDTA, 1 mM DTT, and 10 mM MgCl2) or Cdk5 kinase buffer (50 mM Tris-HCl, pH 7.4, 5 mM MgCl2, 1 mM DTT, and 1 mM EDTA) with 200 μM unlabeled ATP, 10 μCi [γ-32P]ATP, and 50 ng recombinant PKCζ (Upstate Biotechnology, Lake Placid, NY). The reaction was allowed to proceed for 30 min at 32°C for PKCζ assay and at 25°C for Cdk5 measurement. Reactions were stopped by addition of Laemmli sample buffer and boiling for 5 min. Gels were stained with Coomassie Blue, dried, and subjected to autoradiography. As loading controls, 8-μl amounts of immunoprecipitates were subjected to SDS-PAGE, transferred to nylon membranes, stained with Red Ponceau, and visualized using the ECL Western blotting kit (GE Healthcare).
To measure PKCζ or Cdk5 activity during myoblast differentiation, PKCζ was immunoprecipitated from cell lysates. The kinase reaction was performed as described above using histone H1 (3 μg, Sigma-Adrich) as substrate. Phosphate incorporation into histone H1 was visualized by autoradiography of SDS-PAGE gels.
In Vitro Calpain Assay
After phosphorylation by PKCζ, performed as described above, GSTp35/Hiscdk5 was subjected to calpain cleavage assay using a commercial kit (QIA120; Calbiochem) according to the manufacturer's instructions. After incubation with or without recombinant calpain 1 for 5 min at RT, the reaction was stopped by addition of Laemmli buffer. Samples were separated by SDS-PAGE and subjected to immunoblotting using appropriate antibodies.
Preparation of Brain Extracts and Calpain Activation In Vitro
The brains of 2-d-old FVB-n mice were isolated and homogenized in lysis buffer (20 mM HEPES, pH 7.4, 5 mM KCl, 1.5 mM MgCl2, 1 mM DTT, and 1 mM EGTA), using a Teflon grinder (Sato et al., 2007). Each homogenate was centrifuged at 13,000 × g for 25 min at 4°C. Pellets were resuspended in 100 μl lysis buffer, sonicated, and centrifuged, as described above. Supernatants from each sample were divided into two parts: control and test. To induce calpain activity, 4 mM CaCl2 was added to a sample, and incubation for 1 h at 37°C followed. The reaction was stopped by addition of EGTA to 10 mM and boiling in Laemmli sample buffer for 5 min. C2C12 myoblasts transfected with p35 wild type (WT) and p35 S33A were treated as above described to induce calpain activity. p35 processing was analyzed using Western blotting.
Statistics
Quantitative experiments were analyzed using Student's t test. All p values were obtained using two-sided tests using the GraphPad Prism program (San Diego, CA).
RESULTS
PKCζ Is Required for Myoblast Differentiation
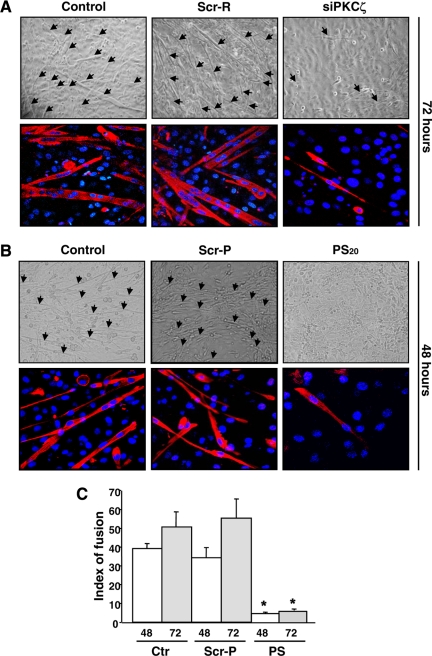
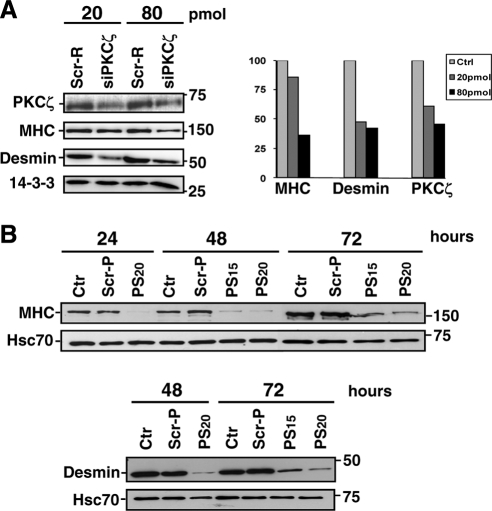
We have previously shown that Cdk5 is a critical regulator of myogenic differentiation (Sahlgren et al., 2003), but it is still unclear how Cdk5 activation is regulated and how Cdk5 interacts with other signaling pathways during differentiation. Because PKCζ has been implicated as a regulator of differentiation (Wooten et al., 2000; Mansat de Mas et al., 2002), we investigated whether PKCζ was involved in Cdk5-mediated myogenic differentiation (Sahlgren et al., 2003). To address this question, we down-regulated PKCζ using siRNA or inhibited PKCζ activity employing a specific PKCζ PS inhibitor (Eichholtz et al., 1993; Zhou et al., 1997; Fishelevich et al., 2006) in both mouse primary myoblasts and the C2C12 myoblast cell line (Figures 1 and 2). Differentiation was induced by reducing the serum content in the medium (2% vol/vol) and was monitored over 72 h by measuring the formation of multinucleated myotubes and the expression of specific differentiation markers, MHC and desmin. Down-regulation of PKCζ by siRNA (siPKCζ) inhibited both primary myoblast fusion (Figure 1A) as well as expression of MHC and desmin (Figures 1A and 2A), whereas scrambled siRNA (Scr-R) had no such effect (Figures 1A and 2A). Likewise, the blocking of PKCζ activity with 15–20 μM PS inhibitor also prevented the differentiation of primary myoblasts, whereas the inactive scrambled peptide (Scr-P) had no effect (Figures 1B and 2B). The fusion index, depicting the number of nuclei within multinucleated myotubes at 48 and 72 h of differentiation, further confirms the significant inhibitory effect of PS inhibitor (Figure 1C). In a similar manner, the inhibition of PKCζ efficiently blocked myogenesis in C2C12 myoblasts (Supplemental Figure 1, A and B), demonstrating that the effect was identical in the two cellular model systems. To confirm that the observed effect was PKCζ-specific, we assessed differentiation in the presence of Calphostin C, an inhibitor of both nPKCs and cPKCs, and found that Calphostin C had no effect on differentiation (Supplemental Figure 1C), compared with siPKCζ or the PKCζ PS inhibitor, both of which completely blocked differentiation. Taken together, our results reveal an essential and specific regulatory role for atypical PKCζ during myogenic differentiation.
Figure 1.
Inhibition of PKCζ expression and activity prevents the formation of myotubes. (A) The down-regulation of PKCζ by siRNA abrogated the myoblast fusion and myotube formation. Primary myoblasts were transfected with PKCζ siRNA (siPKCζ) or scrambled siRNA (Scr-R) and induced to differentiate. At 72 h of differentiation, cells were fixed and double-stained with anti-MHC antibody, and DAPI, to detect differentiated cells and nuclei, respectively. The arrows in the phase-contrast images indicate myotubes. The bottom panels shows fluorescence labeling of MHC (red) and DNA (DAPI, blue), visualized by confocal microscopy. The images reveal a pronounced decrease in cell fusion and myotube formation as well as in MHC expression, when PKCζ is down-regulated. (B) The inhibition of PKCζ activity by a pseudosubstrate inhibitor peptide (PS) blocked the fusion of myoblast to multinucleated myotubes. Mouse primary myoblasts were induced to differentiate in the presence of either the PKCζ inhibitor peptide (PS; 15 or 20 μM) or the scrambled peptide (Scr-P; 20 μM). After 48 h of differentiation, cells were fixed and double-stained with antibodies against MHC and DAPI. Similarly to PKCζ down-regulation by siRNA (Figure 1A), PKCζ inhibition prevented myotube formation. (C) The fusion indexes of mouse primary myoblasts were determined in the presence of both the inhibitor peptide (PS, 20 μM) and the scrambled peptide (Scr-P, 20 μM). The fusion index was determined as a ratio of the number of nuclei in myotubes (showing at least two nuclei) to the total number of nuclei in six randomly chosen microscopic fields. The data are shown as means ± SEM (n = 3; *p < 0.05; Student's t test).
Figure 2.
Inhibition of PKCζ expression and activity prevents myoblast differentiation. (A) The down-regulation of PKCζ by siRNA prevented the myoblast differentiation as indicated by the reduced amount of differentiation markers. Primary myoblasts were transfected with PKCζ siRNA (siPKCζ) or scrambled siRNA (Scr-R) and induced to differentiate. At the indicated time points of differentiation, cells were harvested for Western blot analysis. Desmin and MHC were used as markers of differentiation, whereas 14-3-3 served as a loading control. PKCζ down-regulation induced a decrease in expression of the differentiation markers MHC and desmin, as shown in the histogram. (B) The Inhibition of PKCζ activity by a pseudosubstrate inhibitor peptide (PS) blocked the myoblast differentiation. Mouse primary myoblasts were induced to differentiate in the presence of either the PKCζ inhibitor (PS; 15 or 20 μM) or the scrambled peptide (Scr-P; 20 μM). Cells were harvested at the indicated time points of differentiation and subjected to Western blot analysis. Increasing desmin and MHC expression levels indicated the progress of differentiation, whereas Hsc70 served as a loading control. Similarly to the PKCζ down-regulation by siRNA (Figure 2A), the inhibition of PKCζ activity prevented the myoblast differentiation.
PKCζ Activity Is Up-Regulated during Myogenic Differentiation
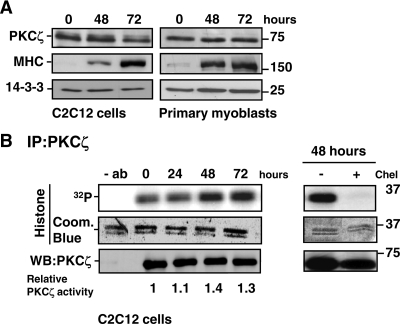
Because the down-regulation and inhibitor experiments indicated a key role for PKCζ in myogenesis, we examined PKCζ expression during the differentiation of both C2C12 cells and mouse primary myoblasts (Figure 3A). Cells were harvested 48 and 72 h after the induction of differentiation, the progress of which was verified by blotting against the differentiation marker, MHC. Immunoblotting revealed that PKCζ levels were relatively stable during differentiation (Figure 3A). As PKCζ expression was constant, we wanted to determine whether PKCζ activity was regulated during differentiation. Indeed, although some activity was detected in proliferating myoblasts, we observed a significant increase in PKCζ activity at 48 h of differentiation (Figure 3B). The PKC inhibitor chelerythrine blocked this activity (Figure 3B), confirming the specificity of the kinase activity assay.
Figure 3.
Expression and activity of PKCζ during myogenesis. (A) The protein levels of PKCζ remained stable during myoblast differentiation. C2C12 cells and primary myoblasts were induced to differentiate, and cells were harvested at the indicated time points. The progress of differentiation was assessed by monitoring the expression of MHC, whereas 14-3-3 served as a loading control. (B) PKCζ activity was up-regulated during myoblast differentiation. C2C12 cells were shifted to differentiation medium, and PKCζ was immunoprecipitated from the cell lysates prepared at the indicated time points. The activity of immunoprecipitated PKCζ was analyzed using histone H1 as a substrate. As a negative control, cell extracts were incubated for 15 min with the PKC inhibitor chelerythrine (Chel, 5 μM) before the activity assay. Coomassie Blue staining was utilized to verify that equal amounts of substrate were used in each reaction, whereas immunoblot analysis confirmed that equal amounts of PKCζ were precipitated from each cell lysate. The blots were scanned on a densitometer to quantitate the amount of PKCζ and the degree of histone 1 phosphorylation. The ratio of 32P-histone 1 and PKCζ at each time point was calculated and normalized to the control values at time point 0 h. The data are representative of three independent experiments.
PKCζ Modulates Cdk5 Activity
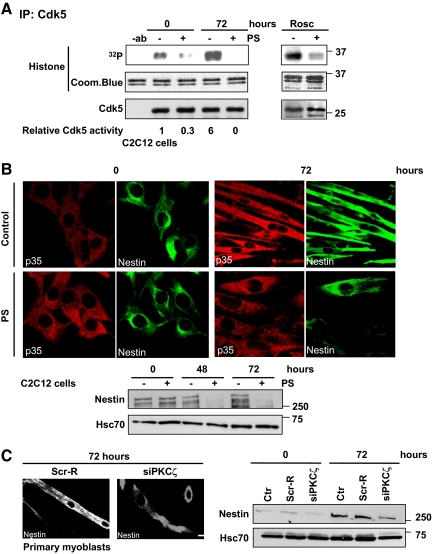
Previous studies have confirmed that Cdk5 is required for myoblast differentiation (Lazaro et al., 1997; Philpott et al., 1997; Sahlgren et al., 2003). Moreover, activation of PKCζ and Cdk5 in differentiating myoblasts showed similar kinetics. To get an indication of the hierarchy between these two kinases, we tested whether roscovitine, a Cdk5 inhibitor, affected PKCζ. However, Cdk5 inhibition had no obvious effect on PKCζ activity (Supplemental Figure 2A), but still efficiently inhibited myoblast differentiation, as indicated by the loss of troponin expression (Supplemental Figure 2B), in line with our previous observations (Sahlgren et al., 2003). As Cdk5 inhibition impaired myoblast differentiation without affecting PKCζ activity, we reasoned that PKCζ is likely to act upstream of Cdk5. To test this hypothesis, we determined whether Cdk5 activity during C2C12 cell differentiation was altered in the presence of the PKCζ-specific PS inhibitor. Cells were harvested at the indicated time points and Cdk5 activity was analyzed by in vitro kinase assay. The results showed that Cdk5 activity was blocked by the PS inhibitor (Figure 4A), demonstrating that PKCζ has the ability to regulate Cdk5 activity and lies upstream of Cdk5 in the sequence of events leading to myoblast differentiation. The specificity of the assay was confirmed using the Cdk5 inhibitor roscovitine (Figure 4A).
Figure 4.
PKCζ modulates Cdk5 activity in vivo and affects the reorganization of nestin filaments during differentiation. (A) The Cdk5 activity was inhibited upon PKCζ inhibition. C2C12 cells were induced to differentiate for 72 h in the presence or absence of the PKCζ pseudosubstrate inhibitor peptide (PS). Cell lysates were subjected to the immunoprecipitation of endogenous Cdk5 and the Cdk5 kinase activity assays were performed using histone H1 as substrate (left). The numbers represent the relative induction in the Cdk5 activity during differentiation in the presence or absence of the inhibitor peptide PS. The data are representative of three independent experiments. As a negative control, cell lysates were preincubated with the Cdk5 inhibitor roscovitine (Rosc; 5 μM) for 15 min before kinase activity assay (right). Immunoblotting confirmed that equal amounts of Cdk5 were immunoprecipitated from each cell lysate. In addition, Coomassie Blue staining showed that equal amounts of histone 1 substrate were used in each reaction. (B) PKCζ activity was necessary for the reorganization of nestin filaments and the regulation of nestin protein levels during differentiation. C2C12 myoblasts were induced to differentiate for 72 h in the presence or absence of the inhibitor peptide (PS). The samples were fixed and double-stained with antibodies recognizing nestin and p35. At 72 h of differentiation, nestin filaments were aligned parallel to myotubes and colocalized with p35. However the Cdk5-mediated reorganization of nestin filaments during differentiation was disturbed when PKCζ activity was inhibited (scale = 10 μm, as in panel C). In parallel cell lysates were prepared from the same experiment, proteins were resolved by SDS-PAGE and analyzed by Western blotting for nestin and Hsc70, the latter serving as a loading control. The immunoblot revealed pronounced decreases in nestin protein levels when PKCζ was inhibited. (C) The down-regulation of PKCζ severely impaired nestin reorganization and stability during differentiation of primary myoblasts. Mouse primary myoblasts were transfected with PKCζ siRNA (siPKCζ) or scrambled siRNA (Scr-R) and induced to differentiate for 72 h. Samples were fixed, stained with anti-nestin antibody (scale bar, 10 μm), and analyzed by Western blotting. The results confirmed alterations in nestin remodeling and turnover observed during differentiation when PKCζ was down-regulated.
PKCζ Regulates the Expression and Reorganization of Nestin
We previously showed that the IF protein nestin acts as a scaffold for Cdk5 (Sahlgren et al., 2003, 2006). In addition, Cdk5 has the ability to regulate its own scaffold, thereby enabling the characteristic reorganization of nestin filaments during myotube formation (Sahlgren et al., 2003). Thus, we wanted to determine whether inhibition of PKCζ affected Cdk5-mediated reorganization of nestin filaments in differentiating myoblasts (Figure 4, B and C; Supplemental Figure 3A). In agreement with our previous observations (Sahlgren et al., 2003), nestin filaments were reorganized, in concert with Cdk5 activation, into longitudinal fibers during differentiation. In contrast, in the presence of the PS inhibitor, nestin reorganization was severely disturbed in both C2C12 cells and in primary myoblasts (Figure 4B; Supplemental Figure 3A). Notably, similar morphological alterations of the nestin network were also observed when PKCζ was down-regulated by siRNA (siPKCζ) in mouse primary myoblasts (Figure 4C). These morphological perturbations of the nestin filament network after inhibition of PKCζ were identical to those previously observed upon Cdk5 inhibition (Sahlgren et al., 2003). This result further supported the proposed function of PKCζ as an upstream regulator of the Cdk5 signaling pathway. Nestin expression, turnover, and stability are also regulated by Cdk5-mediated phosphorylation of nestin (Sahlgren et al., 2003; unpublished results). To investigate the effect of PKCζ inhibition on Cdk5-mediated regulation of nestin in greater detail, nestin protein levels were analyzed in differentiating C2C12 and mouse primary myoblasts (Figure 4, B and C; Supplemental Figure 3B). Inhibition of PKCζ-Cdk5 activity led to a remarkable decrease in nestin protein levels in both C2C12 cells and in primary myoblasts (Figure 4, B and C; Supplemental Figure 3B). These results, employing nestin organization, expression, and stability as a downstream readout of Cdk5 activity, reinforce the hypothesis that PKCζ acts as a regulator of Cdk5 and that the PKCζ–Cdk5 axis is crucial for proper myoblast fusion and simultaneous reorganization and turnover of the nestin cytoskeleton.
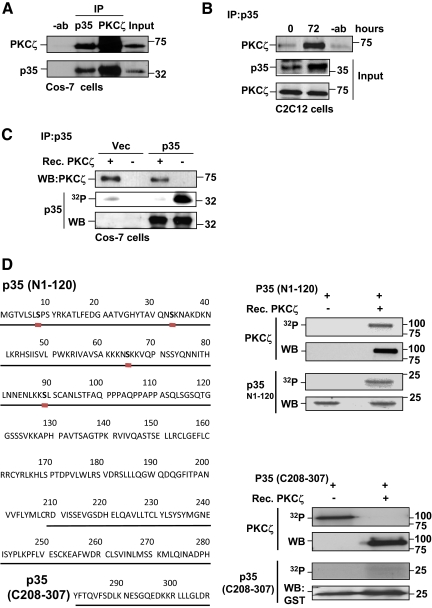
The Cdk5 Activator, p35, Interacts with and Is Phosphorylated by PKCζ
Cdk5 is activated by association with the protein activator p35. As our data positioned PKCζ upstream of Cdk5, PKCζ could influence Cdk5 activity through regulation of p35. To test the possibility of a direct interaction between PKCζ and p35, we performed coimmunoprecipitation experiments. PKCζ was found to be associated with p35 in Cos-7 cells and C2C12 myoblasts (Figure 5, A and B). Interestingly, this association was rather weak in undifferentiated C2C12 cells but was remarkably increased during differentiation (Figure 5B), in concert with raised PKCζ and Cdk5 activities (Figures 3B and 4A). Similar results were obtained using mouse primary myoblasts.
Figure 5.
p35 interacts with and is a target for PKCζ. (A) PKCζ interacted with p35 in Cos-7 cells. Cos-7 cells were transfected with plasmids encoding p35 and PKCζ. Cells were lysed and subjected to immunoprecipitation using either anti-PKCζ or anti-p35 antibodies. Immunoprecipitated samples were separated on SDS-PAGE together with input controls and immunoblotted for PKCζ and p35. The results revealed an interaction between p35 and PKCζ. (B) An interaction between p35 and PKCζ was detected in differentiating myoblasts. C2C12 cells were induced to differentiate, lysed, and subjected to p35 immunoprecipitation. Western blot analysis of immunoprecipitates indicated that p35 and PKCζ interacted during differentiation. (C) p35 was phosphorylated by PKCζ. Cos-7 cells were transfected with p35 or an empty vector. 48 h later, cells were lysed, and p35 was immunoprecipitated. An in vitro phosphorylation assay with recombinant PKCζ was performed using immunoprecipitated p35 as a substrate. The immunoblots demonstrate the amounts of p35 and PKCζ and the autoradiograph the degree of phosphorylation in the different reactions. (D) The N-terminus of p35 contains several putative PKCζ phosphorylation sites (the ProteinScan program was helpful in the prediction of putative phosphorylation sites; see http://156.40.231.198/ProteinScan/ScanProteinForPKCSitesPage.aspx). The N-terminus of p35 was specifically phosphorylated in vitro by PKCζ. In vitro phosphorylation assays were performed with recombinant PKCζ using either truncated N-terminal (N1-120) or C-terminal (C208-307) p35 peptide as a substrate. Proteins were resolved by SDS-PAGE, and the presence of indicated proteins in phosphorylation reactions was monitored by Western blotting. Autoradiographs demonstrate the phosphorylation of p35 peptides as well as the autophosphorylation of PKCζ (indicating kinase activity). Remarkably, only the N-terminus of p35 (N1-120) was specifically phosphorylated by PKCζ.
Given the observed interaction between PKCζ and p35, we explored whether p35 was directly targeted by PKCζ. Judging from the phosphorylation motifs in p35, it contains several potential sites for PKCζ -mediated phosphorylation in its N-terminal region (Figure 5D), but none after Ser-89. By in vitro phosphorylation assay using immunoprecipitated p35 as substrate, we showed that recombinant PKCζ efficiently phosphorylated p35 (Figure 5C). We performed more detailed phosphorylation analysis using two different truncated p35 peptides as substrates. It was observed that the N-terminal (N1-120) portion of p35, containing the predicted phosphorylation sites, was phosphorylated by PKCζ, whereas the C-terminus (C208-307) was not (Figure 5D). To identify the specific phosphorylation sites, a recombinant p35 (Sakaue et al., 2005; Yamada et al., 2007) was phosphorylated in vitro by PKCζ and analyzed by mass spectrometry (Schroeder et al., 2004; Olsen et al., 2005). Serine 33 was identified as a target of PKCζ (Supplemental Figure 4). To further confirm the in vivo relevance of serine 33 phosphorylation, we conducted an in vivo 32P-phosphorylation and phosphopeptide-mapping experiment (Kochin et al., 2006). HEK 293 cells were transfected with constitutively active PKCζ together with either WT p35 or S33A-mutated p35. The transfected cells were metabolically labeled with 32P-orthophosphate, and p35 was immunoprecipitated from the cell lysate, followed by separation on SDS-PAGE and tryptic phosphopeptide mapping (Supplemental Figure 5). Although the in vivo phosphopeptide map of p35 is complex (as it contains the specific Cdk5 sites and a number of other sites for different kinases, with a complexity exceeding that outlined in the literature; data not shown), the comparison of the 2D phosphopeptide maps of WT and S33A-mutated p35 demonstrated loss of the S33-specific phosphopeptide in the sample from S33A-mutated p35 (Supplemental Figure 5B, encircled). This analysis provides additional evidence of the serine 33 being phosphorylated in vivo. Here we demonstrate for the first time that the Cdk5 activator p35 interacts with and is a novel target for PKCζ.
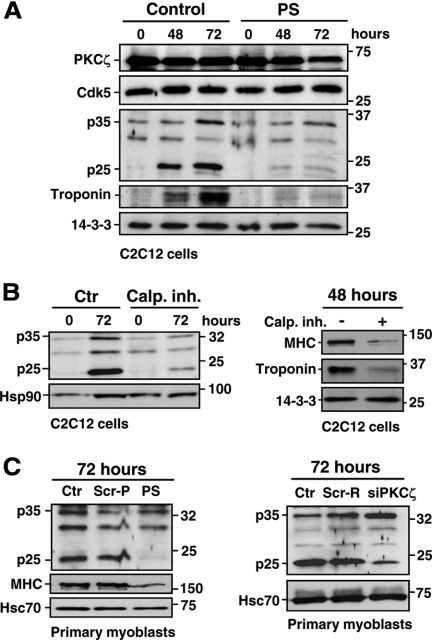
PKCζ Activity Is Essential for Calpain-mediated Cleavage of p35 during Myoblast Differentiation
p35 has been shown to be cleaved by calpains in neuronal cells, leading to generation of a more stable fragment, p25 (Kusakawa et al., 2000). This event has been associated with neurodegenerative processes, as the constitutive hyperactivation of Cdk5 by p25 leads to harmful phosphorylation events (reviewed in Cruz and Tsai., 2004; Lee et al., 2000). Intriguingly, despite the seemingly harmful role of p25, there are reports indicating that p25 is likely to be involved also in normal physiological processes (Fischer et al., 2005; see Discussion for further details). Incited by these observations, we studied whether generation of p25 could be involved in the Cdk5 activation that we had observed during myogenic differentiation. Indeed, in further support of p25 being physiologically relevant, we observed that p35 was cleaved to p25 during differentiation of both C2C12 cells and mouse primary myoblasts (Figure 6A; Supplemental Figure 6A). The identity of the p25 fragment was verified using CaCl2-treated brain lysates as a positive control (Supplemental Figure 6B) and was further confirmed by showing that the p35/p25 bands disappeared upon treatment with a blocking peptide against a p35 antibody that recognizes both the cleaved and uncleaved forms of the protein (Supplemental Figure 6C). Generation of p25 has previously been indicated to be calpain-dependent. To test whether the generation of p25 was calpain-dependent in differentiating myoblasts, we induced the differentiation with or without a calpain inhibitor (calpain inhibitor III). The calpain inhibitor efficiently prevented the formation of the p25 fragment (Figure 6B, left panel), establishing the requirement of calpain also in the differentiation-mediated p25 generation. The physiological requirement for calpain in generating p25 was illustrated by experiments showing that the calpain inhibitor completely blocked differentiation (Figure 6B, right panel) at the same time as it was blocking the generation of p25. Interestingly, this observation is in accordance with previous studies that have indicated an involvement of calpain in myogenic differentiation (Barnoy et al., 1998, 2005; Dourdin et al., 1999; Liang et al., 2006). These results show that p35 is physiologically cleaved by calpains during myoblast differentiation, without inducing cell death and that p25 is an effector molecule generated by calpain.
Figure 6.
PKCζ activity regulates the calpain-mediated cleavage of p35. (A) The calpain-mediated cleavage of p35 during differentiation was prevented by the PKCζ inhibition. C2C12 cells were induced to differentiate in the presence or absence of the pseudosubstrate inhibitor peptide (PS; 20 μM). Cells were harvested at the indicated time points and the corresponding lysates were subjected to immunoblotting using the indicated antibodies. The immunoblot reveals that p35 is cleaved to p25 fragment during the differentiation and that this cleavage is inhibited in the presence of the inhibitor peptide PS. (B) The inhibition of calpain activity by the calpain inhibitor III prevented the p25 generation and inhibited the differentiation of C2C12 myoblasts. C2C12 cells were induced to differentiate in the presence of the calpain inhibitor III (15 μM) and harvested at the indicated time points. Cell lysates were analyzed by Western blotting using the indicated antibodies. (C) The inhibition of PKCζ activity and expression prevents the p35 processing to p25. Primary myoblasts were induced to differentiate in the presence or absence of the inhibitor peptide (PS) or a scrambled peptide (Scr-P; 20 μM; left), or, alternatively, after the transfection with PKCζ siRNA (siPKCζ) or scrambled siRNA (Scr-R; 60 pmol; right). Cell lysates were immunoblotted with the indicated antibodies. Troponin and MHC were used as markers of differentiation, whereas Hsc70, Hsp90, and 14-3-3 served as loading controls.
As aPKC has been shown to activate calpains (Castellani et al., 2006; Xu and Deng 2006), we hypothesized a link between the activities of PKCζ, calpain, and Cdk5, in differentiating myoblasts. Moreover, the predicted PKCζ phosphorylation sites in p35 are located close to the calpain cleavage site. Accordingly, we found that p35 cleavage was prevented by the PS inhibitor during differentiation of both C2C12 and primary myoblasts (Figure 6, A and C). Likewise, the calpain-mediated cleavage of p35 was blocked by siRNA-mediated inhibition of PKCζ expression (siPKCζ) in primary myoblasts (Figure 6C). These results suggest that PKCζ uses calpain-mediated cleavage of p35 to amplify and sustain Cdk5 activation during differentiation.
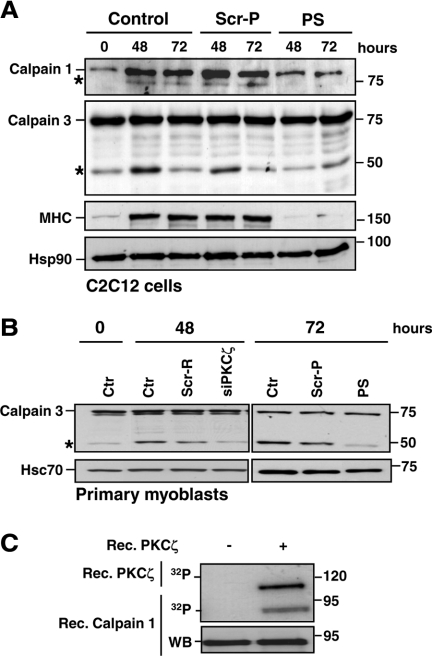
PKCζ Regulates Calpain Activity during Differentiation
Considering the striking effect of PKCζ on p35 processing, we speculated that PKCζ could modulate the p35 cleavage also through direct activation of calpains. To explore this idea, we studied the effect of the PS inhibitor on calpain activity. Mouse C2C12 and primary myoblasts were subjected to differentiation in the presence or absence of the PS inhibitor, and the autocatalytic cleavage of calpains, required for calpain activation, was assessed by Western blot analysis. In support of observations identifying PKCι (an aPKC closely related to PKCζ), as a regulator of calpains (Xu and Deng, 2006), the inhibition of PKCζ also impaired the autocleavage (indicating activation) of both ubiquitous calpain 1 and muscle-specific calpain 3 in C2C12 cells (Figure 7A). Identical effects on calpain 3 cleavage were seen using PKCζ siRNA (siPKCζ) in primary myoblasts (Figure 7B). To further confirm the importance of PKCζ for calpain activation, a luciferase-based calpain activity assay was performed. Similarly to the Western blot results, the inhibition of PKCζ resulted in an overall decrease in calpain activity (Supplemental Figure 7A; Sorimachi et al., 1989). Moreover, we were able to demonstrate that recombinant PKCζ phosphorylates calpain 1 (Figure 7C) and calpain 3 (Supplemental Figure 7B). The prominent interaction between PKCζ, and calpain 3 in myoblasts (Supplemental Figure 7C) further emphasizes a key role of PKCζ as a regulator of calpains. Interestingly, PKCζ inhibition diminished cleavage of another established calpain substrate, RhoA (Kulkarni et al., 2002; Castellani et al., 2006; Supplemental Figure 7D), suggesting that in addition to the generation of p25, PKCζ is likely to affect calpain-mediated cleavage of a number of other calpain substrates involved in muscle differentiation.
Figure 7.
PKCζ regulates the calpain activity during differentiation. (A) PKCζ depletion inhibited the activation of calpains. C2C12 cells were induced to differentiate in the presence of either the pseudosubstrate inhibitor peptide (PS) or scrambled peptide (Scr-P; 20 μM). Samples were harvested at the indicated time points and analyzed by immunoblotting using the indicated antibodies. The activation of calpains during differentiation was monitored by the formation of the auto-cleavage products (denoted by asterisk) of calpain 1 and the muscle-specific isoform, calpain 3. MHC was used as a marker of differentiation, whereas Hsp90 served as a loading control. The immunoblots indicate significant reduction in calpain activation during differentiation when PKCζ activity was inhibited. (B) Mouse primary myoblasts were induced to differentiate after transfection with PKCζ siRNA (siPKCζ) or scrambled siRNA (Scr-R) or in the presence of the pseudosubstrate inhibitor peptide (PS) or a scrambled peptide (Scr-P). Samples were harvested at the indicated time points and subjected to immunoblotting using the indicated antibodies. Hsc70 served as a loading control. The results verified the observations presented in A establishing PKCζ as a regulator of calpains. (C) Calpain 1 is phosphorylated in vitro by PKCζ. A phosphorylation assay was performed with recombinant PKCζ using recombinant calpain 1 as a substrate. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. First, the phosphorylation of calpain 1 as well as the PKCζ autophosphorylation, indicating kinase activity, were detected by autoradiography and, second, the membranes were subjected to Western blot analysis.
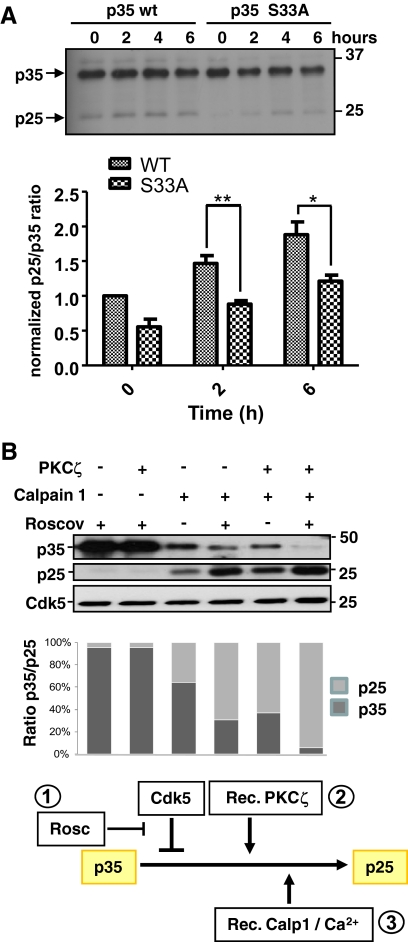
PKCζ Phosphorylation Promotes Calpain-mediated Cleavage of p35
Having shown that p35 was a substrate of PKCζ and that PKCζ activity was necessary for the cleavage of p35 during differentiation, we wanted to determine whether the phosphorylation of p35 by PKCζ promoted its own calpain-mediated cleavage. To verify the role of PKCζ-induced phosphorylation of p35 on serine 33, we performed site-directed mutagenesis to replace serine 33 with alanine residue. The susceptibility of the mutated p35 to the calpain-mediated processing was assessed using two different experimental settings: 1) treatment of C2C12 myoblasts transfected with WT p35 or with p35 S33A with a Ca2+ ionophore or 2) treatment of p35 WT or p35 S33A-transfected C2C12 cell lysates with CaCl2. The Western blot analysis of both experiments revealed p35 S33A to be more resistant to calpain-mediated cleavage than WT p35 (Figure 8A; Supplemental Figure 8). Moreover, to further demonstrate the role of PKCζ on p35 cleavage, we preincubated the Cdk5/p35 complex with recombinant PKCζ, followed by an incubation with recombinant calpain 1 and assessed the p35 cleavage by Western blotting. As shown in Figure 8B, the PKCζ-mediated phosphorylation of p35 significantly promoted the cleavage induced by calpain 1 (cf. lanes 3 and 5). In accordance with previous studies suggesting that Cdk5 phosphorylation protects p35 from calpain-mediated processing (Kamei et al., 2007), Cdk5 inhibition by roscovitine resulted in an accumulation of p25 (lane 4). Here we show that PKCζ appears insensitive to Cdk5-mediated inhibition of p35 cleavage by calpains. These results identify PKCζ as a new master regulator of the p35/Cdk5 complex. The rationale of how the signaling complex would work, as judged from the results of the three different treatments and their combinations is outlined in the scheme below Figure 8B. A more general model of the results in this study is presented in Figure 9. Our findings profile PKCζ as a major upstream regulator of the Cdk5 signaling complex, which is essential for the progression of myogenic differentiation. PKCζ is postulated to have a threefold effect: phosphorylation of p35 into a calpain cleavage-permissive form, boosting calpain activity, and suppressing the autoinhibitory effect of Cdk5 on calpain-mediated p35 cleavage.
Figure 8.
p35 phosphorylation by PKCζ regulates the calpain-mediated cleavage of p35. (A) PKCζ-induced serine 33 phosphorylation of p35 promotes the p35 cleavage. Site-directed mutagenesis was used to replace serine 33 with alanine residue in p35. Plasmids encoding the WT and the S33A-mutated p35 were transfected to C2C12 myoblasts. To activate calpains in proliferating, nondifferentiating C2C12 myoblasts the calcium ionophore A23187 was utilized for the indicated time periods. The generation of p25 was assessed by Western blot analysis. The quantitative data represents mean values of three independent experiments. Mutation of serine 33 resulted in a decrease in the p25 formation indicating that the PKCζ-mediated phosphorylation promotes the calpain-mediated cleavage of p35. (B) PKCζ facilitated the calpain 1–mediated cleavage of p35 and overcame the inhibitory effect of Cdk5. An in vitro assay was conducted to investigate the effect of PKCζ- or Cdk5-mediated phosphorylation on the calpain dependent cleavage of p35. First a recombinant GST-p35/His-Cdk5 complex was incubated either 1) alone (lane 3), 2) with roscovitine (4 μM; lanes 1 and 4) to inhibit the Cdk5-dependent p35 phosphorylation, 3) with recombinant PKCζ (lanes 2 and 5), or 4) with both roscovitine and PKCζ (lane 6). Subsequently, samples were incubated with or without recombinant calpain 1 for 5 min at RT to induce the cleavage of p35. Samples were separated by SDS-PAGE and immunoblotted using anti-p35 antibody. The Cdk5 immunoblot served as a loading control. The quantitative analysis demonstrates the p35-p25 ratios. The results show that calpain 1 treatment alone did not produce as much p25 (lane 3) as when the Cdk5/p35 complex was prephosphorylated with PKCζ (lane 5). The inhibition of Cdk5 activity promoted the generation of p25 depicting the autoinhibitory effect stated in the literature (lane 4; Kamei et al., 2007). Interestingly, PKCζ was insensitive to the Cdk5-induced inhibition and largely increased the calpain-mediated p25 formation. The calpain-dependent generation of the p25 fragment was maximal in the presence of PKCζ and of the Cdk5 inhibitor roscovitine (lane 6). The first two lanes serve as a controls, proving that p25 is not generated in the absence of calpains. The rationale of how the PKCζ-controlled signaling complex would work, as judged from the presented results of the three different treatments and their combinations, is outlined in the scheme below the bottom panel.
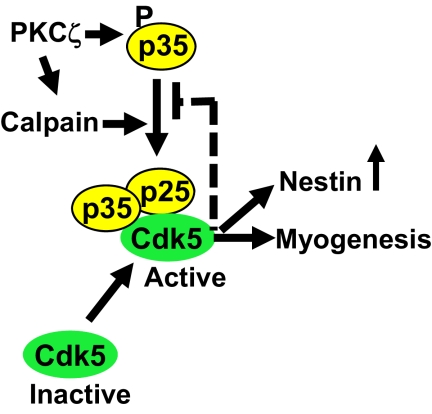
Figure 9.
A tentative model for PKCζ signaling during muscle differentiation. The up-regulation of PKCζ activity during myoblast differentiation promotes the activation of Cdk5 in a calpain-dependent manner. PKCζ phosphorylates the Cdk5 activator protein, p35, and calpains. Consecutively, calpains are activated and trigger the cleavage of the phosphorylated p35 to its more stable fragment, p25, leading to a sustained activation of Cdk5. PKCζ is able to break the autoinhibitory loop, where Cdk5 restrains the p35 processing and p25 generation by phosphorylating p35. Thus, PKCζ is a major upstream regulator of Cdk5 kinase activity essential for the progression of the myogenic differentiation.
DISCUSSION
Skeletal muscle differentiation is a complex, multistep process characterized by the irreversible withdrawal of proliferating myoblasts from the cell cycle, the expression of muscle-specific genes, and the fusion of plasma membranes to enable formation of multinucleated myotubes. Myogenesis is tightly regulated and involves the activation of a number of important signaling determinants orchestrating early differentiation events that lead to the fusion of myoblasts into myotubes and formation of skeletal muscle (reviewed in Stewart and Rittweger, 2006).
Our studies implicate, for the first time, an essential role for aPKCs in myogenesis and demonstrate that PKCζ functions as a critical upstream regulator of the Cdk5 signaling complex in differentiating myoblasts. PKCζ was found to be necessary for both the activity of Cdk5 and subsequent myoblast differentiation. Typical morphological alterations in differentiating myocytes, such as parallel orientation, cell fusion, and formation of myotubes, were completely suppressed by inhibition of PKCζ. Furthermore, PKCζ was essential for reorganization and turnover of the Cdk5 scaffold, nestin (Sahlgren et al., 2003). The latter results are in agreement with a role for PKCζ as an upstream regulator of Cdk5, as Cdk5 is involved in maintaining nestin network organization (Sahlgren et al., 2003) and in modulating nestin stability (our unpublished data).
In the context of the existing PKCζ−/− mouse model there is scarce information about possible effects on the muscle development. The PKCζ−/− mouse has been characterized only in terms of phenotypes related to lymphoid organs and abnormalities in the regulation of NF-κB transcriptional activity (Leitges et al., 2001), the possible effects on other cell types and tissues have not been considered to any greater extent. In addition, there is a distinct possibility that aPKCs could compensate for each other, as the deletion of the atypical lamba and iota PKCs produces an embryonically lethal phenotype (Suzuki et al., 2003), suggesting that aPKCs may compensate for loss of individual isoforms. In light of our results, the PKCζ−/− mouse should obviously be carefully reexamined in terms of possible effects on muscles and myocardium.
Unlike the other Cdks, which require both binding of cyclin proteins and phosphorylation for activation, Cdk5 is activated by association with a protein distantly related to cyclins, p35. p35 expression, in turn, is regulated by phosphorylation events, modifying both subcellular localization and stability, as well as by calpain-mediated cleavage (Kusakawa et al., 2000; Saito et al., 2003). The latter modification leads to the generation of a p25 fragment, the half-life of which is three- to fivefold longer than that of p35. The formation of p25 enables both amplified and spatially less restricted Cdk5 activation as p25 lacks the myristoylation signal, resulting in redistribution of the cleaved protein from the membrane to the cytosol and nucleus (Patrick et al., 1998; Saito et al., 2003; O'Hare et al., 2005; Wei et al., 2005). Until now, the Cdk5/p25 complex has been mainly associated with detrimental effects in neurodegenerative diseases (Patrick et al., 1999; Shelton and Johnson, 2004; Smith et al., 2006). However, despite the accumulated evidence of p25 as potential harmful effector molecule, there are reports implicating that p25 is involved also in normal physiological processes. Transient expression of p25 in hippocampus induces an increase in NMDA (N-methyl-d-aspartate) signaling, spine density, and the number of synapses, and facilitates learning and memory. In contrast, prolonged p25 activity leads to a neuronal loss and severe cognitive defects (Fischer et al., 2005). This discovery shows that p25 is involved as a Cdk5 regulator during normal homeostasis and demonstrates the importance of a tightly controlled spatiotemporal regulation of p25. In agreement with a role for p25 in normal physiological neuronal processes, our study reveals that p35 is cleaved to the p25 fragment during myoblast differentiation and that this cleavage is correlated with an increase in Cdk5 activity. Although p25 generation has usually been linked to apoptotic events, it is becoming increasingly evident that apoptosis and differentiation, two seemingly distinct processes, share several key features. For example, actin fiber disassembly, caspase-3 activation, and increased activity of matrix metalloproteinases are indispensable for membrane fusion occurring during both myoblast differentiation and apoptosis (Fernando et al., 2002). Hence, formation of the Cdk5/p25 complex, with increased subcellular distribution and elevated activity compared with Cdk5/p35, may be crucial for phosphorylation events most likely shared by both processes. Moreover, it has been demonstrated that nuclear localization of Cdk5 defines the post-mitotic state of neurons (Zhang et al., 2008) and that PKCζ, possessing functional import and export signals, undergoes rapid cytonuclear shuttling within neurons (Neri et al., 1999). Therefore, it is tempting to speculate that both increased PKCζ activity and p25 formation are required for nuclear activity of Cdk5, thereby prohibiting differentiating myoblasts from reentering the cell cycle.
p35 is proteolytically cleaved by calpains (Kusakawa et al., 2000). Here, we show that the muscle-specific calpain isoform, calpain 3, interacts with and is a substrate for PKCζ. In addition, we demonstrate that the overall calpain activity is drastically reduced by PKCζ inhibition. Interestingly, a recent publication by Xu and Deng (2006) showed that another aPKC, PKCι, phosphorylated and activated calpains 1 and 2 in human lung cancer cells. In muscle cells, calpains mediate cleavage of various structural proteins, such as talin, desmin, and dystrophin. These cleavage events are necessary for both intracellular proteolysis and the cytoskeletal reorganization required for cell fusion (Dargelos et al., 2002). We demonstrate a novel interaction between p35 and PKCζ in differentiating myoblasts and show that PKCζ phosphorylates p35 on Ser-33, independently of Cdk5, thereby generating a more cleavage-prone p35. Cdk5-mediated phosphorylation has been shown to protect p35 from calpain-induced cleavage (Kamei et al., 2007), whereas PKCζ-mediated phosphorylation of p35 promotes p35 cleavage and overrides the Cdk5-mediated inhibition of such cleavage. These results outline a regulatory mechanism in which PKCζ is a key player, functioning to balance Cdk5/p35 and Cdk5/p25 activities. Such a mechanism is essential for both spatial and temporal control of Cdk5 activity. Considering that both PKCζ and Cdk5 are involved in neuronal differentiation (Coleman and Wooten, 1994; Cruz and Tsai, 2004), it would be worthwhile to assess the role of the PKCζ/Cdk5 signaling pathway in this particular context. Our results describing a role for PKCζ in regulation of calpain activity and p25 generation may have ramifications for the etiology of p25-associated neurodegenerative processes.
Our observations thus point to an essential role of PKCζ in the regulation of overall calpain activity, required for p35 cleavage and muscle differentiation. Although we and others have demonstrated the significance of Cdk5 in myogenesis (Lazaro et al., 1997, Sahlgren et al., 2003), very little is known about the upstream signaling pathway(s) controlling Cdk5. Our work highlights a crucial role for PKCζ during muscle differentiation, in orchestrating both calpain activity and Cdk5 signaling, processes that synergistically promote myogenesis (Figure 9). Although further work is required for a detailed understanding of PKCζ regulatory functions, we reveal for the first time cross-talk between PKCζ and the Cdk5/p35 signaling complex, and describe how these two major signaling pathways work together to control muscle development.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. J. Blenis for providing us with PKCζ-encoding plasmids and Helena Saarento for excellent technical assistance. Dr. Nick Morrice and Robert Gourlay are acknowledged for expert help with the p35 phosphorylation analysis. This work was supported by the Academy of Finland, the Sigrid Jusélius Foundation, the Research Institute of the Åbo Akademi University, and the Foundation of the Åbo Akademi University.
Abbreviations used:
- Cdk5
cyclin-dependent kinase 5
- IF
intermediate filament
- MHC
myosin heavy chain
- PKC
protein kinase C
- PS
pseudosubstrate inhibitor peptide
- Scr-P
scrambled peptide
- Scr-R
scrambled RNAi.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-10-0847) on March 3, 2010.
REFERENCES
- Barnoy S., Glaser T., Kosower N. S. The calpain-calpastatin system and protein degradation in fusing myoblasts. Biochim. Biophys. Acta. 1998;1402:52–60. doi: 10.1016/s0167-4889(97)00144-4. [DOI] [PubMed] [Google Scholar]
- Barnoy S., Maki M., Kosower N. S. Overexpression of calpastatin inhibits L8 myoblast fusion. Biochem. Biophys. Res. Commun. 2005;332:697–701. doi: 10.1016/j.bbrc.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Berra E., Diaz-Meco M. T., Dominguez I., Municio M. M., Sanz L., Lozano J., Chapkin R. S., Moscat J. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell. 1993;74:555–563. doi: 10.1016/0092-8674(93)80056-k. [DOI] [PubMed] [Google Scholar]
- Castellani L., Salvati E., Alema S., Falcone G. Fine regulation of RhoA and Rock is required for skeletal muscle differentiation. J. Biol. Chem. 2006;281:15249–15257. doi: 10.1074/jbc.M601390200. [DOI] [PubMed] [Google Scholar]
- Cheung Z. H., Ip N. Y. Cdk5, mediator of neuronal death and survival. Neurosci. Lett. 2004;361:47–51. doi: 10.1016/j.neulet.2003.12.117. [Review] [DOI] [PubMed] [Google Scholar]
- Cicero S., Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J. Neurosci. 2005;25:9658–9668. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman E. S., Wooten M. W. Nerve growth factor-induced differentiation of PC12 cells employs the PMA-insensitive protein kinase C-zeta isoform. J. Mol. Neurosci. 1994;5:39–57. doi: 10.1007/BF02736693. [DOI] [PubMed] [Google Scholar]
- Cruz J. C., Tsai L. H. A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease. Curr. Opin. Neurobiol. 2004;14:390–394. doi: 10.1016/j.conb.2004.05.002. Review. [DOI] [PubMed] [Google Scholar]
- Dargelos E., Dedieu S., Moyen C., Poussard S., Veschambre P., Brustis J. J., Cottin P. Characterization of the calcium-dependent proteolytic system in a mouse muscle cell line. Mol. Cell Biochem. 2002;231:147–154. doi: 10.1023/a:1014421017461. [DOI] [PubMed] [Google Scholar]
- Dhavan R., Tsai L. H. A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [Review] [DOI] [PubMed] [Google Scholar]
- Dourdin N., Balcerzak D., Brustis J. J., Poussard S., Cottin P., Ducastaing A. Potential m-calpain substrates during myoblast fusion. Exp. Cell Res. 1999;246:433–442. doi: 10.1006/excr.1998.4325. [DOI] [PubMed] [Google Scholar]
- Eichholtz T., de Bont D. B., de Widt J., Liskamp R. M., Ploegh H. L. A myristoylated pseudosubstrate peptide, a novel protein kinase C inhibitor. J. Biol. Chem. 1993;268:1982–1986. [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Fernando P., Kelly J. F., Balazsi K., Slack R. S., Megeney L. A. Caspase 3 activity is required for skeletal muscle differentiation. Proc. Natl. Acad. Sci. USA. 2002;99:11025–11030. doi: 10.1073/pnas.162172899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Sananbenesi F., Pang P. T., Lu B., Tsai L. H. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Fishelevich R., Malanina A., Luzina I., Atamas S., Smyth M. J., Porcelli S. A., Gaspari A. A. Ceramide-dependent regulation of human epidermal keratinocyte CD1d expression during terminal differentiation. J. Immunol. 2006;176:2590–2599. doi: 10.4049/jimmunol.176.4.2590. [DOI] [PubMed] [Google Scholar]
- Fu A. K., Fu W. Y., Cheung J., Tsim K. W., Ip F. C., Wang J. H., Ip N. Y. Cdk5 is involved in neuregulin-induced AChR expression at the neuromuscular junction. Nat. Neurosci. 2001;4:374–381. doi: 10.1038/86019. [DOI] [PubMed] [Google Scholar]
- Gao L., Joberty G., Macara I. G. Assembly of epithelial tight junctions is negatively regulated by Par6. Curr. Biol. 2002;12((3)):221–225. doi: 10.1016/s0960-9822(01)00663-7. [DOI] [PubMed] [Google Scholar]
- Guo H., Gu F., Li W., Zhang B., Niu R., Fu L., Zhang N., Ma Y. Reduction of protein kinase C zeta inhibits migration and invasion of human glioblastoma cells. J. Neurochem. 2009;109:203–213. doi: 10.1111/j.1471-4159.2009.05946.x. [DOI] [PubMed] [Google Scholar]
- Hirai T., Chida K. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J. Biochem. 2003;133:1–7. doi: 10.1093/jb/mvg017. [Review] [DOI] [PubMed] [Google Scholar]
- Hofmann J. The potential for isoenzyme-selective modulation of protein kinase C. FASEB J. 1997;11:649–669. doi: 10.1096/fasebj.11.8.9240967. [Review] [DOI] [PubMed] [Google Scholar]
- Kamei H., Saito T., Ozawa M., Fujita Y., Asada A., Bibb J. A., Saido T. C., Sorimachi H., Hisanaga S. Suppression of calpain-dependent cleavage of the CDK5 activator p35 to p25 by site-specific phosphorylation. J. Biol. Chem. 2007;282:1687–1694. doi: 10.1074/jbc.M610541200. [DOI] [PubMed] [Google Scholar]
- Kochin V., Imanishi S. Y., Eriksson J. E. Fast track to a phosphoprotein sketch - MALDI-TOF characterization of TLC-based tryptic phosphopeptide maps at femtomolar detection sensitivity. Proteomics. 2006;6:5676–5682. doi: 10.1002/pmic.200600457. [DOI] [PubMed] [Google Scholar]
- Kulkarni S., Goll D. E., Fox J. E. Calpain cleaves RhoA generating a dominant-negative form that inhibits integrin-induced actin filament assembly and cell spreading. J. Biol. Chem. 2002;277:24435–24441. doi: 10.1074/jbc.M203457200. [DOI] [PubMed] [Google Scholar]
- Kusakawa G., Saito T., Onuki R., Ishiguro K., Kishimoto T., Hisanaga S. Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J. Biol. Chem. 2000;275:17166–17172. doi: 10.1074/jbc.M907757199. [DOI] [PubMed] [Google Scholar]
- Lazaro J. B., Kitzmann M., Poul M. A., Vandromme M., Lamb N. J., Fernandez A. Cyclin dependent kinase 5, cdk5, is a positive regulator of myogenesis in mouse C2 cells. J. Cell Sci. 1997;110:1251–1260. doi: 10.1242/jcs.110.10.1251. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Kwon Y. T., Li M., Peng J., Friedlander R. M., Tsai L. H. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Leitges M., Sanz L., Martin P., Duran A., Braun U., García J. F., Camacho F., Diaz-Meco M. T., Rennert P. D., Moscat J. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol. Cell. 2001;8:771–780. doi: 10.1016/s1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- Lew J., Qi Z., Huang Q. Q., Paudel H., Matsuura I., Matsushita M., Zhu X., Wang J. H. Structure, function, and regulation of neuronal Cdc2-like protein kinase. Neurobiol. Aging. 1995;16:263–268. doi: 10.1016/0197-4580(95)00014-6. [DOI] [PubMed] [Google Scholar]
- Liang Y. C., Yeh J. Y., Forsberg N. E., Ou B. R. Involvement of mu- and m-calpains and protein kinase C isoforms in L8 myoblast differentiation. Int. J. Biochem. Cell Biol. 2006;38:662–670. doi: 10.1016/j.biocel.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Liu Q., Ning W., Dantzer R., Freund G. G., Kelley K. W. Activation of protein kinase C-zeta and phosphatidylinositol 3′-kinase and promotion of macrophage differentiation by insulin-like growth factor-I. J. Immunol. 1998;160:1393–1401. [PubMed] [Google Scholar]
- Mansat de Mas V., de Thonel A., Gaulin V., Demur C., Laurent G., Quillet-Mary A. Protein kinase C-zeta overexpression induces erythroid phenotype in the monocytic leukaemia cell line U937. Br. J. Haematol. 2002;118:646–653. doi: 10.1046/j.1365-2141.2002.03625.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y., Yamauchi J., Chan J. R., Okada A., Tomooka Y., Hisanaga S., Tanoue A. Cdk5 regulates differentiation of oligodendrocyte precursor cells through the direct phosphorylation of paxillin. J. Cell Sci. 2007;120:4355–4366. doi: 10.1242/jcs.018218. [DOI] [PubMed] [Google Scholar]
- Neri L. M., Martelli A. M., Borgatti P., Colamussi M. L., Marchisio M., Capitani S. Increase in nuclear phosphatidylinositol 3-kinase activity and phosphatidylinositol (3,4,5) trisphosphate synthesis precede PKC-zeta translocation to the nucleus of NGF-treated PC12 cells. FASEB J. 1999;13:2299–22310. [PubMed] [Google Scholar]
- Nguyen C., Hosokawa T., Kuroiwa M., Ip N. Y., Nishi A., Hisanaga S., Bibb J. A. Differential regulation of the Cdk5-dependent phosphorylation sites of inhibitor-1 and DARPP-32 by depolarization. J. Neurochem. 2007;103:1582–1593. doi: 10.1111/j.1471-4159.2007.04868.x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;7:484–496. [Review] [PubMed] [Google Scholar]
- O'Hare M. J., Kushwaha N., Zhang Y., Aleyasin H., Callaghan S. M., Slack R. S., Albert P. R., Vincent I., Park D. S. Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. J. Neurosci. 2005;25:8954–8966. doi: 10.1523/JNEUROSCI.2899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J. V., de Godoy L.M.F., Li G., Macek B., Mortensen P., Pesch R., Makarov A., Lange O., Horning S., Mann M. Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell. Proteom. 2005;4:2010–2021. doi: 10.1074/mcp.T500030-MCP200. [DOI] [PubMed] [Google Scholar]
- Patrick G. N., Zhou P., Kwon Y. T., Howley P. M., Tsai L. H. p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway. J. Biol. Chem. 1998;273:24057–24064. doi: 10.1074/jbc.273.37.24057. [DOI] [PubMed] [Google Scholar]
- Patrick G. N., Zukerberg L., Nikolic M., de la Monte S., Dikkes P., Tsai L. H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Philpott A., Porro E. B., Kirschner M. W., Tsai L. H. The role of cyclin-dependent kinase 5 and a novel regulatory subunit in regulating muscle differentiation and patterning. Genes Dev. 1997;11:1409–1421. doi: 10.1101/gad.11.11.1409. [DOI] [PubMed] [Google Scholar]
- Rosales J., Han B., Lee K. Y. Cdk7 functions as a cdk5 activating kinase in brain. Cell Physiol. Biochem. 2003;13:285–296. doi: 10.1159/000074543. [DOI] [PubMed] [Google Scholar]
- Sahlgren C. M., Mikhailov A., Vaittinen S., Pallari H. M., Kalimo H., Pant H. C., Eriksson J. E. Cdk5 regulates the organization of Nestin and its association with p35. Mol. Cell. Biol. 2003;23:5090–5106. doi: 10.1128/MCB.23.14.5090-5106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlgren C. M., Pallari H. M., He T., Chou Y. H., Goldman R. D., Eriksson J. E. A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. EMBO J. 2006;25:4808–4819. doi: 10.1038/sj.emboj.7601366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue F., Saito T., Sato Y., Asada A., Ishiguro K., Hasegawa M., Hisanaga S. Phosphorylation of FTDP-17 mutant tau by cyclin-dependent kinase 5 complexed with p35, p25, or p39. J. Biol. Chem. 2005;280:31522–31529. doi: 10.1074/jbc.M504792200. [DOI] [PubMed] [Google Scholar]
- Saito T., Onuki R., Fujita Y., Kusakawa G., Ishiguro K., Bibb J. A., Kishimoto T., Hisanaga S. Developmental regulation of the proteolysis of the p35 cyclin-dependent kinase 5 activator by phosphorylation. J. Neurosci. 2003;25:8954–8966. doi: 10.1523/JNEUROSCI.23-04-01189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., et al. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron. 2002;35:907–920. doi: 10.1016/s0896-6273(02)00857-7. [DOI] [PubMed] [Google Scholar]
- Sato K., Zhu Y. S., Saito T., Yotsumoto K., Asada A., Hasegawa M., Hisanaga S. Regulation of membrane association and kinase activity of Cdk5–p35 by phosphorylation of p35. J. Neurosci. Res. 2007;85:3071–3078. doi: 10.1002/jnr.21438. [DOI] [PubMed] [Google Scholar]
- Schnack C., Hengerer B., Gillardon F. Identification of novel substrates for Cdk5 and new targets for Cdk5 inhibitors using high-density protein microarrays. Proteomics. 2008;8:1980–1986. doi: 10.1002/pmic.200701063. [DOI] [PubMed] [Google Scholar]
- Schroeder M. J., Shabanowitz J., Schwartz J. C., Hunt D. F., Coon J. J. A neutral loss activation method for improved phosphopeptide sequence analysis by quadrupole ion trap mass spectrometry. Anal. Chem. 2004;76:3590–3598. doi: 10.1021/ac0497104. [DOI] [PubMed] [Google Scholar]
- Seo H. R., Kim J., Bae S., Soh J. W., Lee Y. S. Cdk5-mediated phosphorylation of c-Myc on Ser-62 is essential in transcriptional activation of cyclin B1 by cyclin G1. J. Biol. Chem. 2008;283:15601–15610. doi: 10.1074/jbc.M800987200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton S. B., Johnson G. V. Cyclin-dependent kinase-5 in neurodegeneration. J. Neurochem. 2004;88:1313–1326. doi: 10.1111/j.1471-4159.2003.02328.x. Review. [DOI] [PubMed] [Google Scholar]
- Smith P. D., Mount M. P., Shree R., Callaghan S., Slack R. S., Anisman H., Vincent I., Wang X., Mao Z., Park D. S. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J. Neurosci. 2006;26:440–447. doi: 10.1523/JNEUROSCI.2875-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorimachi H., Imajoh-Ohmi S., Emori Y., Kawasaki H., Ohno S., Minami Y., Suzuki K. Molecular cloning of a novel mammalian calcium-dependent protease distinct from both m and mu-types. Specific expression of the mRNA in skeletal muscle. J. Biol. Chem. 1989;264:20106–20111. [PubMed] [Google Scholar]
- Stewart C. E., Rittweger J. Adaptive processes in skeletal muscle: molecular regulators and genetic influences. J. Musculoskelet. Neuronal. Interact. 2006;6:73–86. [Review] [PubMed] [Google Scholar]
- Suzuki A., Yamanaka T., Hirose T., Manabe N., Mizuno K., Shimizu M., Akimoto K., Izumi Y., Ohnishi T., Ohno S. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J. Cell Biol. 2001;52:1183–1196. doi: 10.1083/jcb.152.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Akimoto K., Ohno S. Protein kinase C lambda/iota (PKClambda/iota): a PKC isotype essential for the development of multicellular organisms. J. Biochem. 2003;133(1):9–16. doi: 10.1093/jb/mvg018. [Review] [DOI] [PubMed] [Google Scholar]
- Ways D. K., Posekany K., deVente J., Garris T., Chen J., Hooker J., Qin W., Cook P., Fletcher D., Parker P. Overexpression of protein kinase C-zeta stimulates leukemic cell differentiation. Cell Growth Differ. 1994;5:1195–1203. [PubMed] [Google Scholar]
- Wei F. Y., Nagashima K., Ohshima T., Saheki Y., Lu Y. F., Matsushita M., Yamada Y., Mikoshiba K., Seino Y., Matsui H., Tomizawa K. Cdk5-dependent regulation of glucose-stimulated insulin secretion. Nat. Med. 2005;11:1104–1108. doi: 10.1038/nm1299. [DOI] [PubMed] [Google Scholar]
- Wooten M. W., Seibenhener M. L., Neidigh K. B., Vandenplas M. Mapping of atypical protein kinase C within the nerve growth factor signaling cascade: relationship to differentiation and survival of PC12 cells. Mol. Cell. Biol. 2000;20:4494–4504. doi: 10.1128/mcb.20.13.4494-4504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten M. W., Seibenhener M. L., Mamidipudi V., Diaz-Meco M. T., Barker P. A., Moscat J. The atypical protein kinase C interacting protein p62 is a scaffold for NFkB. J. Biol. Chem. 2001;276:7709–7712. doi: 10.1074/jbc.C000869200. [DOI] [PubMed] [Google Scholar]
- Xu L., Deng X. Protein kinase Ciota promotes nicotine-induced migration and invasion of cancer cells via phosphorylation of micro- and m-calpains. J. Biol. Chem. 2006;281:4457–4466. doi: 10.1074/jbc.M510721200. [DOI] [PubMed] [Google Scholar]
- Yamada M., Saito T., Sato Y., Kawai Y., Sekigawa A., Hamazumi Y., Asada A., Wada M., Doi H., Hisanaga S. Cdk5–p39 is a labile complex with the similar substrate specificity to Cdk5–p35. J. Neurochem. 2007;102:1477–1487. doi: 10.1111/j.1471-4159.2007.04505.x. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cicero S. A., Wang L., Romito-Digiacomo R. R., Yang Y., Herrup K. Nuclear localization of Cdk5 is a key determinant in the postmitotic state of neurons. Proc. Natl. Acad. Sci. USA. 2008;105:8772–8777. doi: 10.1073/pnas.0711355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Seibenhener M. L., Wooten M. W. Nucleolin is a protein kinase C-zeta substrate. Connection between cell surface signaling and nucleus in PC12 cells. J. Biol. Chem. 1997;272:31130–31137. doi: 10.1074/jbc.272.49.31130. [DOI] [PubMed] [Google Scholar]
- Zukerberg L. R., Patrick G. N., Nikolic M., Humbert S., Wu C. L., Lanier L. M., Gertler F. B., Vidal M., Van Etten R. A., Tsai L. H. Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase up-regulation, and neurite outgrowth. Neuron. 2000;26:633–634. doi: 10.1016/s0896-6273(00)81200-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.